Abstract
Adipokine adiponectin (APN) has been recently reported to play a role in regulating bone mineral density (BMD). To explore the mechanism by which APN affects BMD, we investigated BMD and biomechanical strength properties of the femur and vertebra in sham-operated (Sham) and ovariectomized (OVX) APN knockout (KO) mice as compared to their operated wild-type (WT) littermates. The results show that APN deficiency has no effect on BMD but induces increased ALP activity and osteoclast cell number. While OVX indeed leads to significant bone loss in both femora and vertebras of WT mice with comparable osteogenic activity and a significant increase in osteoclast cell number when compared to that of sham control. However, no differences in BMD, ALP activity and osteoclast cell number were found between Sham and OVX mice deficient for APN. Further studies using bone marrow derived mesenchymal stem cells (MSCs) demonstrate an enhanced osteogenic differentiation and extracellular matrix calcification in APN KO mice. The possible mechanism for APN deletion induced acceleration of osteogenesis could involve increased proliferation of MSCs and higher expression of Runx2 and Osterix genes. These findings indicate that APN deficiency can protect against OVX-induced osteoporosis in mice, suggesting a potential role of APN in regulating the balance of bone formation and bone resorption, especially in the development of post-menopausal osteoporosis.
Introduction
Fat mass is closely associated with both bone turnover and density. Adiponectin, the most abundant protein derived from the adipocytes [1], has been reported to contribute to this relationship. Previous studies have suggested that APN and its receptors are expressed in bone-forming cells [2]. However, the manner in which APN affects bone formation remains unclear both in vivo and in vitro. Some studies reveal that human osteoblasts treated with APN display an increased proliferation, differentiation and increased mineralization, suggesting a positive effect of APN on bone formation [3], [4]. While the bone marrow cells isolated from APN-deficient mice have reduced osteogenesis as compared to WT mice [5]. In addition, C57BL/6J mice treated with an adiponectin-adenovirus for 2 weeks show an increase in trabecular bone, a decreased number of osteoclasts and reduced circulating N-telopeptides of type collagen (NTX) as compared to LacZ-adenovirus-treated mice [6]. These results suggest that APN positively regulates bone mass.
However, other studies suggest adiponectin seems to exert a negative net effect on bone mass and to be an independent predictor of lower bone mass [7]–[12]. Clinical studies show that an increasing level of APN is associated with a significantly decreased whole body bone mineral content (BMC) and BMD, indicating a negative association between APN and BMD [7], [8]. Studies with mouse models have further confirmed the role of APN in negative regulation of BMD. The data from APN transgenic mice indicate that the elevated circulating APN could inhibit the acquisition of bone mass in growing mice and result in decreased biomechanical measures of functional strength that are surrogate measures of susceptibility to fractures [9]. In agreement with these results, trabecular bone volume and trabecular number are increased at 14-week of age by 30% and 38% in APN KO male C57BL/6J mice, respectively [10]. Meanwhile, another study using 8-week old transgenic male mice in which APN is specifically expressed in the liver reveals the differences neither in BMD of femur, tibia or vertebrae, nor in bone formation or resorption parameters as compared to their WT littermates [13].
In order to investigate the function of APN in bone metabolism, we generated APN KO mice [14], [15]. Although we did not find any significant differences in bone formation between the two genotypes under physiological conditions, we believed that APN would play an important role in some pathological processes related to bone metabolism. In this study, we measured BMD and biomechanical strength properties of femur and vertebra in ovariectomized and sham-operated APN KO mice as compared to their operated WT littermates. We also evaluated the proliferation, differentiation, and mineralization ability of the MSCs from APN KO and WT mice. Our data indicate that APN deficiency suppresses bone loss under the condition of ovary removal, probably as a result of increased proliferation of MSCs and higher expression of Runx2 and Osterix genes.
