Abstract
Background
MyD88 is an adaptor molecule in Toll-like receptor and interleukin 1 receptor signaling implicated in tumorigenesis through proinflammatory mechanisms. We have recently reported that MyD88 also directly promotes optimal activation of the Ras/Erk pathway. Here we investigate MyD88 implication in the maintenance of the transformation of Ras-dependent tumors.
Methods
RNA interference was used to inhibit MyD88 expression in the colon cancer cell lines HCT116 and LS513. Apoptosis, DNA damage, p53 function, ERCC1 levels, and Ras and inflammatory signaling pathways were analyzed. Using in vitro assays and xenotransplantation in nude mice (five per group), HCT116 tumor growth was assessed following MyD88 knockdown in presence or absence of chemotherapy.
Results
MyD88 exerts antiapoptotic functions in colon cancer cells via the Ras/Erk, but not the NF-κB, pathway. MyD88 inhibition leads to defective ERCC1-dependent DNA repair and to accumulation of DNA damage, resulting in cancer cell death via p53. Furthermore, we show that knocking down MyD88 sensitizes cancer cells to genotoxic agents such as platinum salts in vitro and in vivo. Indeed, HCT116 tumor growth following treatment with a combination of suboptimal MyD88 inhibition and suboptimal doses of cisplatin (fold tumor increase = 5.4±1.6) was statistically significantly reduced in comparison to treatment with doxycycline alone (12.4±3.1) or with cisplatin alone (12.5±2.6) (P = .005 for both, one-sided Student t test).
Conclusions
Collectively, these results indicate a novel and original link between inflammation, DNA repair, and cancer, and provide further rationale for MyD88 as a potential therapeutic target in Ras-dependent cancers, in the context of concomitant genotoxic chemotherapy.
To ensure recognition of a broad variety of pathogens, the innate immune system has evolved, through a number of receptors, a strategy to recognize a limited number of conserved pathogen-associated molecular patterns. These receptors include the Toll-like receptors (TLRs), which are transmembrane receptors expressed in a variety of immune, but also epithelial and transformed cells (1). TLRs are connected to the cell signaling machinery via intracellular adaptor molecules. The first such adaptor molecule to be discovered was MyD88, which has an N-terminal death domain (DD), which recruits downstream signaling molecules (2). MyD88 is also an adaptor of the interleukin 1 receptor (IL-1R) family signaling. Activation of the TLR/IL-1R signaling pathway activates the major inflammatory transcription factor NF-kB by allowing its nuclear translocation.
Inflammation is recognized as a promoter of carcinogenesis (3). Predictably, MyD88 was shown to play a role in tumorigenesis via TLR and IL-1 proinflammatory mechanisms (4). We have recently shown that MyD88 operates as an adaptor connecting inflammatory signaling pathways with the Ras oncogenic signaling pathway. Specifically, we showed that MyD88 is required for Ras-dependent cell signaling and transformation (5). Here we show in a panel of Ras-dependent colon cancer cell lines that, in addition to its role in tumor initiation, MyD88 plays an important role in the survival of Ras-transformed cells. We demonstrate that MyD88 is required for the expression of the major DNA repair enzyme ERCC1, and therefore for efficient DNA repair, and that knocking down MyD88 sensitizes colon cancer cells to genotoxic agents such as platinum salts in vitro and in vivo. These results indicate a novel and original link between inflammation, DNA repair, and cancer.
Materials and Methods
Cell Lines
Lovo, Sw48, LS513, and LS174T colon cancer cell lines were authenticated by short tandem repeat profiling by American Type Culture Collection and expanded upon receipt. They were cultured in Dulbecco’s modified Eagle medium/10% fetal calf serum (FCS; Invitrogen, Saint-Aubin, France). HCT116 p53+/+, HCT116 p53–/– cells, obtained from P. Hainaut (Lyon, France) were ascertained at thawing based on their differential expression of p53 and p21. HCT116 cells being highly unstable, only early-passage isolates (maximum of five) were used, and all key functional data were confirmed with another cell line (LS513). Culture was performed in McCoy medium (Invitrogen, Saint-Aubin, France)/10% FCS.
Small Interfering RNA (siRNA) and Short Hairpin RNA (shRNA) Sequences
siRNA and shRNA was purchased from Thermo-Fisher (Waltham, MA). MyD88 siRNA sequence 1: 5′-GGAAUGUGACUUCCA GACCUU-3′, MyD88 siRNA sequence 2: 5′-AUUUGCACUCAG CCUCUCUUUUU-3′. p53 siRNA: 5′-CAAUGGUUCACUGAA GACC-3′. p65 (RelA) siRNA: 5′-GAUCAAUGGCUACACAGG A-3′. shMyD88: Sense CGGACCCTAAATCCAATAGAAA. Spacer: TAGTGAAGCCACAGATGTA. Antisense: TTTCTATT GGATTTAGGGTCCT.
Transfection
Cell (250000) transfections with siRNA were performed using 3–5 μg Lipofectamine 2000 (Invitrogen, Saint-Aubin, France). MyD88 siRNA was used at 100nM, p53 siRNA at 200nM, and p65 (RelA) at 20nM. DNA transfections were performed using Fugene 6 (Roche, Basel, Switzerland) at a ratio of 1:3 (DNA/Fugene) with 1 µg of DNA per well.
shRNA Induction
A total of 250000 cells of HCT116 p53+/+ or p53–/– stably expressing a doxycycline-inducible nonsilencing or human shMyD88 were treated with doxycycline (Sigma, Saint-Quentin, France) at 4 µg/mL. Cells were in the desired experiment 48 hours after transfection.
