Abstract
Matrix metalloproteinases (MMPs) are zinc dependent endopeptidases that can be released from neurons in an activity dependent manner to play a role in varied forms of learning and memory. MMP inhibitors impair hippocampal long term potentiation (LTP), spatial memory, and behavioral correlates of drug addiction. Since MMPs are thought to influence LTP through a β1 integrin dependent mechanism, it has been suggested that these enzymes cleave specific substrates to generate integrin binding ligands. In previously published work, we have shown that neuronal activity stimulates rapid MMP dependent shedding of intercellular adhesion molecule-5 (ICAM-5), a synaptic adhesion molecule expressed on dendrites of the telencephalon. We have also shown that the ICAM-5 ectodomain can interact with β1 integrins to stimulate integrin dependent phosphorylation of cofilin, an event that occurs with dendritic spine maturation and LTP. In the current study, we investigate the potential for the ICAM-5 ectodomain to stimulate changes in α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate receptor (AMPAR) dependent glutamatergic transmission. Single cell recordings show that the ICAM-5 ectodomain stimulates an increase in the frequency, but not the amplitude, of AMPA mini excitatory post synaptic currents (mEPSCs). With biotinylation and precipitation assays, we also show that the ICAM-5 ectodomain stimulates an increase in membrane levels of GluA1, but not GluA2, AMPAR subunits. In addition, we observe an ICAM-5 associated increase in GluA1 phosphorylation at serine 845. Concomitantly, ICAM-5 affects an increase in GluA1 surface staining along dendrites without affecting an increase in dendritic spine number. Together these data are consistent with the possibility that soluble ICAM-5 increases glutamatergic transmission and that post-synaptic changes, including increased phosphorylation and dendritic insertion of GluA1, could contribute. We suggest that future studies are warranted to determine whether ICAM-5 is one of a select group of synaptic CAMs whose shedding contributes to MMP dependent effects on learning and memory.
Introduction
Matrix metalloproteinases (MMPs) are a family of structurally related enzymes that can be released from cells as pro- and active forms. They were named for their ability to process proteins of the extracellular matrix but are now appreciated to act on a variety of soluble molecules and cell surface receptors as well [1]. While studies of MMPs in the CNS have generally focused on the potential for pathologically elevated enzyme levels to stimulate blood brain barrier breakdown or cellular injury, recent evidence suggests that physiological levels of select MMPs can play a critical role in normal CNS function and learning and memory in particular [2–4]. For example, several groups have shown that MMPs are important to spatial learning and memory, and to correlates of the maladaptive memory that underlies addiction [5,6]. Previous studies have also shown that MMP inhibitors can impair LTP [7,8].
Consistent with a role for MMPs in learning and memory, expression and release of the enzymes can be increased by neuronal activity [9–12]. Such release may be rapid, in that MMP dependent shedding of a neuronal substrate occurs within several minutes of N-methyl-D-aspartic acid (NMDA) application [11]. Published studies suggest that preformed MMPs exist in perisynaptic stores [12,13], and in non neural cells, stimulated release can follow from a soluble NSF attachment protein receptor (SNARE) dependent mechanism [14]. If a similar mechanism occurs in neurons, MMP release might be facilitated by stimuli that evoke SNARE dependent release of select neurotransmitters. A recent study has also shown that glutamate stimulates transport of MMP-9 mRNA to dendrites, and that neuronal activity stimulates local translation and release of the enzyme [15].
The ability of MMPs to influence long term potentiation and hippocampal dependent memory likely involves structural changes to the post synaptic element of glutamatergic synapses [16]. More than 90% of excitatory synapses terminate on dendritic spines [17], and long lasting facilitation of neurotransmission has been linked to increases in the size of spines and associated increases in the number of glutamate receptors [18–20]. Consistent with the potential for MMPs to influence dendritic spines, at least one MMP has been shown to increase spine size [21]. The means by which MMPs exert their effects on dendritic spines and LTP are, however, not completely understood. Previous studies suggest that the engagement of β1 integrins may contribute [8]. Integrins including β1 are expressed at the synapse, integrin activation plays a role in LTP, and integrin antagonists can block MMP-dependent LTP and spine enlargement [8,21–27]. Engagement of β1 integrin receptors has been shown to stimulate src kinase dependent phosphorylation of NMDA receptors [23], and may also stimulate the actin polymerization that underlies spine expansion [22].
In terms of how MMP activity stimulates integrin dependent effects, one possibility is that MMPs cleave specific synaptic cell adhesion molecules (CAMs) to generate integrin binding ligands. Varied CAMs are known to possess integrin binding domains [28], and several of these are CAMs are enriched at the glutamatergic synapse [29]. CAMs are also well localized to be MMP substrates, in that their proximity to sites of MMP release may allow them to be cleaved before MMPs are bound by endogenously expressed MMP inhibitors, or tissue inhibitors of metalloproteinases (TIMPs).
In a previously published study, we have shown that neuronal activity stimulates rapid MMP-dependent cleavage of the synaptic cell adhesion molecule intercellular adhesion molecule-5 (ICAM-5), an adhesion molecule that is highly expressed on dendrites of the telencephalon [11,30]. Earlier studies had shown that ICAM-5 shedding was associated with spine maturation [29,30]. These studies, which focused on developmental spine maturation and evaluated spine morphology many hours following NMDA-stimulated ICAM-5 cleavage [29], had suggested that the shedding of ICAM-5 might disrupt N and C terminal interactions of the full length molecule that are important to filopodial maintenance [31]. While shedding may therefore allow for spine expansion, a non-mutually exclusive possibility, and one that we have focused on in recent studies [32], is that the shed ectodomain can bind to unengaged post synaptic integrins to stimulate dendritic actin polymerization and spine expansion.
In a previous publication [33], we have shown that the ectodomain of ICAM-5 can interact with β1 integrins to stimulate phosphorylation of cofilin, an event associated with dendritic actin polymerization. The question of whether soluble ICAM-5 dependent effects are significant enough to influence dendritic levels of glutamate receptors and AMPA mEPSCs is addressed in the current study.
Materials and Methods
Cell Culture
All experimental procedures were approved by and performed in agreement with policies of the Georgetown University Animal Care and Use Committee (GUACUC). Every effort was made to minimize suffering. Hippocampal tissue was harvested from embryonic day 18 Sprague-Dawley rats using a protocol modified from [34]. Briefly, hippocampal tissue was finely chopped and digested with 0.1% trypsin as well as by mechanical trituration. Cells were plated onto cell culture-ware previously treated with poly-d-lysine and laminin (Sigma, St. Louis, MO), at an approximate density of 150 cells/mm2. Cultures were maintained in Neurobasal A medium with B27 (Invitrogen, Carlsbad, CA), with bi-weekly changes, and stored in a humidified 5% CO2 and 95% O2 incubator at 37°C. Experiments were performed on cultures at 14 days in vitro (DIV).
Reagents
Recombinant ICAM-5 was purchased from R & D Systems, Minneapolis, MN and reconstituted in sterile phosphate buffered saline just prior to use. This construct contains the major portion of the ICAM-5 ectodomain (leu 31-arg828). Antibodies to GluA1 were purchased from Millipore (AB1504, C terminal epitope) and Calbiochem (PC246, N terminal epitope for live surface staining). The anti-phospho-GluA1 was from from R & D Systems (PPS008), the anti-GluA2 from BD pharmingen (live surface staining) and Millipore/Chemicon (Western blot following biotinylation/precipitation), and the anti-PSD95 from Millipore/Chemicon (MAB1598). The pEGFP construct was commercially obtained (Clonetech), and Lipofectamine 2000 was purchased from Invitrogen.