Materials and Methods
Animal Studies
All procedures were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine. The APN KO mouse model in which the APN gene is null was generated previously in our lab [14], [15]. Briefly, a targeting vector was constructed by replacing the mouse APN genomic fragment covering exon2 and exon3, with the pGK-neo gene cassette. Genomic DNA from mouse tails was used for genotyping by PCR. The mRNA expression level of APN was identified using RT-PCR and its protein level was detected using an APN ELISA kit (RayBiotech Inc., Norcross, GA). The primer sequences are listed in Table 1. Congenic C57BL/129S1 APN KO and WT mice were housed under l2-h light/12-h dark cycles in a controlled environment with 40–50% relative humidity at 22°C. All experimental procedures were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were individually caged, given free access to regular water and chow (Shanghai SLRC laboratory animal LLC, Shanghai, China, national standard number of laboratory animals’ nutrients for formula feeds is GB 14924.3-2010) before and after ovariectomy surgery. Age-matched (6–8 weeks of age) KO female mice and their WT littermates were weighed and then underwent OVX or sham-operation. The number of mice in WT.Sham, WT.OVX, KO.Sham and KO.OVX was 5, 6, 6, and 7, respectively. All surgeries were performed by one person under sodium pentobarbital anesthesia, and all efforts were made to minimize animal suffering and to reduce the number of animals used. 10 weeks post-surgery, mice were weighed again and then euthanized by cervical dislocation. The left femora and the L2–5 vertebrae with the skin and muscle removed were kept in physiological saline and stored at −20°C for detection of BMD and for biomechanics procedures. For protein and RNA analysis, the right femora, L1 vertebrae and tibiae were frozen in liquid nitrogen, and stored at −80°C until use.
Table 1. List of the primers used in the study.
| Productc | Sequence | Size (bp) |
| WT allele 1 | Up: GAGAGTCCTGAGTATTATCCACACG | 4540 |
| Low: TATTTTTTACTGCCGCTATCTGG | ||
| MT allele 1 | Up: GCTACCGGTGGATGTGGAAT | 3680 |
| Low: CTGCTCTTGCCCCAAACTGA | ||
| WT allele 2 | Up: GTAGATGCAGATCTTTGGAGTGGA | 691 |
| Low: AATCATGGTACTGAGTGCTTTAGAA | ||
| MT allele 2 | Up: TGGGGATTGGAGAGATGGCTTA | 904 |
| Low: TTTGAGGGGACGACGACAGTATC | ||
| β-actin | Up: ACGGCCAGGTCATCACTATTG | 351 |
| Low: CCTGCTTGCTGATCCACATCT | ||
| APN | Up: ACCAGGCCGTGATGGCAGAGAT | 839 |
| Low: TGTGAAGCCCCCATACCAAATGTG | ||
| ALP | Up: TCGGGACTGGTACTCGGATAA | 779 |
| Low: CTGGTAGTTGTGAGCGTAAT | ||
| OC | Up: CTCTGTCTCTCTGACCTCACAG | 360 |
| Low: GGAGCTGCTGTGACATCCATAC | ||
| Col1α | Up: GAGGCATTAAGGGTCATCGTGG | 761 |
| Low: CATTAGGCGCAGGAAGGTCAGC | ||
| Osterix | Up: GGCAAGAGGTTCACTCGTTC | 497 |
| Low: GTCTGACTGGCCTCCTCTTC | ||
| RUNX2 | Up: GAGGTACCAGAT GGGACTGTG | 103 |
| Low: TCGTTGAACCTTGCTACTTGG |
Measurement of Bone Mineral Density by DEXA
Bone mineral density (BMD, mg/cm2) was measured for the left femora and L2–5 vertebrae by dual-energy X-ray absorptiometry (DEXA) using a PIXImus Instrument (GE Lunar, Madison, WI).
Bone Biomechanical Testing
Prior to biomechanical testing, BMD of the femora and L2–5 vertebrae was measured, as described above. Then the femora were used for the three-point bend testing that was performed by a computer-controlled mechanical testing machine (Instron-5543, USA) equipped with a 500N M-SI sensor (Celtron) under the following conditions: sample space, 9 mm and plunger speed, 1.8 mm/min at room temperature. The load-deformation curve was plotted, and based on this curve, the bone biomechanical indices, including the energy, maximum load and maximum stress were calculated. The L3 vertebra was loaded to failure by compression testing using the same equipment. The parameters were obtained via a computer-assisted analyzer installed in the load cell.