Flow Cytometry
For apoptosis, 200000 Lovo, Sw48, LS513, LS174T, HCT116 p53+/+, and HCT116 p53–/– cells were stained with Annexin V–fluorescein isothiocyanate (FITC) and propidium iodide (Apoptosis Detection Kit, Abcys, Courtaboeuf, France). In brief, cells were pelleted, then resuspended in Annexin V-FITC (2.5 µL/condition) and propidium iodide (1 µg/mL). DNA damage was detected by pS139 H2AX (Millipore, Darmstadt, Germany). Cells were fixed in paraformaldehyde 4%, then permeabilized in Triton 0.5%. Cells were incubated with the primary antibody at 1/800, then with an AlexaFluor 488–coupled secondary antibody at 1/800. Fluorescence was read on a BD FACScalibur and analyzed using CellQuest (Becton Dickinson, Franklin Lakes, NJ).
In Vitro Combination Assays
In vitro combination tests were performed on the HCT116 p53+/+ or p53–/– shMyD88 cell lines, where MyD88 extinction is obtained after 48 hours of doxycycline treatment. Cells were left untreated, or were treated with doxycycline alone, with chemotherapy alone, or with both. The agents and concentrations used are listed in the legend to Table 1. Viability was measured by an MTS assay (Promega, Madison, WI). Data were analyzed using CompuSyn software (Paramus, NJ).
Table 1.
Combination index for various combinations of MyD88 silencing (doxycycline treatment) and chemotherapy agents in HCT116 p53+/+ or p53–/– cells expressing a doxycycline-inducible MyD88 short hairpin RNA (shMyD88)
| Drug* | HCT116 p53+/+ shMyD88 | HCT116 p53–/– shMyD88 | ||
|---|---|---|---|---|
| Combination | ED50 | ED90 | ED50 | ED90 |
| Doxycycline + cisplatin | 0.81 | 0.55 | 0.784 | 0.33 |
| Doxycycline + oxaliplatin | 1.32 | 0.35 | 2.57 | 0.79 |
| Doxycycline + etoposide | 1.30 | 3.88 | 6.94 | 1.54 |
| Doxycycline + paclitaxel | 1.72 | 6.15 | 3.18 | 3.32 |
* The concentrations used are as follows: doxycycline: 0, 1, 2, 4, 8, and 16 µg/mL; cisplatin: 0, 6.2, 12.5, 25, 50, and 100 µM; oxaliplatin: 0, 8.7, 17.5, 35, 70, and 140 µM; etoposide: 0, 520.5, 1041, 2082, 4164, and 8328 nM; paclitaxel: 0, 4.5, 9, 18, 36, and 72 nM. ED50 = effective dose that kills 50% of the cells; ED90 = effective dose that kills 90% of the cells.
Xenografts
Cells were subcutaneously injected into the flank of 6-week-old female BALB/c-nude mice (Charles River, Les Oncins, France). When tumors reached 200mm3, mice were given 3% glucose in drinking water; or doxycycline (Sigma, Saint-Quentin, France) at 2mg/mL in drinking water containing 3% glucose. For in vivo combination assays, mice were given 3% glucose in drinking water; doxycycline at 1mg/mL in drinking water containing 3% glucose, cisplatin (i.p.) at 0.5mg/kg every two days; or both doxycycline and cisplatin at the concentrations and modes of adminis tration described above. Tumor volume was measured every three days with an electronic caliper. Mice were killed when their tumors reached 2000mm3 or when moribund. Mice were housed in ANICAN, our specific pathogen-free animal facility. The experiments were performed in accordance with European Union guidelines and validated by the local Animal Ethics Evaluation Committee (CECCAPP).
Immunohistochemistry
Tunnel analysis was performed on paraffin sections using the In Situ Cell Death Detection Kit (Roche Diagnostics) as per the manufacturer’s instructions.
Statistical Analysis
All experiments in this study were performed at least three times except xenograft experiments, which were carried out twice with five mice in each group, with similar results. Statistical tests were done using Student t test. A P value of <.05 was considered statistically significant. All statistical tests were one-sided because the assumption was made in advance that the results would only be in one direction. This assumption was proven correct by the data. Synergistic interactions were calculated using the Compusym software (Paramus, NJ) at 90% efficient death level.
Results
MyD88 Silencing and Cancer Cell Viability In Vitro
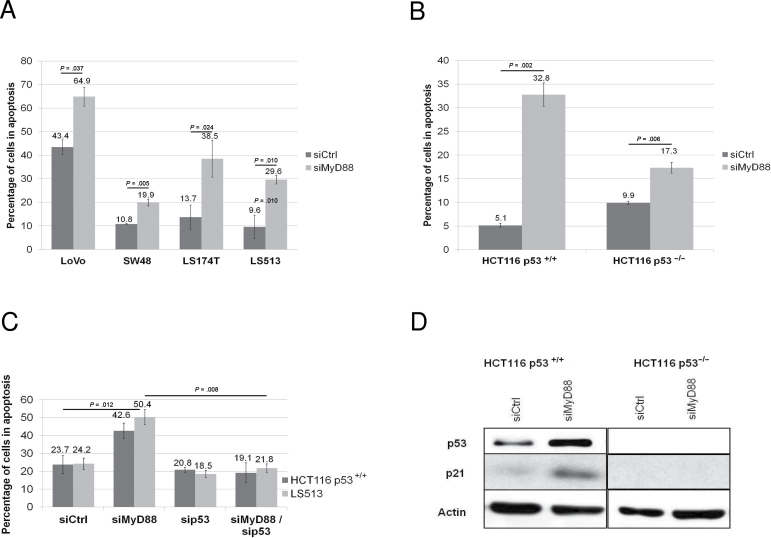
To investigate the role for MyD88 in the maintenance of transformation of Ras-dependent tumor cells, we performed siRNA experiments targeting MyD88 in colon cancer cell lines bearing activating mutations in the Ras oncogene and a functional p53 (6,7). As shown in Figure 1A, MyD88 silencing (controls in Supplementary Figure 1A, available online) induces apoptosis in all four colon cancer cell lines (all RNA interference extinction controls are found in the Supplementary Material, available online). To investigate a possible implication of p53 in the apoptosis observed upon MyD88 silencing, we used the isogenic HCT116 colon cancer cell lines (p53+/+ and p53–/–) (8). We observed that MyD88 silencing (controls in Supplementary Figure 1B, available online) induces substantially higher apoptosis levels in HCT116 p53+/+ compared to HCT116 p53–/– cells (Figure 1B). To ascertain that the difference in MyD88 knockdown-mediated apoptosis in these two lines is exclusively due to their p53 status, we knocked down MyD88 and p53, either separately or simultaneously, in HCT116 p53+/+ but also in LS513 cell lines. We observed that simultaneous extinction of MyD88 and p53 (controls in Supplementary Figure 1C, available online) completely reversed the apoptosis caused by MyD88 silencing in both cell lines, thereby confirming that this apoptosis is strictly dependent on p53 (Figure 1C).