Cell stimulation
ICAM-5 treated cultures received 1-2.5 µg/ml (as noted) recombinant protein for 60 min prior to analysis. It was previously established that this concentrations in this range stimulated an increase in phospho-cofilin [33].
Single Cell Recordings
Ruptured-patch whole-cell voltage-clamp recordings were obtained from cultured hippocampal neurons at DIV 12-14 for examination of glutamate receptor responses. Pyramidal cells were selected using a 60X water immersion objective with a long working distance (2 mm) and high numerical aperture (1.0). Recording electrodes (4-6 MΩ tip resistance) were pulled on a vertical pipette puller from borosilicate glass capillaries (Wiretrol II, Drummond, Broomall, PA) and filled with an internal solution containing (in mM): 145 K-gluconate, 10 HEPES, 5 ATP-Mg, 0.2 GTP-Na, and 0.5 EGTA, adjusted to pH 7.2 with KOH. Extracellular solution was perfused at a rate of 2.0-2.8 ml/minute and contained (in mM): 145 NaCl, 5 KCl, 1 CaCl2, 5 HEPES, 5 glucose, 26 sucrose and 0.25 mg/L phenol red, adjusted to pH to 7.4 with NaOH. Voltage-clamp recordings were performed at a holding potential of -70 mV using either a Multiclamp 700B or an Axopatch 1-D amplifier (Molecular Device Co., Sunnyvale CA, USA).
Miniature excitatory postsynaptic currents (mEPSCs) were isolated by local application of 25 μM of bicuculline methobromide (BMR) and 0.5 μM tetrodotoxin (TTX) through the “Y tube” method [35]. The AMPA receptor antagonist NBQX (1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide disodium salt hydrate) was applied in a subset of recordings to verify events were AMPA receptor mediated. All drug-containing stock solutions were diluted to the desired working concentration in the extracellular solution.
Currents were low pass filtered at 2 kHz and digitized at 5-10 kHz using a Dell computer equipped with Digidata 1322A data acquisition board and pCLAMP9 software (Molecular Devices). Events were identified using a semi-automated threshold based mini detection software (Mini Analysis, Synaptosoft Inc., Fort Lee, NJ) and were visually confirmed. mEPSC averages were based on >100 events in each recording.
Sample preparation and Western blot
Western blot was performed on lysates as previously described [33]. Lysates from cultured cells were prepared via the addition of lysis buffer [50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% sodium dodecyl sulfate, 1% NP-40, 0.5% sodium deoxycholate, 0.2 mM phenylmethylsulfonyl fluoride, 0.5 mM dithiothreitol, 1× protease inhibitor cocktail (Sigma P8340)]. The mixture was placed into a microfuge tube, sonicated for 10 s, kept on ice for 20 min, and then spun at 14 000 rpm for 15 min at 4°C in a microcentrifuge. The quality of transfer was verified by Ponceau staining and molecular weights were inferred by comparison to prestained markers (BioRad).
For analysis of surface GluA1 and GluA2, surface proteins were first biotinylated, and then pulled down to be analyzed by Western blot. Cultures were treated for 1 hour after which they were washed twice with cold phosphate-buffered saline and incubated in PBS containing 1 mg/ml EZ-Link Sulfo-NHS-Biotin (Pierce) for 30 min at 4 °C. The biotinylation reaction was stopped by washing cells with quenching solution (PBS/100 mM glycine). Cells were incubated in quenching solution for a total of 20 min. Cells were then solubilized at 4 °C in a lysis buffer, containing 150 mM NaCl, 1 mM EDTA, and 100 mM Tris-HCl, pH 7.4 1% Triton X-100, and protease inhibitor cocktail (Roche). To clear lysates, samples were spun at 16 000 × g at 4 °C for 20 min. A small portion of cleared lysate was saved for analysis, as a lysate fraction. The remaining lysate was incubated with avidin beads (Pierce) at 4 °C overnight. After incubation, beads were pelleted by centrifugation at 16 000 × g for 15 min, and the supernatant was saved as the intracellular fraction. The beads were washed once in lysis buffer, and then twice in lysis buffer containing high salt (500 mM NaCl), and once again in lysis buffer containing low salt (50 mM NaCl). Biotinylated proteins were eluted with SDS sample buffer, containing 100 mM mercaptoethanol. The integrity of the cell membrane during biotinylation was tested by immunoblotting with an anti-actin antibody.
Immunocytochemistry, Microscopy, and Image Analysis
Live cell surface staining was performed as described [36–38]. Briefly, neurons were transfected with pEGFP 24 h before initiation of experiments. At the completion of each experiment, neurons were incubated with primary antibody for 10 minutes then lightly fixed for 5 minutes in 4% paraformaldehyde (nonpermeabilizing conditions). After fixation, antibody labeled GluA1 or GluA2 was detected with Alexa Fluor linked secondary antibody.
For quantitative studies of surface staining, spine and filopodia analysis and puncta numbers, secondary dendrites from 16–32 pyramidal neurons were evaluated per experimental group. Each group included three replicate cover slips. 20 µm segments beginning 10 microns from the soma were evaluated. Quantitation of surface signal and puncta were performed as previously described [36,37]. Spines and filopodia were defined as structures having a length of 0.2-2 µm and greater than 2 µm respectively.
Results
I. Soluble ICAM-5 stimulates an increase in mEPSC frequency
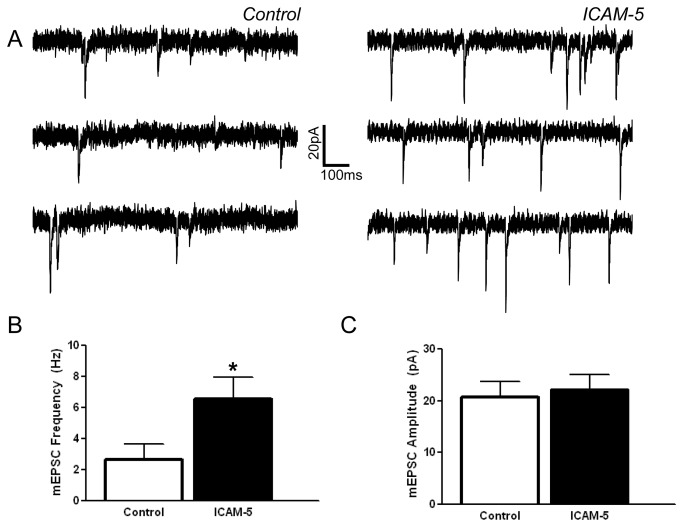
ICAM-5 is expressed on dendrites of the telencephalon and shed in a neuronal activity dependent manner. Its shedding by MMPs generates an N terminal fragment containing the major portion of the ectodomain including integrin binding domains [11,26]. In previous studies, we have shown that soluble ICAM-5 co-immunoprecipitates with β1 integrins [33]. We have also shown that it can stimulate a β1 integrin dependent increase in action potential frequency, an endpoint that can be associated with changes including, but not limited to, altered frequency or amplitude of AMPAR mEPSCs [32]. Herein we have evaluated the potential for soluble ICAM-5 to influence AMPAR mEPSC recordings in hippocampal neurons. Representative tracings are shown in Figure 1A. As shown in Figure 1B, we see an ICAM-5 stimulated increase in the frequency of AMPAR mEPSCs. Of interest, an increase in the amplitude of mEPSCs is not observed (Figure 1C).
Figure 1. The ICAM-5 ectodomain affects an increase in mini excitatory post synaptic current (mEPSC) frequency.