Bone Histomorphometry and Histological Analysis
The left femora and L1 vertebrae with the skin and muscle removed were fixed in 10% formaldehyde for a week. Then they were rinsed with ddH2O and decalcified in 10% EDTA-2Na in 0.1 M Tris buffer (pH 7.3) for 4 weeks at room temperature. After washed with phosphate buffer, the decalcified specimens were then embedded in paraffin and sectioned with a microtome. H&E staining was performed to study the histological changes. Tartrate-resistant acid phosphatase, a marker enzyme for osteoclasts, was detected using enzyme histochemistry with naphthol AS-MX phosphate (Sigma–Aldrich Corp.).
ALP Activity Assay
Frozen L1 vertebrae were homogenized in 0.5% NETN buffer containing protease inhibitor cocktail (Sigma, St. Louis, MO, USA) with a homogenizer at 4°C. After centrifuging at 12,000 g for 10 minutes at 4°C, the supernatant protein was quantified using BCA protein assay reagent (Pierce, Rockford, IL). ALP activity was quantified using an ALP detection kit (Nanjing Jiancheng Biotechnology Institute, Nanjing, China) and a spectrophotometer (Bio-Rad, Hercules, CA, USA) at a wavelength of 520 nm. Each value was normalized against the protein amount in the reaction, and expressed as “U/gprot”, according to the manufacture’s instruction.
Semi-quantitative RT-PCR Analysis
Differential gene expression was detected by semi-quantitative RT-PCR. Total RNA was isolated from frozen lumbar using the TRIzol reagent according to the manufacturer’s protocol (Invitrogen, Carlsbad, CA, USA). Semi-quantitative RT-PCR was performed with cDNA reverse-transcribed from 1 µg of total RNA using AMV reverse transcriptase (Takara, Otsu, Japan). The cycle threshold values were normalized to the expression of the housekeeping gene β-actin. Band density was scanned and calculated.
Bone Marrow Cell Cultures
The number of adherent mesenchymal stem cell (MSC) colonies was determined by the following established procedures [16]. Briefly, bone marrow cells were collected from the femora and tibiae of 8-week-old APN KO female mice and WT littermates. For colony-forming assays, cells were plated at a density of 5×106 cells/well in a 12-multi-well plate and cultured for 8 days in α-MEM (Gibco, CA, USA) containing 10% FBS, 100 units/ml penicillin and 100 µg/ml streptomycin. Media was changed every third day. On day 8, cells were fixed with 4% paraformaldehyde, stained with 0.5% crystal violet in 4% paraformaldehyde. The number of colonies was then counted. Colonies of less than 2 mm in diameter and faintly stained colonies were ignored [17]. MSC proliferation assay was performed by cytometry. Cells with different genotype were plated at same density in a 12-multi-well plate. On days 6, 7 and 8, cells were collected by trypsin digestion and cell number was counted using a Beckman Coulter Vi-cell XR Cell Viability Analyzer.
In vitro ALP Activity and Ca2+ Content
For ALP and Ca2+ analysis, cells were plated at a density of 2×107 cells/well in a 6-multi-well plate in α-MEM containing 10% FBS. After 8 days of culture, cells were washed twice with PBS and lysed in 0.5% NETN buffer containing a protease inhibitor cocktail (Sigma). ALP activity and Ca2+ content in the lysate were measured using different kits (Jiancheng Technology) and the protein content was determined using BCA protein assay reagent (Pierce Biotechnology). ALP activity and Ca2+ content were measured using certain amount of protein and normalized against the protein amount in the reaction, and expressed as “U/gprot” and “mmol/gprot”, respectively.
For the analysis of osteogenesis, bone marrow cells were plated at a density of 5×106 cells/well in a 12-multi-well plate in α-MEM containing 10% FBS and cultured for 8 days. Cells were further induced for 3 days with added ascorbic acid, dexamethasone and β-glycerophosphate. On day 12, culture plates were rinsed with PBS, fixed with 4% paraformaldehyde, and stained with 2% Alizarin red S (pH 4.0) (Sigma) [5]. For mRNA assays, cells were plated at a density of 2×107 cells/well in a 6-multi-well plate and cultured for 8 days in α-MEM containing 10% FBS. Total RNA was extracted from the cultures, using the TRIzol reagent RNA extraction protocol according to manufacturer’s instructions (Invitrogen). Semi-quantitative RT-PCR was performed by using cDNA reverse-transcribed from 1 µg of total RNA. PCR cycles varied from 26 to 34 cycles. Band density was scanned and calculated.