Figure 1.
Effect of MyD88 silencing p53-dependent apoptosis. A) Apoptosis of colon cancer cell lines upon MyD88 silencing by small interfering RNA (siRNA). B) Apoptosis of HCT116 p53+/+ or p53–/– cell lines upon MyD88 silencing by siRNA. C) Apoptosis of HCT116 p53+/+ or LS513 (p53WT) transfected with MyD88-specific siRNA (siMyD88) in presence or absence of p53-specific siRNA. D) Western blot analysis of the expression of p53 and p21 proteins in HCT116 p53+/+ or p53–/– cells upon MyD88 silencing by siRNA. Each experiment was performed three independent times, with each condition done in triplicate. Error bars indicate standard deviation. For statistical analysis, one-sided Student t test was used, with P values indicated in the figure. siCtrl = non-silencing siRNA.
Moreover, an overexpression of p53 and p21 proteins was observed in HCT116 p53+/+ upon MyD88 silencing (Figure 1D). The stabilization of p53 and the overexpression of p21 are well-known signatures of the activation of the p53 pathway (7). Furthermore, to rule out the possibility that the observed apoptosis is dependent on the MyD88 siRNA sequence used and off-target effects, we performed MyD88 silencing experiments using a second siRNA sequence targeting MyD88, and obtained similar results to those obtained with the first siRNA sequence (Supplementary Figure 1D, available online).
MyD88 Silencing and Tumor Growth In Vivo
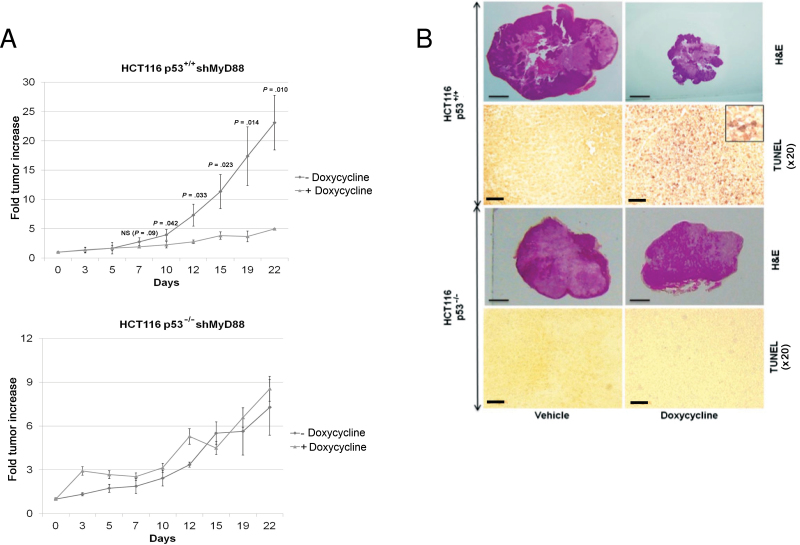
We then investigated the in vivo relevance of our in vitro observations. In order to avoid potential hurdles generally associated with systemic injection of siRNA into mice, we generated by viral transduction HCT116 p53+/+ or p53–/– cell lines stably expressing a doxycycline-inducible nonsilencing shRNA (shNS) or MyD88 shRNA (shMyD88). First, the four resulting HCT116 cell lines were tested for apoptosis induction upon doxycycline treatment. As shown in Supplementary Figure 2A (available online), apoptosis induction was restricted to HCT116 cell lines expressing shMyD88, but not shNS. We then performed xenograft experiments in which HCT116 p53+/+ or p53–/– cell lines expressing shMyD88 were implanted subcutaneously into nude mice. Only in HCT116 p53+/+ cells, a marked reduction in tumor growth (fold tumor increase: 4.9±0.1 vs 23.1±4.7; P = .01) (Figure 2A), accompanied by TUNEL staining (Figure 2B), was observed upon doxycycline administration, indicating that p53 is required for MyD88 knockdown-mediated apoptosis of cancer cells in vivo. To rule out the possibility that the different outcomes of MyD88 knockdown are due to the differences in growth kinetics (Figure 2A), this parameter was measured in five cell lines used in this study. We found that HCT116 p53–/– and LS513 cells had similarly slow rates of proliferation (Supplementary Figure 2B, available online). However, the susceptibility of LS513 cells is similar to that of HTC116 p53+/+, and not that of p53–/–, cells (Figure 1, A and C). This makes it highly unlikely that the difference in the proapoptotic effect of MyD88 inhibition between p53+/+ and p53–/– cells is due to differences in growth kinetics.
Figure 2.
Effect of MyD88 silencing on p53-dependent tumor inhibition in vivo. A) Growth of HCT116 p53+/+ or p53–/– cells expressing doxycycline-inducible MyD88 short hairpin RNA (shMyD88) implanted subcutaneously in nude mice, treated or not treated with doxycycline. B) Hematoxylin and eosin (H&E; scale bar = 3mm), and TUNEL staining (scale bar = 100 μm) on tumor sections from mice described in (A). Inset, high magnification of the TUNEL staining in HCT116 p53+/+ tumors, showing nuclear staining. Each experiment was performed twice, with five mice per group. Error bars indicate standard deviation. For statistical analysis, one-sided Student t test was used, with P values indicated in the figure.