Stimulation of rat hippocampal neurons with 1 µg/ml ICAM-5 ectodomain (60 min. pretreatment) is associated with an increase in mEPSC frequency. In these experiments, 1,468 events from 11 control cells and 2353 events from 16 ICAM-5 stimulated cells were evaluated using standard techniques [74]. Representative tracings are shown in (A) while the average mEPSC frequency is shown in (B) and amplitude in (C). The difference between mEPSC frequency in control and ICAM treated neurons was significant (*p < 0.05, Student’s t test).
II. Soluble ICAM-5 stimulates an increase in surface levels of GluA1
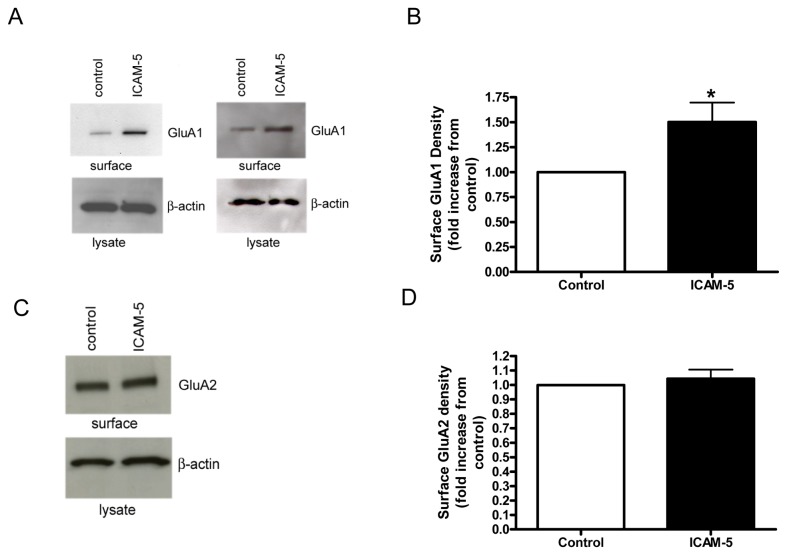
Several studies suggest that MMPs can potently and rapidly modulate the structure of dendritic spines in particular, with overall effects likely influenced by the particular MMP family member, its concentration, and the maturity of the system studied [21,39,40]. A potential post synaptic contribution to the change in mEPSC frequency could follow from an increase in the number of AMPA responsive synapses. We therefore tested ICAM-5 for its ability to influence surface levels of GluA1 and 2. As shown in Figure 2A, ICAM-5 was associated with an increase in surface levels of the GluA1 receptor subunit. Figure 2B shows results of densitometric analysis from replicate experiments. While there was variability in the increase, there was an ICAM-5 associated increase in each experiment. Actin levels in lysates did not differ, nor did total lysate levels of GluA1 (not shown). In figure 2C, surface protein preparations that had shown ICAM-5 associated changes in GluA1 were also examined for GluA2. While this subunit is also important to LTP [20], at one hour post treatment we did not observe an associated increase in surface levels of GluA2.
Figure 2. The ICAM-5 ectodomain stimulates an increase in surface levels of the glutamate receptor subunit GluA1.
Rat hippocampal neurons were unstimulated (control) or stimulated for 60 min. with 1 µg/ml of the ICAM-5 ectodomain (R & D Systems). Surface proteins were then biotinylated, and biotinylated proteins pulled down to be analyzed by Western blot. As can be appreciated, ICAM-5 was associated with an increase in surface GluA1 (A). Blots from separate experiments are shown. Densitometric analysis showing the fold increase in GluA1 band intensity in ICAM-5 versus control treated cultures in shown in (B). The mean and standard error for the fold increase from 6 replicate experiments is shown, and the difference between control and ICAM-5 groups is significant at p < 0.1 (*p=0.05). A representative blot for GluA2 in surface protein preparations is shown in (C), and densitometric analysis showing the fold change in GluA2 band intensity from 3 replicate experiments follows in (D). The mean and standard error for the fold change from 3 replicate experiments is shown, and the difference between control and ICAM-5 groups is not significant (p= 0.6).
III. Soluble ICAM-5 is associated with an increase in the phosphorylation of GluA1 at serine 845
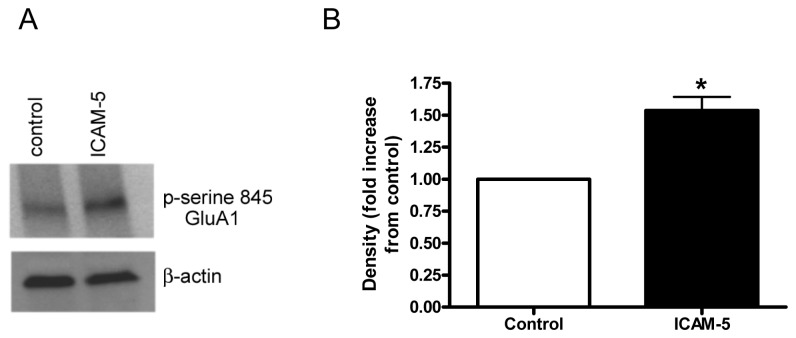
Phosphorylation of GluA subunits can influence receptor function and subunit localization 845 [41–43]. GluA1 phosphorylation sites include serine 897 and serine 845, with the latter typically stimulated by PKA. PKA dependent phosphorylation has been linked to activity dependent synaptic incorporation of the subunit [42], and the serine 845 site linked to fear memory [44]. Importantly, β1 integrin agonists have recently been shown to associate with Gαs and activate cAMP/PKA [45]. In figure 3, we show results from experiments that examined the ability of soluble ICAM-5 to stimulate an increase in the serine 845 phosphorylation of GluA1. Results from representative Western blot are shown in Figure 3A, and results from densitometric analysis of blots from 5 experiments are shown in Figure 3B.
Figure 3. Phosphorylation of GluA1 at serine-845 is increased by soluble ICAM-5.
Rat hippocampal neurons were unstimulated (control) or treated for 60 min. with 1 µg/ml of soluble ICAM-5 and lysates tested by Western blot for phospho-serine 845 GluA1. A representative blot is shown in (A) while densitometric analysis of blots from 5 experiments using distinct cultures is shown in (B). The mean and standard error for the fold increase is shown, and the fold increase is significant at p < 0.1 (*p= 0.06).
IV. ICAM-5 affects an increase in GluA1 surface staining along dendrites
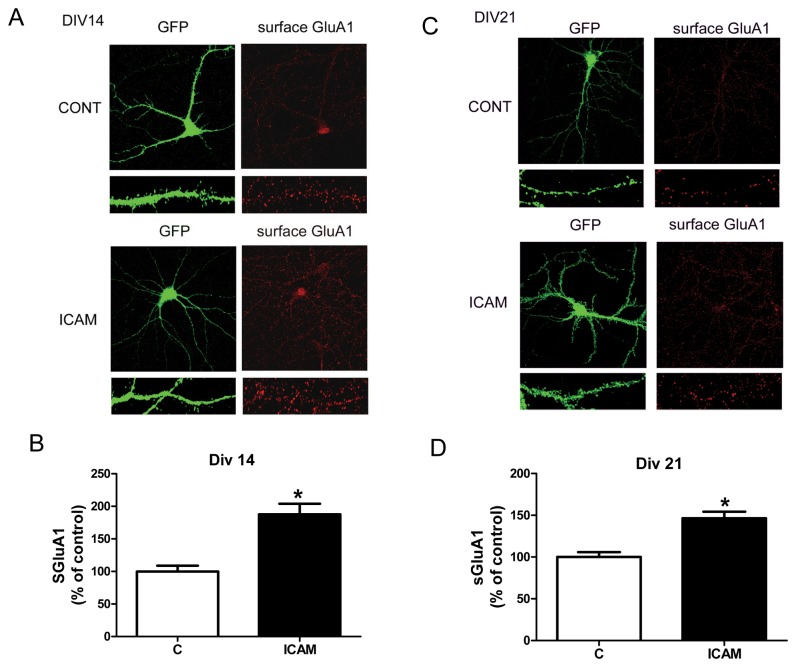
An increase in surface GluA1 as detected by biotinylation and precipitation assays is not localization specific. To determine whether surface GluA1 increased along dendrites in particular, surface labeling studies were performed according to established methods [37]. Results are shown in Figure 4 and demonstrate an increase in the intensity of GluA1 along proximal dendritic spines in ICAM-5 treated cultures at both 14 and 21 DIV. The intensity of GluA2 was not increased by ICAM-5, showing instead a non significant decrease at DIV 14 (not shown).