Statistical Analysis
The results are expressed as mean±standard deviation (SD) from at least 3 independent experiments. The statistical analysis was performed using Student t test or analysis of variance (ANOVA) with post hoc Scheffe testing when appropriate (SPSS v19). P<0.05 was considered statistically significant.
Results
Deficiency of APN Inhibits OVX-induced Osteoporosis in Mice
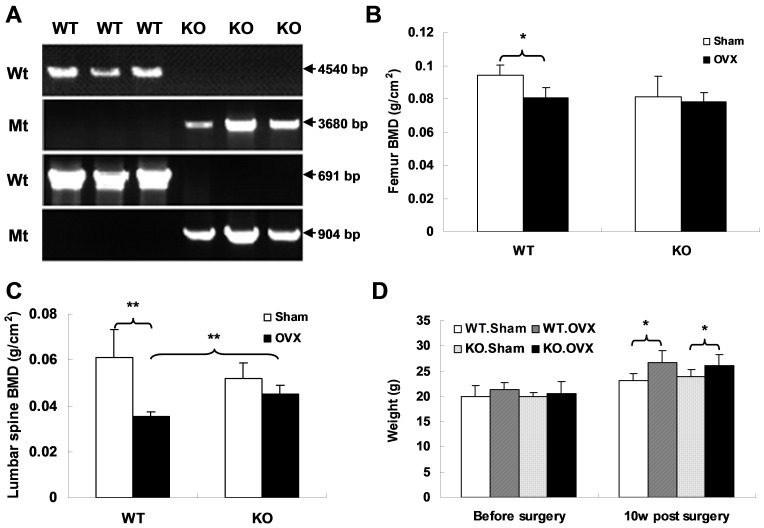
The APN KO mouse model was generated by the homologous recombination method. Exons 2 and 3 of the APN gene were replaced with the pGK-neo gene. Genomic DNA from mouse tails was used for genotyping by PCR reactions with two different pairs of primers (Figure 1A). To investigate the role of APN in bone metabolism, we analyzed BMD in APN KO mice, as well as in their age- and sex-matched WT littermates. We found that there is no significant difference in BMD between APN KO and WT mice (data not shown). We therefore performed OVX or sham surgery when the mice were 8 weeks old. 10 weeks after surgery, several indices were measured to evaluate the bone metabolism upon OVX between two different genotypes of mice. We found that the BMD of both femora and vertebrae decreased significantly in WT mice after OVX surgery as compared to those with sham surgery (p<0.05, Figure 1B, 1C). However, no significant differences were found in either femora or vertebrae of the APN KO mice between OVX and sham surgery groups (p>0.05, Figure 1B, 1C). To test whether OVX has any effect on body weight, APN KO and WT mice were weighed before and 10 weeks post surgery. The results show that no differences were found in body weight between APN KO and WT mice before OVX surgery, but body weight was augmented after OVX surgery both in WT and KO mice (WT.OVX vs WT.Sham = 26.6±2.45 g vs 23.12±1.49 g, p = 0.035; KO.OVX vs KO.Sham = 26.68±1.80 g vs 23.83±1.56 g, p = 0.018, Figure 1D).
Figure 1. OVX results in significant bone loss in WT but not APN KO mice.
(A) Two-pair primers were used for PCR genotyping. The products derived from both WT and targeted alleles were amplified with expected size, respectively. (B, C) BMD of femora and L2–5 vertebrae was evaluated by the DEXA method (WT.Sham, n = 5; WT.OVX, n = 6; KO.Sham, n = 6; KO.OVX, n = 7). (D) Change in body weight among the four groups was assessed either before or 10 weeks post surgery. Data is expressed as mean ± SD. *indicates p<0.05, **indicates p<0.01.