Mechanisms of Apoptosis Following MyD88 Silencing
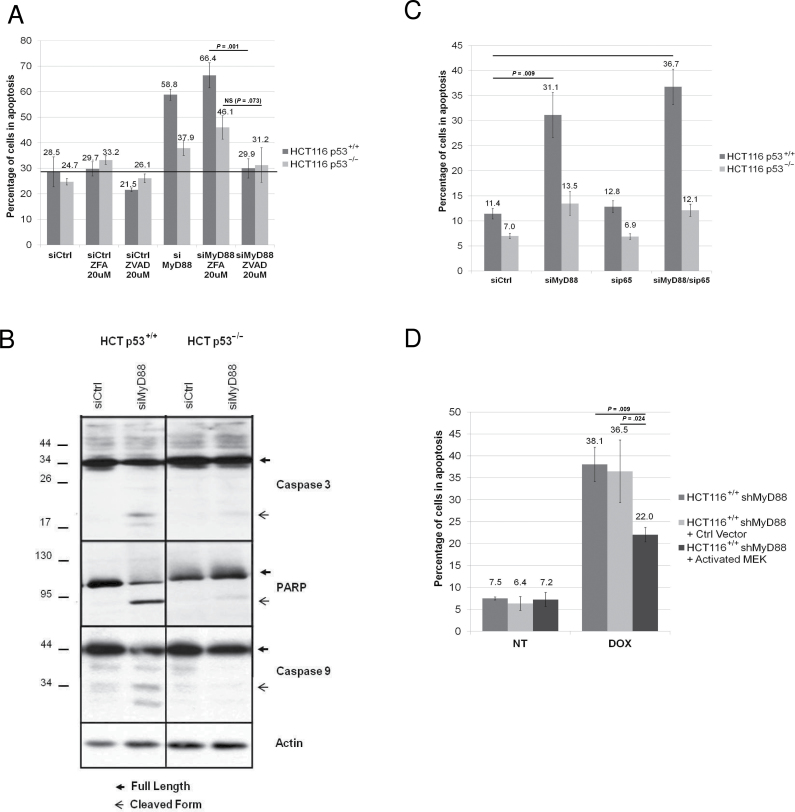
We then silenced MyD88 using siRNA in HCT116 p53+/+ and p53–/– cell lines in the presence or absence of Z-VAD-FMK, a general caspase inhibitor. We show that Z-VAD treatment statistically significantly inhibits the apoptosis (Figure 3A) induced by MyD88 silencing (controls in Supplementary Figure 3A) in the HCT116 p53+/+ cell line (29.9%±3.8% vs 66.4%±4.9%; P = .001), indicating that this apoptosis is caspase-dependent. This is consistent with the observation that cleavage of caspase 3 and 9, as well as of PARP, is only detected upon MyD88 silencing in the HCT116 p53+/+, but not in p53–/– cells (Figure 3B), further underscoring a role for p53 in apoptosis induction.
Figure 3.
MyD88 extinction, Erk-MAPK signaling, and caspase-dependent apoptosis. A) Apoptosis of HCT116 p53+/+ or p53–/– cell lines upon MyD88 silencing by small interfering RNA (siRNA) and treatment with either caspase inhibitor ZVAD or its control (ZFA). B) Western blot analysis of the expression of caspase 9, caspase 3, and PARP proteins in HCT116 p53+/+ or p53–/– cells upon MyD88 silencing by siRNA. C) Apoptosis analysis of HCT116 p53+/+ or p53–/– cells upon silencing of MyD88 and/or NF-κB p65. D) Apoptosis of HCT116 p53+/+ expressing a doxycycline-inducible MyD88 short hairpin RNA (shMyD88), transfected with either an empty vector or with constitutively activated MEK, after treatment or no treatment (NT) with doxycycline (DOX). Each experiment was performed three independent times, with each condition done in triplicate. Error bars indicate standard deviation. For statistical analysis, one-sided Student t test was used, with P values indicated in the figure. NS = not significant; siCtrl = non-silencing siRNA; siMyD88 = MyD88-specific siRNA.
We had previously shown that MyD88 is implicated in two major pathways: the TLR/IL-1R pathway known to predominantly activate NF-κB, and the canonical Ras pathway, which activates the Erk/MAP kinase (5). To address whether the apoptosis induced upon MyD88 silencing is due to the resulting inhibition of the Ras pathway and/or of the NF-κB pathway, we first inhibited in HCT116 p53+/+ and p53–/– cells the NF-κB pathway by silencing p65 RelA, and found that this did not lead to apoptosis (Supplementary Figure 3D, available online), suggesting that the NF-κB pathway is not implicated in the apoptosis observed upon MyD88 silencing. This was confirmed by experiments in which following MyD88 knockdown (controls in Supplementary Figure 3B, available online), apoptosis is shown to be independent of the presence or absence of p65 (Figure 3C). In contrast, treatment of HCT116 p53+/+ cells with the MEK inhibitor U0126 induced apoptosis (data not shown), consistent with earlier reports that the inhibition of the canonical Ras pathway induces cell death (9). To directly address the implication of the Ras/Erk pathway in MyD88 silencing-induced apoptosis, we performed complementation assays in which we bypass the inhibition of the Ras pathway induced by MyD88 silencing by concomitantly expressing a constitutively active form of MEK (controls in Supplementary Figure 3C, available online). Figure 3D shows that this statistically significantly reduces apoptosis (22.0%±1.7% vs 38.1%±3.9%; P = .009), indicating that this apoptosis is at least partly due to the inactivation of the MAP kinase canonical pathway following loss of MyD88.