Figure 4. Soluble ICAM-5 affects an increase in GluA1 surface staining along dendrites in particular.
Figure 4 shows data from live cell surface staining for GluA1 in control and ICAM-5 treated hippocampal neurons at 14 and 21 DIV. Indicated cultures were treated with 2.5 µg/ml soluble ICAM-5 and surface staining performed 1 hour later. Representative images are shown in A and C, while quantitative data is shown in B and D. The mean and standard error for percent control values were 100 +/- 8.8, n=21 for the DIV 14 control group; 187.5 +/- 16.4, n=16 for the DIV 14 ICAM-5 group; 100 +/- 5.8, n=25 for the DIV 21 control group; and 146.3 +/- 7.9, n=25 for the DIV 21 ICAM-5 group. Differences in GluA1 staining between control and ICAM-5 treated cultures are significant at p< 0.01 (*) at both 14 and 21 DIV.
V. Soluble ICAM-5 does not affect an increase in dendritic spine number
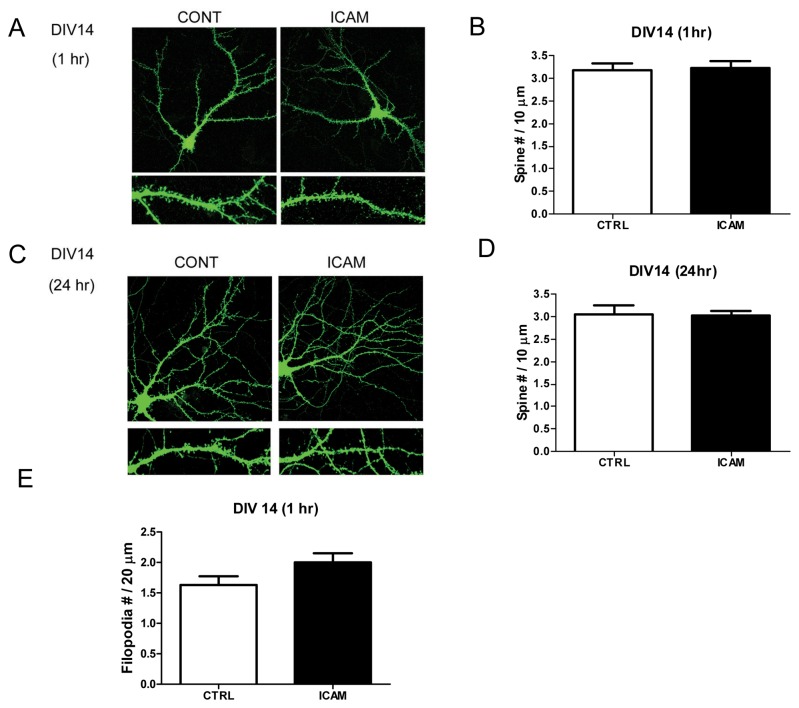
An increase in mEPSC frequency and dendritic surface levels of GluA1 could follow, at least in some part, from an increase in the insertion of GluA1 into existing, but GluA lacking and thus post synaptically silent, synapses. While increased staining for GluA1 along dendrites could follow from increased insertion along the shaft and/or increased insertion into existing synapses, a non-mutually exclusive possibility that could contribute to increased staining and an increase in the number of AMPAR responsive synapses would be an increase in spine number with GluA1 entering newly formed spines. Of interest is that in certain brain regions, de novo spines can form quickly [46]. Therefore, we also tested sICAM-5 for its effects on dendritic spine number. As shown in Figure 5, however, ICAM-5 did not stimulate a significant increase in spine number. Of interest, is that a non-significant trend towards an increase in filopodia was observed.
Figure 5. Soluble ICAM-5 does not increase spine number.
Figure 5 shows results from and experiment that compared dendritic spine number in control and ICAM-5 treated cultures. In cultures that were treated for 1h or 24h with 2.5 µg/ml ICAM-5, there was no significant difference in spine number (A–D). There was, however, a non-statistically significant trend towards an increase in dendritic filopodia, defined as protrusions greater than 2 µm in length, in cultures treated for 1h with ICAM-5 (E). The mean and standard error for spine number was 3.172 +/- 0.15, n=30 for the 1h control group; 3.23 +/- 0.149, n=32 for the 1h ICAM-5 group; 3.065 +/- 0.2, n=24 for the 24 h control group; and 3.027 +/-0.11, n=31 for the 24h ICAM-5 group. The mean and standard error for filopodia number were 1.6 +/- 0.14, n=27 for the control group and 2 +/- 0.15, n=27 for the ICAM-5 group.
VI. Schematic representation of MMP-dependent ICAM-5 signaling at the synapse
In figure 6 we show a hypothetical model in which MMPs are rapidly released from preformed peri-synaptic stores to cleave ICAM-5 at a membrane proximal site. The released N terminal fragment can bind unengaged integrins to stimulate intracellular signaling cascades leading to increased phosphorylation and membrane insertion of GluA1 subunits. Following ectodomain shedding, the C terminal fragment of ICAM-5 could undergo additional processing followed by internalization and degradation. It is worth noting that following MMP or A disintegrin and metalloproteinase (ADAM) mediated shedding, select CAMs are further processed by gamma secretase. Intracellular domains (ICDs) thus generated may be degraded or, in some cases, influence gene transcription [47,48].
Figure 6. Schematic representation of MMP-dependent ICAM-5 signaling at the synapse.