Biomechanical properties of APN KO mice and their WT littermates were further measured by a three-point bending test and a compression test. We found there were no differences of energy, maximum load or maximum stress of vertebrae and femora between two genotypes under physiological conditions with sham operation. Following OVX, WT mice showed a significant reduction in these biomechanical parameters (Table 2), indicating that bone strength in WT mice was reduced because of the surgery. However, OVX treatment did not result in statistically significant differences in these parameters in APN KO mice, as compared with sham operated mice (Table 2). This result indicates the protection of bone strength by APN deletion upon OVX surgery. Taken together, these results demonstrate that WT mice, but not KO mice have an osteoporosis phenotype after OVX. In other words, APN deficiency significantly inhibits OVX induced osteoporosis development.
Table 2. Biomechanical parameter changes in APN KO and WT mice upon OVX surgery.
| Energy (mJ) | Maximum Load(N) | Maximum Stress (Mpa) | ||||
| lumbar | femur | lumbar | femur | lumbar | femur | |
| WT.Sham | 24.39±2.49 | 4.45±0.13 | 50.69±10.50 | 23.33±0.90 | 0.59±0.01 | 105.03±5.41 |
| WT.OVX | 14.24±2.82** | 3.06±0.18** | 28.69±4.67** | 17.53±1.99** | 0.38±0.04** | 92.87±9.60* |
| KO.Sham | 20.92±8.04 | 3.31±0.53 | 47.74±2.53 | 21.04±0.79 | 0.64±0.06 | 160.25±10.07 |
| KO.OVX | 20.53±6.64 | 3.48±0.67 | 42.07±4.11 | 22.62±3.04 | 0.54±0.05 | 146.73±1.86 |
Note: Mean values ± SD are shown. WT.Sham, n = 5; WT.OVX, n = 6; KO.Sham, n = 6; KO.OVX, n = 7.
indicates significant differences between WT.OVX and WT.Sham mice at p<0.05,
indicates significant differences between WT.OVX and WT.Sham mice at p<0.01.
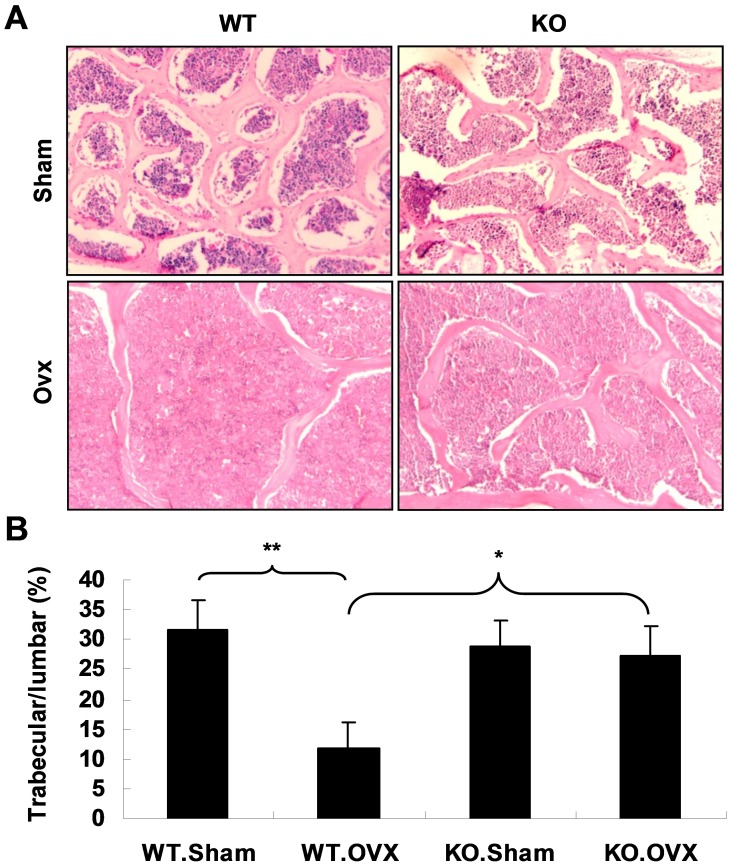
More evidence was obtained on bone histomorphometry to confirm the result. The H&E staining was performed on the sections of L1 vertebra. It was found that the average surface area of bone trabeculae dramatically decreased in WT.OVX mice compared with WT.Sham mice (Figure 2A, left, 2B). However, there is no significant difference in the ratio of surface area of bone trabeculae to that of L1 vertebrae of APN KO mice between sham and OVX operations (Figure 2A, right, 2B).