MyD88 and DNA Repair
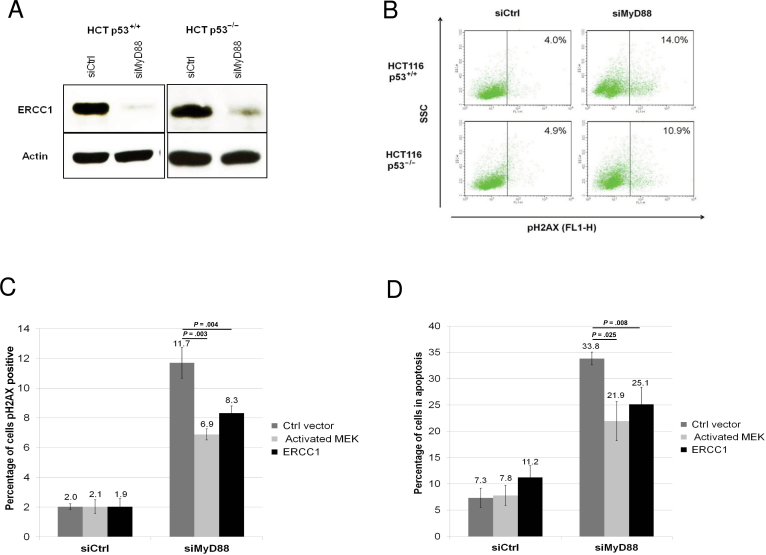
Previous studies have established a link between the inhibition of Ras pathway and the accumulation of DNA damage (10). A direct role for Erk was reported in the transcription of ERCC1 DNA repair enzyme (11). We therefore analyzed the levels of ERCC1 in HCT116 p53+/+ or p53–/– cells and observed in both cell lines a decrease in the expression of this enzyme (Figure 4A) upon MyD88 silencing. Next, we reasoned that the down-regulation of a major DNA repair enzyme such as ERCC1 should lead to the accumulation of DNA damage. Indeed, we show an increase in H2AX S139 phosphorylation—an indicator of DNA damage (12)—in both HCT116 p53+/+ or p53–/– cell lines (Figure 4B) upon MyD88 silencing (controls in Supplementary Figure 4A). To address whether ERCC1 downregulation and accumulation of DNA damage are causally related, we performed complementation assays in which the inhibition of ERCC1 production induced by MyD88 silencing is bypassed by reexpressing ERCC1 in HCT116 p53+/+ cells. As shown in Figure 4, C and D, this is indeed the case, as ectopically expressing ERCC1 in the cells in which MyD88 was silenced (controls in Supplementary Figure 4B) statistically significantly reverses both the accumulation of DNA damage (pH2AX positive cells: 8.3%±0.5% vs 11.7%±1.0%; P = .004) and apoptosis (25.1%±3.3% vs 33.8%±1.2%; P = .008), respectively. A statistically substantial level of apoptosis remains in cells complemented with MEK or ERCC1. This could be due to the fact that in these experiments MyD88 inhibition occurred in virtually all cells, which express a stably expressed lentiviral vector, whereas the complementing genes were introduced using lipophilic transfection, which is relatively inefficient—typically in the order of 30%–50% in our hands.
Figure 4.
MyD88 silencing, ERCC1, and DNA damage. A) Western blot analysis of the expression of DNA repair enzyme ERCC1 in HCT116 p53+/+ or p53–/– cells upon MyD88 silencing by small interfering RNA (siRNA). B) Fluoroescence-activated cell sorting (FACS) analysis of DNA damage foci accumulation as revealed by the phosphorylation of the S139 of histone H2AX, in HCT116 p53+/+ or p53–/– cells upon MyD88 silencing by siRNA. C) Quantification of DNA damage foci accumulation (pS139 H2AX), by FACS analysis in HCT116 p53+/+ cells upon MyD88 silencing by siRNA, and transfection with either an empty vector, with a constitutively activated MEK, or with ERCC1. D) Apoptosis of HCT116 p53+/+ cells upon MyD88 silencing by siRNA, and transfection with either an empty vector, or with a constitutively activated MEK, or with ERCC1. Each experiment was performed three independent times, with each condition done in triplicate. Error bars indicate standard deviation. For statistical analysis, one-sided Student t test was used, with P values indicated in the figure. Ctrl = control; siCtrl = non-silencing siRNA; siMyD88 = MyD88-specific siRNA.
Combination of MyD88 Inhibition and Genotoxic Agents
Platinum-based agents (eg, cisplatin and oxaliplatin) are frequently used in colon cancer therapy. These genotoxic agents induce DNA damage that is preferentially repaired by the nucleotide excision repair (NER) DNA pathway (13). Because decreasing ERCC1 expression by MyD88 silencing reduces the NER pathway, leading to accumulation of DNA damage, we hypothesized that MyD88 silencing should synergize with these genotoxic drugs for the induction of apoptosis. We therefore performed in vitro combination assays using shMyD88 HCT116 (p53+/+ and p53–/–) cells treated with doxycycline (MyD88 silencing) in combination with different doses of chemotherapeutic agents (cisplatin, oxaliplatin, paclitaxel, and etoposide). Combination indexes were calculated using the Compusym software at a 90% efficient death level. If the combination index is less than 1, this indicates a synergy between the two molecules. We show that MyD88 silencing synergizes in a p53-independent manner (Table 1), and allows drug-dose reduction (Table 2), with cisplatin and oxaliplatin but not with etoposide (an inhibitor of topoisomerase II), or with paclitaxel (an inhibitor of tubulin depolymerization) (Table 1).
Table 2.