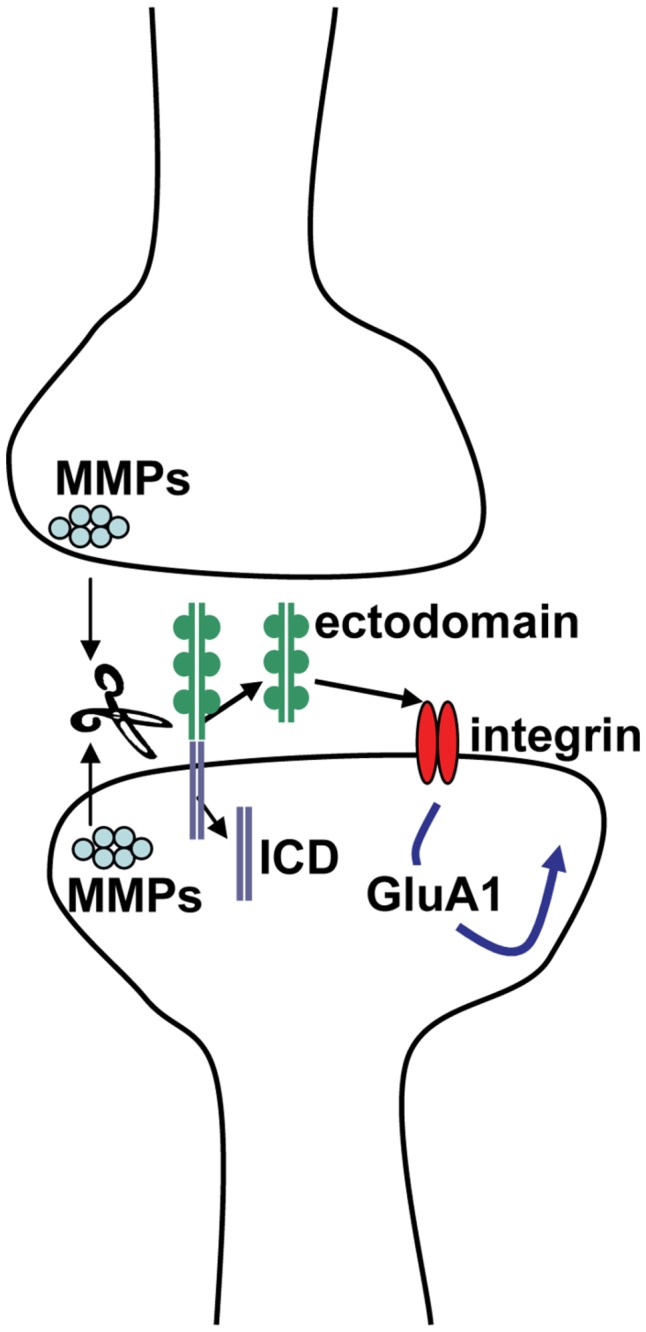
In figure 6 we show a hypothetical model in which MMPs are rapidly released from preformed peri-synaptic stores to cleave ICAM-5 (green and lavender) at a membrane proximal site. The released N terminal fragment can bind unengaged integrins (red ovals) to stimulate intracellular signaling cascades leading to increased phosphorylation and membrane insertion of GluA1 subunits. Following ectodomain shedding, the C terminal fragment of ICAM-5 could undergo additional processing followed by internalization and degradation. It is worth noting that following MMP or ADAM mediated shedding, select CAMs are further processed by intramembranous proteolysis. ICDs thus generated may be degraded or, in some cases, influence gene transcription.
Discussion
Previous studies have shown that MMPs play a role in varied forms of learning and memory (reviewed in 2,3,49–52). Though MMPs cleave varied relevant substrates, including pro-neurotrophins and insulin like growth factor binding proteins [53,54], several studies suggest that their potential to generate integrin-binding ligands likely represents an important means by which they enhance neurotransmission [7,8,21,33]. For example, β1 integrin signaling has been implicated in MMP dependent effects on LTP [7,8].
CAMs represent an important class of integrin binding ligands, and their shed ectodomains may interact with previously unengaged integrins to stimulate enhanced NMDAR subunit phosphorylation/function and/or actin polymerization with dendritic spine expansion [28]. In previous work, we have shown that neuronal activity stimulates rapid MMP dependent shedding of the ICAM-5 ectodomain [11], and that recombinant ectodomain can stimulate β1 integrin dependent phosphorylation of cofilin [33], an event permissive for dendritic actin polymerization and observed with spine expansion and LTP [21]. We have also observed that soluble ICAM-5 stimulates a β1 integrin dependent increase action potential frequency in hippocampal neurons, an endpoint that may be associated with pre and/or post synaptic changes including an increase in the amplitude or frequency of AMPA mEPSCs [32].
In the present study, we find that soluble ICAM-5 stimulates an increase in the frequency, but not the amplitude, of AMPAR mini EPSCs. This is an effect that could follow, at least in part, from post synaptic changes associated with an increase in the number of responsive units. We did not, however, observe an ICAM-5 associated increase in spine number nor in PSD-95 positive puncta (not shown). Another possibility to account for frequency increases would be unsilencing of previously silent, GluA deficient, synapses. It has been suggested that GluA1 containing receptors in particular are inserted into dendritic spines during unsilencing of synapses by electrical stimulation [55], and prior studies have shown that unsilencing of synapses can be associated with increased AMPAR mEPSC frequency [56]. A study focused on cofilin mediated actin dynamics with cLTP also showed an increase in GluA1 insertion into spines that was associated with an increase in mEPSC frequency [57]. Of interest is that a large portion of the spines (50%) that showed increased GluA1 insertion were not measurably enlarged. Post synaptically silent synapses are relatively prevalent in DIV 14 neuronal cultures [58], and also occur in mature brain [59]. As opposed to an increase in mEPSC amplitude, an increase in frequency might be expected if ICAM-5 were to have predominant effects on relatively thin, AMPAR-deficient spines. One possibility is that β1 integrins are more highly expressed, or present in a more avid form, on relatively less mature spines. These integrins do localize to synapses in CA1 where they are concentrated postsynaptically [24]. In a recent study, however, while β1 integrins were observed on the heads of filopodia, expression was more robust on mature spines [26]. Avidity and ligand binding availability issues as a function of maturity have yet to be fully explored. While integrin dependent effects on actin dynamics in spines from DIV 14 hippocampal neurons from E (15,16) mouse embryos have been demonstrated [25], experiments with acute hippocampal slices prepared at early and later (P21 versus P42) post natal stages suggest that integrin dependent effects on dendritic arbor and synapse stability may be important at relatively later post natal ages [60,61]. ICAM-5 expression as a function of maturity should also be considered. In vivo, ICAM-5 expression is higher on thin spines than it is on relatively mature mushroom spines [29]. Thus, in vivo shedding might be more likely to present greater agonist levels to relatively thin spines.
The lack of a substantial effect of ICAM-5 on the amplitude of AMPA mEPSCs in the present study is also of interest. While mechanisms including activation of previously silent synapses could contribute to frequency changes, ICAM-5 dependent actin polymerization and spine expansion may not have occurred to an extent sufficient to measurably increase amplitude. Whether ICAM-5 has appreciable effects on the size of small and/or large spines remains to be determined. Of interest is that ICAM-5 did stimulate a non-significant trend towards an increase in filopodia formation, and though filopodia would not be expected to contribute to the increase in mEPSC frequency, this trend is consistent with previous reports showing that select MMPs and integrin binding ligands may influence the morphology of dendritic protrusions [25,62] In particular, a recent study showed that chemical LTP could stimulate rapid, MMP-dependent, development of spine head protrusions and that spines with protrusions gained post synaptic GluAs [62]. It is also of interest that theta burst stimulation has been linked to changes in the morphology of dendritic protrusions with a short lived increase in dendritic filopodia at 30 minutes post-stimulation, and an increase in the width of existing spines that is notable at 2h post-stimulation [63].
It should be mentioned that an increase in mini frequency can also reflect an increase in the probability of neurotransmitter release. We could not perform paired pulse facilitation (PPF) experiments with neuronal cultures and due to the relatively large size of soluble ICAM-5, paired pulse experiments in slices were not pursued. Previous studies that have examined β1 integrin signaling in synaptic plasticity have demonstrated defects in AMPAR transmission and LTP [64,65] but no changes in PPF [65]. Of interest, mice with a postnatal knock down of β1 had a phenotype similar to animals with knock outs of GluA1 [65]. Thus, while we cannot conclusively rule out the possibility that ICAM-5 might also stimulate a change in transmitter release probability, our subsequent experiments followed up on the potential for ICAM-5 to stimulate changes in the phosphorylation and surface expression of GluA1, a subunit that has been linked to post synaptic effects including activation of post synaptically silent synapses [56,66].