Dose reduction indexes (DRIs) for doxycycline (MyD88 inhibition) and chemotherapy agents upon combination of the two in HCT116 p53+/+ or p53–/– cells expressing a doxycycline-inducible MyD88 short hairpin RNA (shMyD88) in vitro
| Treatment | DRI | |||
|---|---|---|---|---|
| Doxycycline | Chemotherapy | Doxycycline | Chemotherapy | |
| Doxycyclin–cisplatin | 7.4 | 2.4 | 3.8 | 14.4 |
| Doxycyclin–oxaliplatin | 4.4 | 8.2 | 2.3 | 2.7 |
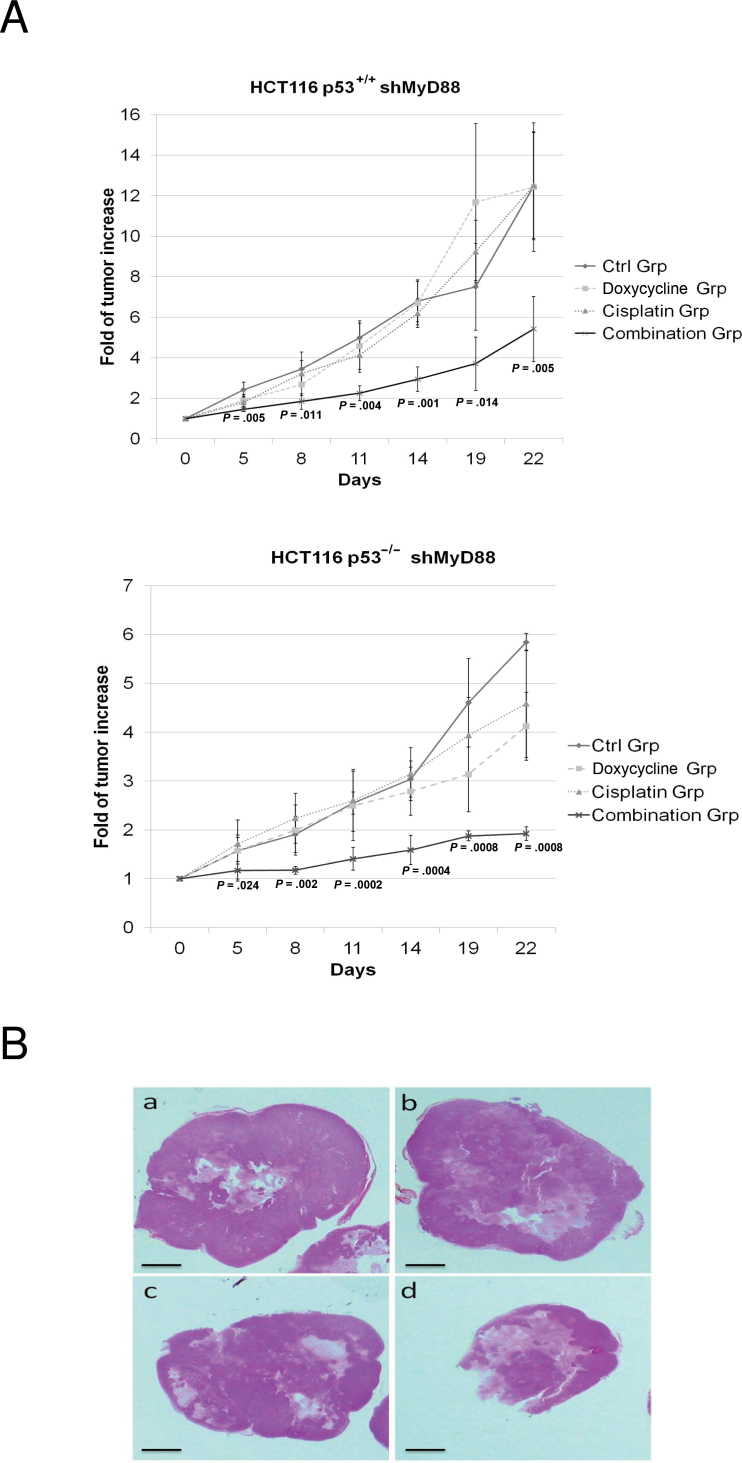
Next, we sought to investigate the relevance in vivo of the synergy observed in vitro. HCT116 p53+/+ or p53–/– cells expressing doxycycline-inducible MyD88-specific shRNA were implanted subcutaneously into nude mice. HCT116 p53+/+ tumor growth following treatment with a combination of suboptimal MyD88 inhibition and suboptimal doses of cisplatin (fold tumor increase = 5.4±1.6) was statistically significantly reduced in comparison to treatment with doxycycline alone (fold tumor increase = 12.4±3.1) or with cisplatin alone (fold tumor increase = 12.5±2.6) (P = .005, one-sided Student t test). Similar results were obtained in HCT116 p53–/– (Figure 5).
Figure 5.
Effect of MyD88 inhibition on cancer cell sensitivity to genotoxic agents in vivo. A) Analysis of the growth of HCT116 p53+/+ cells expressing a doxycycline-inducible MyD88 short hairpin RNA (shMyD88) implanted subcutaneously into nude mice and treated either with doxycycline at a suboptimal concentration, with cisplatin at suboptimal dose, or with the combination of suboptimal doses of the two agents. The control group (Ctrl) received only drinking water containing 3% of glucose. B) Hematoxylin and eosin staining of representative histological section from the mice above. Each experiment was performed twice, with five mice per group. Error bars indicate standard deviation. For statistical analysis, one-sided Student t test was used, with P values indicated in the figure.
Discussion
We had previously shown that MyD88 is implicated in two major pathways: the TLR/IL-1R pathway known to predominantly activate NF-κB, and the canonical Ras pathway, which activates the Erk MAP kinase (5). Our data indicates that MyD88-dependent, optimal activation of the Ras pathway is essential for the survival of colon cancer cells. Ras-MAPK pathway activation has been linked to efficient DNA repair, predominantly through the enzyme ERCC1, which is mainly implicated in NER (14). Indeed, it is well documented that treatment of cells with MEK inhibitors decreases ERCC1 expression at the mRNA and protein levels. We showed a loss of ERCC1 protein expression following MyD88 extinction in colon cancer cell lines, concomitant with accumulation of DNA damage. At first glance, it may seem intriguing that a defect in the NER pathway—which is implicated in the repair of single-strand DNA breaks—induces an accumulation of double-strand DNA breaks, as revealed by pH2AX staining. A possible explanation is that with the accumulation of unrepaired single-strand breaks on the DNA double helix, a number of these lesions will transform into double-strand breaks and become detectable by the H2AX staining (15).
At this point, p53—when present—is activated by this DNA damage and induces the apoptotic program (Figure 6, A and B). It is worth mentioning here that DNA damage is a cellular stress that induces preferentially the intrinsic apoptotic pathway based on caspase 9 activation (16), which is consistent with our data showing caspase 9 activation upon MyD88 silencing.
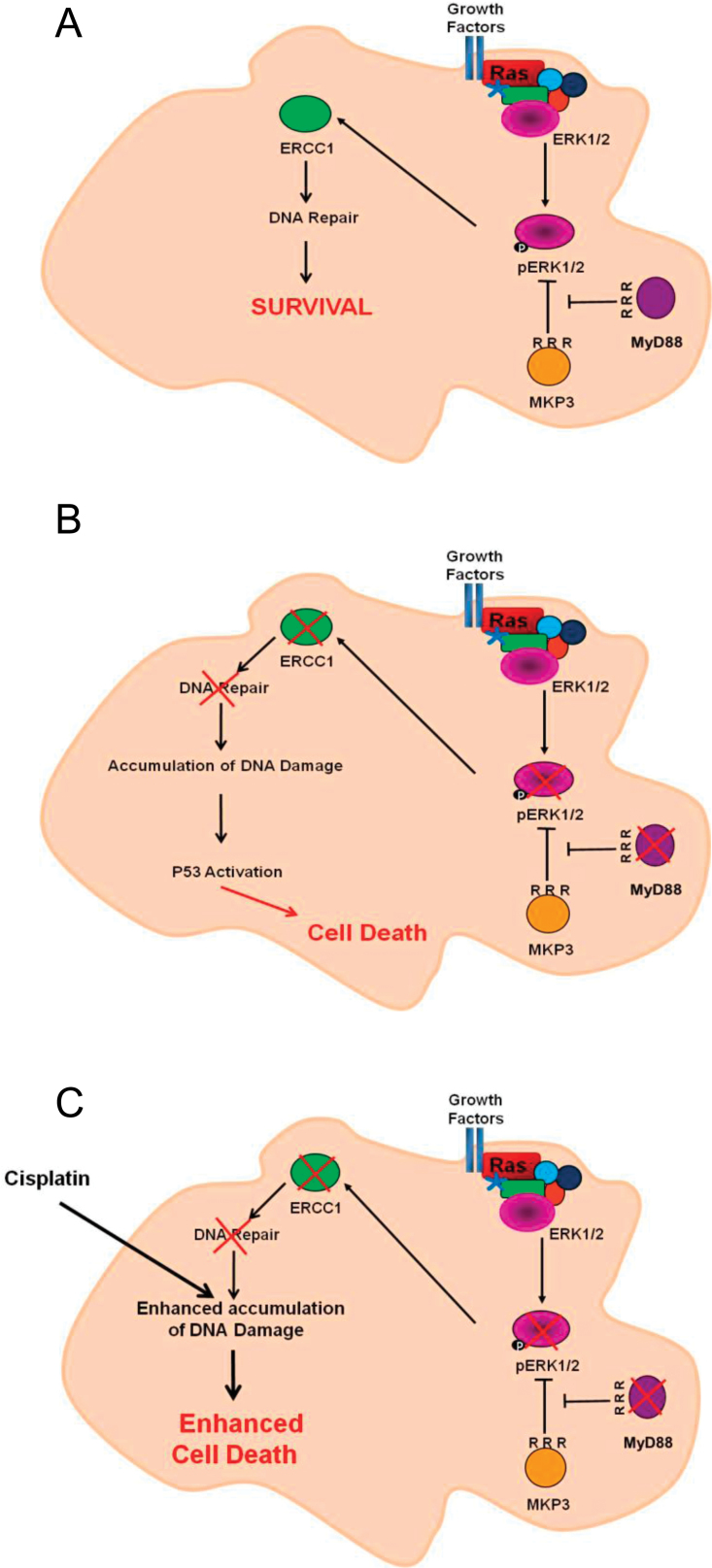
Figure 6.
Model for MyD88 inhibition alone or in combination with cisplatin in Ras-dependent cells. A) Ras pathway optimal activation in presence of MyD88 induces DNA repair enzyme ERCC1, enabling efficient DNA repair mechanisms in cancer cells. B) Absence of MyD88 leads to the inactivation of the canonical Ras/Erk pathway, therefore decreasing the expression of DNA repair enzyme ERCC1 and reducing cellular DNA repair abilities, leading to an accumulation of DNA damage and activating the apoptotic program via p53. C) Combining MyD88 inhibition with cisplatin enhances DNA damage accumulation and increases cell death.
It is noteworthy that the inhibition of MyD88 and p53 appears to be only partially required for triggering apoptosis following inhibition of MyD88 in Figures 1B and 2A, whereas in it appears to be absolutely required in Figure 1C. One possibility is that because the p53–/– HCT116 cell line has been in culture for a long time, it has likely developed compensatory mechanisms that may account for the observed residual apoptosis. This is a different situation to that in cells competent for a given gene, and in which the gene is inactivated and the cells analyzed immediately afterward. Similar considerations apply when analyzing conventional knockout versus inducible conditional knockout mice.