Consistent with a post synaptic locus for mEPSC frequency results, ICAM-5 stimulated an increase in membrane levels of GluA1, and an increase serine 845 phosphorylation of this subunit. Prior studies suggest that serine 845 phosphorylation and synaptic incorporation of GluA1 can occur with LTP [67–70], fear memory [44], and unsilencing of synapses by electrical stimulation [55]. GluA subunit phosphorylation can influence both receptor function and subunit exocytosis [71]. Synaptic incorporation of GluA1 has been shown to increase with LTP as a consequence of subunit movement by lateral diffusion [67]. This is followed, minutes later, by exocytosis of intracellular GluA1 primarily onto the dendritic shaft and potentially to replenish pools for future lateral movement [67]. GluA2 is also important to LTP, and though we did not observe increased membrane levels of GluA2 or examine the phosphorylation or specific function of this subunit, calcium impermeable GluA2 containing receptors could be incorporated into the membrane on a different timescale and/or in response to ICAM-5/integrin independent events that follow LTP induction [72].
While LTP and learning likely increase GluA1 insertion through varied mechanisms including several that are MMP independent, our results suggest that soluble ICAM-5 might also contribute. Though future studies will be necessary to determine mechanisms by which ICAM-5 can influence GluA1 phoshorylation and insertion, integrin signaling has recently been linked to the activation of protein kinase A [45,73], a kinase that can phosphorylate the GluA1 subunit at serine 845 [71]. Non-mutually exclusive mechanisms by which the ICAM-5 ectodomain could contribute to changes in the phosphorylation of GluA1 include integrin dependent phosphorylation of GluN subunits [23] to increase NMDA receptor mediated calcium influx and subsequent GluA1 phosphorylation. Another possibility, though yet untested, is that a kinase more typically linked to integrin signaling, such as Akt, might phosphorylate GluA1.
In summary, we have shown that in DIV 14 neurons, the soluble ICAM-5 ectodomain can increase membrane levels of GluA1 and glutamatergic transmission as determined by a significant increase in the frequency of mEPSCs. If soluble ICAM-5 has the same effects in vivo, it could belong to a subset of synaptic CAMs that are shed in a neuronal activity dependent manner to enhance excitatory neurotransmission. Its in vitro effects and in vivo expression on plasticity spines of the telencephalon make it a molecule of interest that warrants further study. Future studies using CAM cleavage resistant mutants, and unbiased approaches to determine which CAM ectodomains show increased co immunoprecipitation with integrins in the setting of LTP, may be indicated.
Funding Statement
This work was supported in part by the National Multiple Sclerosis Society, the National Institutes of Mental Health (MH096822), and the von Matsch Professorship in Neurological diseases. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McCawley LJ, Matrisian LM (2001) Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol 13: 534-540. doi:10.1016/S0955-0674(00)00248-9. PubMed: 11544020. [DOI] [PubMed] [Google Scholar]
- 2. Milward EA, Fitzsimmons C, Szklarczyk A, Conant K (2007) The matrix metalloproteinases and CNS plasticity: an overview. J Neuroimmunol 187: 9-19. doi:10.1016/j.jneuroim.2007.04.010. PubMed: 17555826. [DOI] [PubMed] [Google Scholar]
- 3. Huntley GW (2012) Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci 13: 743-757. doi:10.1038/nrn3320. PubMed: 23047773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wiera G, Wozniak G, Bajor M, Kaczmarek L, Mozrzymas JW (2013) Maintenance of long-term potentiation in hippocampal mossy fiber - CA3 pathway requires fine-tuned MMP-9 proteolytic activity. Hippocampus 23: 529-543. doi:10.1002/hipo.22112. PubMed: 23418057. [DOI] [PubMed] [Google Scholar]
- 5. Brown TE, Forquer MR, Cocking DL, Jansen HT, Harding JW et al. (2007) Role of matrix metalloproteinases in the acquisition and reconsolidation of cocaine-induced conditioned place preference. Learn Mem 14: 214-223. doi:10.1101/lm.476207. PubMed: 17353546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown TE, Forquer MR, Harding JW, Wright JW, Sorg BA (2008) Increase in matrix metalloproteinase-9 levels in the rat medial prefrontal cortex after cocaine reinstatement of conditioned place preference. Synapse 62: 886-889. doi:10.1002/syn.20562. PubMed: 18792988. [DOI] [PubMed] [Google Scholar]
- 7. Meighan PC, Meighan SE, Davis CJ, Wright JW, Harding JW (2007) Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem 102: 2085-2096. doi:10.1111/j.1471-4159.2007.04682.x. PubMed: 17587312. [DOI] [PubMed] [Google Scholar]
- 8. Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P et al. (2006) Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci 26: 1923-1934. doi:10.1523/JNEUROSCI.4359-05.2006. PubMed: 16481424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Michaluk P, Kolodziej L, Mioduszewska B, Wilczynski GM, Dzwonek J et al. (2007) Beta-dystroglycan as a target for MMP-9, in response to enhanced neuronal activity. J Biol Chem 282: 16036-16041. doi:10.1074/jbc.M700641200. PubMed: 17426029. [DOI] [PubMed] [Google Scholar]
- 10. Pauly T, Ratliff M, Pietrowski E, Neugebauer R, Schlicksupp A et al. (2008) Activity-dependent shedding of the NMDA receptor glycine binding site by matrix metalloproteinase 3: a PUTATIVE mechanism of postsynaptic plasticity. PLOS ONE 3: e2681. doi:10.1371/journal.pone.0002681. PubMed: 18629001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Conant K, Wang Y, Szklarczyk A, Dudak A, Mattson MP et al. (2010) Matrix metalloproteinase-dependent shedding of intercellular adhesion molecule-5 occurs with long-term potentiation. Neuroscience 166: 508-521. doi:10.1016/j.neuroscience.2009.12.061. PubMed: 20045450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilczynski GM, Konopacki FA, Wilczek E, Lasiecka Z, Gorlewicz A et al. (2008) Important role of matrix metalloproteinase 9 in epileptogenesis. J Cell Biol 180: 1021-1035. doi:10.1083/jcb.200708213. PubMed: 18332222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sbai O, Ferhat L, Bernard A, Gueye Y, Ould-Yahoui A et al. (2008) Vesicular trafficking and secretion of matrix metalloproteinases-2, -9 and tissue inhibitor of metalloproteinases-1 in neuronal cells. Mol Cell Neurosci 39: 549-568. doi:10.1016/j.mcn.2008.08.004. PubMed: 18817873. [DOI] [PubMed] [Google Scholar]
- 14. Kean MJ, Williams KC, Skalski M, Myers D, Burtnik A et al. (2009) VAMP3, syntaxin-13 and SNAP23 are involved in secretion of matrix metalloproteinases, degradation of the extracellular matrix and cell invasion. J Cell Sci 122: 4089-4098. doi:10.1242/jcs.052761. PubMed: 19910495. [DOI] [PubMed] [Google Scholar]
- 15. Dziembowska M, Milek J, Janusz A, Rejmak E, Romanowska E et al. (2012) Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci 32: 14538-14547. doi:10.1523/JNEUROSCI.6028-11.2012. PubMed: 23077039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee KF, Soares C, Beique JC (2012) Examining form and function of dendritic spines. Neural Plast: 2012: 704103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nimchinsky EA, Sabatini BL, Svoboda K (2002) Structure and function of dendritic spines. Annu Rev Physiol 64: 313-353. doi:10.1146/annurev.physiol.64.081501.160008. PubMed: 11826272. [DOI] [PubMed] [Google Scholar]
- 18. Kessels HW, Malinow R (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61: 340-350. doi:10.1016/j.neuron.2009.01.015. PubMed: 19217372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Malinow R, Malenka RC (2002) AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103-126. doi:10.1146/annurev.neuro.25.112701.142758. PubMed: 12052905. [DOI] [PubMed] [Google Scholar]
- 20. Kerchner GA, Nicoll RA (2008) Silent synapses and the emergence of a postsynaptic mechanism for LTP. Nat Rev Neurosci 9: 813-825. doi:10.1038/nrg2472. PubMed: 18854855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang XB, Bozdagi O, Nikitczuk JS, Zhai ZW, Zhou Q et al. (2008) Extracellular proteolysis by matrix metalloproteinase-9 drives dendritic spine enlargement and long-term potentiation coordinately. Proc Natl Acad Sci U S A 105: 19520-19525. doi:10.1073/pnas.0807248105. PubMed: 19047646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kramar EA, Lin B, Rex CS, Gall CM, Lynch G (2006) Integrin-driven actin polymerization consolidates long-term potentiation. Proc Natl Acad Sci U S A 103: 5579-5584. doi:10.1073/pnas.0601354103. PubMed: 16567651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bernard-Trifilo JA, Kramar EA, Torp R, Lin CY, Pineda EA et al. (2005) Integrin signaling cascades are operational in adult hippocampal synapses and modulate NMDA receptor physiology. J Neurochem 93: 834-849. doi:10.1111/j.1471-4159.2005.03062.x. PubMed: 15857387. [DOI] [PubMed] [Google Scholar]
- 24. Mortillo S, Elste A, Ge Y, Patil SB, Hsiao K et al. (2012) Compensatory redistribution of neuroligins and N-cadherin following deletion of synaptic beta1-integrin. J Comp Neurol 520: 2041-2052. doi:10.1002/cne.23027. PubMed: 22488504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shi Y, Ethell IM (2006) Integrins control dendritic spine plasticity in hippocampal neurons through NMDA receptor and Ca2+/calmodulin-dependent protein kinase II-mediated actin reorganization. J Neurosci 26: 1813-1822. doi:10.1523/JNEUROSCI.4091-05.2006. PubMed: 16467530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ning L, Tian L, Smirnov S, Vihinen H, Llano O et al. (2013) Interactions between Intercellular Adhesion Molecule-5 (ICAM-5) and beta1 integrins regulate neuronal synapse formation. J Cell Sci 126: 77-89. doi:10.1242/jcs.106674. PubMed: 23015592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pozo K, Cingolani LA, Bassani S, Laurent F, Passafaro M et al. (2012) Beta3 integrin interacts directly with GluA2 AMPA receptor subunit and regulates AMPA receptor expression in hippocampal neurons. Proc Natl Acad Sci U S A 109: 1323-1328. doi:10.1073/pnas.1113736109. PubMed: 22232691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Conant K, Lim ST, Randall B, Maguire-Zeiss KA (2012) Matrix metalloproteinase dependent cleavage of cell adhesion molecules in the pathogenesis of CNS dysfunction with HIV and methamphetamine. Curr HIV Res 10: 384-391. doi:10.2174/157016212802138733. PubMed: 22591362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tian L, Stefanidakis M, Ning L, Van Lint P, Nyman-Huttunen H et al. (2007) Activation of NMDA receptors promotes dendritic spine development through MMP-mediated ICAM-5 cleavage. J Cell Biol 178: 687-700. doi:10.1083/jcb.200612097. PubMed: 17682049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matsuno H, Okabe S, Mishina M, Yanagida T, Mori K et al. (2006) Telencephalin slows spine maturation. J Neurosci 26: 1776-1786. doi:10.1523/JNEUROSCI.2651-05.2006. PubMed: 16467526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Furutani Y, Matsuno H, Kawasaki M, Sasaki T, Mori K et al. (2007) Interaction between telencephalin and ERM family proteins mediates dendritic filopodia formation. J Neurosci 27: 8866-8876. doi:10.1523/JNEUROSCI.1047-07.2007. PubMed: 17699668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niedringhaus M, Chen X, Dzakpasu R, Conant K (2012) MMPs and soluble ICAM-5 increase neuronal excitability within in vitro networks of hippocampal neurons. PLOS ONE 7: e42631. doi:10.1371/journal.pone.0042631. PubMed: 22912716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Conant K, Lonskaya I, Szklarczyk A, Krall C, Steiner J et al. (2011) Methamphetamine-associated cleavage of the synaptic adhesion molecule intercellular adhesion molecule-5. J Neurochem 118: 521-532. doi:10.1111/j.1471-4159.2010.07153.x. PubMed: 21166806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pak DT, Yang S, Rudolph-Correia S, Kim E, Sheng M (2001) Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron 31: 289-303. doi:10.1016/S0896-6273(01)00355-5. PubMed: 11502259. [DOI] [PubMed] [Google Scholar]
- 35. Murase K, Ryu PD, Randic M (1989) Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett 103: 56-63. doi:10.1016/0304-3940(89)90485-0. PubMed: 2476693. [DOI] [PubMed] [Google Scholar]
- 36. Dumanis SB, Cha HJ, Song JM, Trotter JH, Spitzer M et al. (2011) ApoE receptor 2 regulates synapse and dendritic spine formation. PLOS ONE 6: e17203. doi:10.1371/journal.pone.0017203. PubMed: 21347244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoe HS, Lee KJ, Carney RS, Lee J, Markova A et al. (2009) Interaction of reelin with amyloid precursor protein promotes neurite outgrowth. J Neurosci 29: 7459-7473. doi:10.1523/JNEUROSCI.4872-08.2009. PubMed: 19515914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee KJ, Moussa CE, Lee Y, Sung Y, Howell BW et al. (2010) Beta amyloid-independent role of amyloid precursor protein in generation and maintenance of dendritic spines. Neuroscience 169: 344-356. doi:10.1016/j.neuroscience.2010.04.078. PubMed: 20451588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bilousova TV, Rusakov DA, Ethell DW, Ethell IM (2006) Matrix metalloproteinase-7 disrupts dendritic spines in hippocampal neurons through NMDA receptor activation. J Neurochem 97: 44-56. doi:10.1111/j.1471-4159.2006.03701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michaluk P, Wawrzyniak M, Alot P, Szczot M, Wyrembek P et al. (2011) Influence of matrix metalloproteinase MMP-9 on dendritic spine morphology. J Cell Sci 124: 3369-3380. doi:10.1242/jcs.090852. PubMed: 21896646. [DOI] [PubMed] [Google Scholar]
- 41. Goel A, Xu LW, Snyder KP, Song L, Goenaga-Vazquez Y et al. (2011) Phosphorylation of AMPA receptors is required for sensory deprivation-induced homeostatic synaptic plasticity. PLOS ONE 6: e18264. doi:10.1371/journal.pone.0018264. PubMed: 21483826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL et al. (2003) PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci 6: 136-143. doi:10.1038/nn997. PubMed: 12536214. [DOI] [PubMed] [Google Scholar]
- 43. He K, Song L, Cummings LW, Goldman J, Huganir RL et al. (2009) Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc Natl Acad Sci U S A 106: 20033-20038. PubMed: 19892736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clem RL, Huganir RL (2010) Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science 330: 1108-1112. doi:10.1126/science.1195298. PubMed: 21030604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Alenghat FJ, Tytell JD, Thodeti CK, Derrien A, Ingber DE (2009) Mechanical control of cAMP signaling through integrins is mediated by the heterotrimeric Galphas protein. J Cell Biochem 106: 529-538. doi:10.1002/jcb.22001. PubMed: 19170051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kwon HB, Sabatini BL (2011) Glutamate induces de novo growth of functional spines in developing cortex. Nature 474: 100-104. doi:10.1038/nature09986. PubMed: 21552280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A et al. (2003) A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell 114: 635-645. doi:10.1016/j.cell.2003.08.008. PubMed: 13678586. [DOI] [PubMed] [Google Scholar]