The first line of treatment in colon cancer after surgical intervention is the use of chemotherapy, in particular platinum-based agents (eg, cisplatin and oxaliplatin). Given that these agents induce a specific type of DNA damage (bulky T adducts) repaired by the NER pathway (13), and because MyD88 silencing compromises the NER pathway, it was reasonable to expect a synergy between these events in the induction of colon cancer cell apoptosis (Figure 6C). Indeed, multiple recent studies have reported that ERCC1 overexpression is a major resistance mechanism developed by tumors to evade the action of platinum salts such as cisplatin (17). In addition, studies on colon, cervical, and ovarian cancer suggest that ERCC1 levels can predict resistance to cisplatin and oxaliplatin treatment, and that inhibiting ERCC1 resensitizes resistant tumors to cisplatin (18).
Given that MyD88 silencing alone induces only modest apoptosis in absence of p53 (Figures 1–3), it was unexpected that MyD88 silencing should synergize with DNA damage–inducing agents in HCT116 p53–/– to the same extent as in p53+/+ cells. However, it is known that platinum salts are able to induce apoptosis via DNA damage accumulation in cells lacking p53 using various pathways (13).
One limitation of the present study is that only colon cancer cell lines were thoroughly analyzed for the effects of MyD88 inhibition. Cell lines from other cancer types should also be tested to ensure that the mechanisms described here are general. Another limitation is that for practical reason, only cisplatin was used in the combination studies in vivo. It would eventually be useful to test a wider panel of genotoxic agents in conjunction with MyD88 knockdown.
In conclusion, MyD88 plays an important role in promoting the optimal activation of the Ras/Erk survival pathway, which is required for the expression of DNA repair enzymes such as ERCC1, and therefore for efficient DNA repair in cancer cells. Inhibition of MyD88 therefore leads to the accumulation of DNA damage, resulting in cell death via the tumor suppressor protein p53. These observations provide further rationale for the concept of cancer cell addiction to the continuous activation of survival signaling, and point to MyD88 as a promising potential therapeutic target in Ras-dependent cancer cells, in particular in the context of concomitant genotoxic chemotherapy.
Funding
French National Cancer Institute (PLBIO11-071); Ligue Contre le Cancer (C388 –MYD88); Association de Recherche Contre le Cancer (SFI20111203820). The study sponsor(s) had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Supplementary Material
References
- 1. West AP, Koblansky AA, Ghosh S. Recognition and signalling by toll-like receptors. Annu Rev Cell Dev Biol. 2006;22:409–437 [DOI] [PubMed] [Google Scholar]
- 2. Lee MS, Kim YJ. Signalling pathways downstream of pattern-recognition receptors and their crosstalk. Annu Rev Biochem. 2007;76:447–480 [DOI] [PubMed] [Google Scholar]
- 3. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545 [DOI] [PubMed] [Google Scholar]
- 4. Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science. 2007;317(5834):124–127 [DOI] [PubMed] [Google Scholar]
- 5. Coste I, Le Corf K, Kfoury A, et al. Dual function of MyD88 in Ras signalling and inflammation, leading to mouse and human cell transformation. J Clin Invest. 2010;120(10):3663–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gayet J, Zhou XP, Duval A, et al. Extensive characterization of genetic alterations in a series of human colorectal cancer cell lines. Oncogene. 2001;20(36):5025–5032 [DOI] [PubMed] [Google Scholar]
- 7. Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2(8):594–604 [DOI] [PubMed] [Google Scholar]
- 8. Rago C, Vogelstein B, Bunz F. Genetic knockouts and knockins in human somatic cells. Nat Protoc. 2007;2(11):2734–2746 [DOI] [PubMed] [Google Scholar]
- 9. Bessard A, Frémin C, Ezan F, Fautrel A, Gailhouste L, Baffet G. RNAi-mediated Erk2 knockdown inhibits growth of tumor cells in vitro and in vivo. Oncogene. 2008;27(40):5315–5325 [DOI] [PubMed] [Google Scholar]
- 10. Dai Y, Chen S, Pei XY, et al. Interruption of the Ras/MEK/Erk signalling cascade enhances Chk1 inhibitor-induced DNA damage in vitro and in vivo in human multiple myeloma cells. Blood. 2008;112(6):2439–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yacoub A, Park JS, Qiao L, Dent P, Hagan MP. MAPK dependence of DNA damage repair: ionizing radiation and the induction of expression of the DNA repair genes XRCC1 and ERCC1 in DU145 human prostate carcinoma cells in a MEK1/2 dependent fashion. Int J Radiat Biol. 2001;77(10):1067–1078 [DOI] [PubMed] [Google Scholar]
- 12. Sak A, Stuschke M. Use of γH2AX and other biomarkers of double-strand breaks during radiotherapy. Semin Radiat Oncol. 2010;20(4):223–231 [DOI] [PubMed] [Google Scholar]
- 13. Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22(47):7265–7279 [DOI] [PubMed] [Google Scholar]
- 14. Gossage L, Madhusudan S. Current status of excision repair cross complementing-group 1 (ERCC1) in cancer. Cancer Treat Rev. 2007;33(6):565–577 [DOI] [PubMed] [Google Scholar]
- 15. Kuzminov A. Single-strand interruptions in replicating chromosomes cause double-strand breaks. Proc Natl Acad Sci U S A. 2001;98:8241–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roos WP, Kaina B. DNA damage-induced apoptosis: from specific DNA lesions to the DNA damage response and apoptosis. Cancer Lett. 2013;332(2):237–248 [DOI] [PubMed] [Google Scholar]
- 17. Youn CK, Kim MH, Cho HJ, et al. Oncogenic H-Ras up-regulates expression of ERCC1 to protect cells from platinum-based anticancer agents. Cancer Res. 2004;64(14):4849–4857 [DOI] [PubMed] [Google Scholar]
- 18. Liu GY, Qu QX, Mi RR, Qi J. Enhanced cisplatin cytotoxicity by RNA interfering the excision repair cross complementing 1 gene in ovarian cancer cell lines [in Chinese]. Zhonghua Fu Chan Ke Za Zhi. 2006;41(5):339–342 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.