- 48. Jordan BA, Kreutz MR (2009) Nucleocytoplasmic protein shuttling: the direct route in synapse-to-nucleus signaling. Trends Neurosci 32: 392-401. doi:10.1016/j.tins.2009.04.001. PubMed: 19524307. [DOI] [PubMed] [Google Scholar]
- 49. Bajor M, Kaczmarek L (2013) Proteolytic Remodeling of the Synaptic Cell Adhesion Molecules (CAMs) by Metzincins in Synaptic Plasticity. Neurochem Res 38: 1113-1121. doi:10.1007/s11064-012-0919-6. PubMed: 23124395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rivera S, Khrestchatisky M, Kaczmarek L, Rosenberg GA, Jaworski DM (2010) Metzincin proteases and their inhibitors: foes or friends in nervous system physiology? J Neurosci 30: 15337-15357. doi:10.1523/JNEUROSCI.3467-10.2010. PubMed: 21084591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Howell MD, Gottschall PE (2012) Lectican proteoglycans, their cleaving metalloproteinases, and plasticity in the central nervous system extracellular microenvironment. Neuroscience 217: 6-18. doi:10.1016/j.neuroscience.2012.05.034. PubMed: 22626649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Van Hove I, Lemmens K, Van de Velde S, Verslegers M, Moons L (2012) Matrix metalloproteinase-3 in the central nervous system: a look on the bright side. J Neurochem 123: 203-216. doi:10.1111/j.1471-4159.2012.07900.x. PubMed: 22862420. [DOI] [PubMed] [Google Scholar]
- 53. Lee R, Kermani P, Teng KK, Hempstead BL (2001) Regulation of cell survival by secreted proneurotrophins. Science 294: 1945-1948. doi:10.1126/science.1065057. PubMed: 11729324. [DOI] [PubMed] [Google Scholar]
- 54. Larsen PH, DaSilva AG, Conant K, Yong VW (2006) Myelin formation during development of the CNS is delayed in matrix metalloproteinase-9 and -12 null mice. J Neurosci 26: 2207-2214. doi:10.1523/JNEUROSCI.1880-05.2006. PubMed: 16495447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Emond MR, Montgomery JM, Huggins ML, Hanson JE, Mao L et al. (2010) AMPA receptor subunits define properties of state-dependent synaptic plasticity. J Physiol 588: 1929-1946. doi:10.1113/jphysiol.2010.187229. PubMed: 20351044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liao D, Scannevin RH, Huganir R (2001) Activation of silent synapses by rapid activity-dependent synaptic recruitment of AMPA receptors. J Neurosci 21: 6008-6017. PubMed: 11487624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gu J, Lee CW, Fan Y, Komlos D, Tang X et al. (2010) ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci 13: 1208-1215. doi:10.1038/nn.2634. PubMed: 20835250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Abrahamsson T, Gustafsson B, Hanse E (2008) AMPA silencing is a prerequisite for developmental long-term potentiation in the hippocampal CA1 region. J Neurophysiol 100: 2605-2614. doi:10.1152/jn.90476.2008. PubMed: 18799599. [DOI] [PubMed] [Google Scholar]
- 59. Beique JC, Lin DT, Kang MG, Aizawa H, Takamiya K et al. (2006) Synapse-specific regulation of AMPA receptor function by PSD-95. Proc Natl Acad Sci U S A 103: 19535-19540. doi:10.1073/pnas.0608492103. PubMed: 17148601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kerrisk ME, Greer CA, Koleske AJ (2013) Integrin alpha3 Is Required for Late Postnatal Stability of Dendrite Arbors, Dendritic Spines and Synapses, and Mouse Behavior. J Neurosci 33: 6742-6752. doi:10.1523/JNEUROSCI.0528-13.2013. PubMed: 23595732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Warren MS, Bradley WD, Gourley SL, Lin YC, Simpson MA et al. (2012) Integrin beta1 signals through Arg to regulate postnatal dendritic arborization, synapse density, and behavior. J Neurosci 32: 2824-2834. doi:10.1523/JNEUROSCI.3942-11.2012. PubMed: 22357865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Szepesi Z, Bijata M, Ruszczycki B, Kaczmarek L, Wlodarczyk J (2013) Matrix Metalloproteinases Regulate the Formation of Dendritic Spine Head Protrusions during Chemically Induced Long-Term Potentiation. PLOS ONE 8: e63314. doi:10.1371/journal.pone.0063314. PubMed: 23696812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bourne JN, Harris KM (2011) Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus 21: 354-373. doi:10.1002/hipo.20768. PubMed: 20101601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang Z, Shimazu K, Woo NH, Zang K, Muller U et al. (2006) Distinct roles of the beta 1-class integrins at the developing and the mature hippocampal excitatory synapse. J Neurosci 26: 11208-11219. doi:10.1523/JNEUROSCI.3526-06.2006. PubMed: 17065460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD et al. (2006) Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci 26: 223-232. doi:10.1523/JNEUROSCI.4110-05.2006. PubMed: 16399691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Selcher JC, Xu W, Hanson JE, Malenka RC, Madison DV (2012) Glutamate receptor subunit GluA1 is necessary for long-term potentiation and synapse unsilencing, but not long-term depression in mouse hippocampus. Brain Res 1435: 8-14. doi:10.1016/j.brainres.2011.11.029. PubMed: 22197030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Makino H, Malinow R (2009) AMPA receptor incorporation into synapses during LTP: the role of lateral movement and exocytosis. Neuron 64: 381-390. doi:10.1016/j.neuron.2009.08.035. PubMed: 19914186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kopec CD, Li B, Wei W, Boehm J, Malinow R (2006) Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation. J Neurosci 26: 2000-2009. doi:10.1523/JNEUROSCI.3918-05.2006. PubMed: 16481433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC et al. (2000) Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science 287: 2262-2267. doi:10.1126/science.287.5461.2262. PubMed: 10731148. [DOI] [PubMed] [Google Scholar]
- 70. Shi S, Hayashi Y, Esteban JA, Malinow R (2001) Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell 105: 331-343. doi:10.1016/S0092-8674(01)00321-X. PubMed: 11348590. [DOI] [PubMed] [Google Scholar]
- 71. Lee HK, Takamiya K, Han JS, Man H, Kim CH et al. (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112: 631-643. doi:10.1016/S0092-8674(03)00122-3. PubMed: 12628184. [DOI] [PubMed] [Google Scholar]
- 72. Adesnik H, Nicoll RA (2007) Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci 27: 4598-4602. doi:10.1523/JNEUROSCI.0325-07.2007. PubMed: 17460072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lim CJ, Kain KH, Tkachenko E, Goldfinger LE, Gutierrez E et al. (2008) Integrin-mediated protein kinase A activation at the leading edge of migrating cells. Mol Biol Cell 19: 4930-4941. doi:10.1091/mbc.E08-06-0564. PubMed: 18784251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Partridge JG, Janssen MJ, Chou DY, Abe K, Zukowska Z et al. (2009) Excitatory and inhibitory synapses in neuropeptide Y-expressing striatal interneurons. J Neurophysiol 102: 3038-3045. doi:10.1152/jn.00272.2009. PubMed: 19759327. [DOI] [PMC free article] [PubMed] [Google Scholar]