Figure 2. OVX surgery leads to dramatically decreased bone trabeculae in WT but not in APN KO mice.
(A) H&E staining on the sections of L1 vertebra shows significant reduced bone trabeculae in WT mice upon OVX, but not in APN KO mice. Original magnification 100×. (B) The ratios of bone trabeculae to vertebra area were calculated. The values are presented as mean ± SD (n = 3). Trabecular/lumbar means the ratio of bone trabeculae proportion/area to vertebra proportion/area. *indicates p<0.05, **indicates p<0.01.
Deficiency of APN Results in Increased ALP Expression and Activity
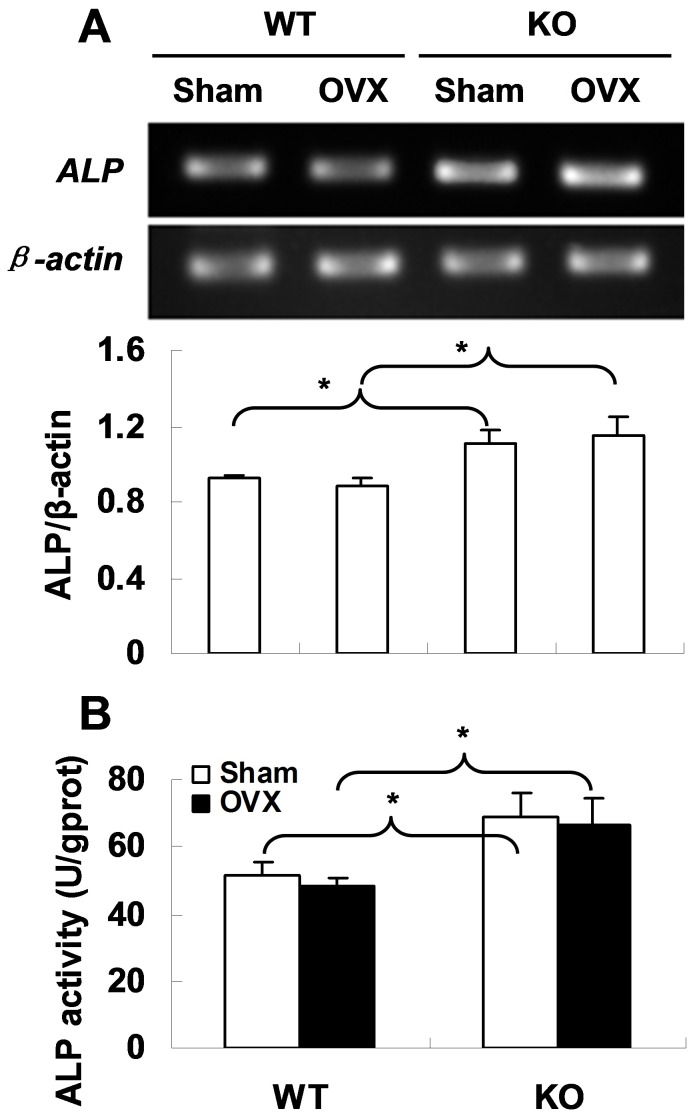
To find out why APN deletion is associated with protection of bone mass, we evaluated the osteoblast activity in mouse vertebrae by testing ALP gene expression and protein activity. The RT-PCR showed a higher ALP mRNA level in APN KO mice as compared to WT mice (Figure 3A). Consistently, ALP activity was found significantly increased in KO mice than that in WT mice (p<0.05, Figure 3B). These findings indicate that APN deficiency could benefit osteogenesis in mice. Interestingly, we found that OVX surgery had no effect on ALP expression and activity in both WT and APN KO mice (Figure 3A, 3B), suggesting that OVX-induced bone loss could be caused by other factors rather than impaired osteogenesis.
Figure 3. Increased ALP expression and activity in APN KO mice.
(A) RT-PCR shows ALP expression in mouse vertebrae of each in four groups (top) and the band density was scanned and calculated (bottom). All reactions were repeated independently more than three times. (B) Increased ALP activity in vertebrae of APN KO mice. ALP activity was normalized to the amount of protein assayed. *indicates p<0.05.
Deficiency of APN Results in Increased Osteoclastogenesis
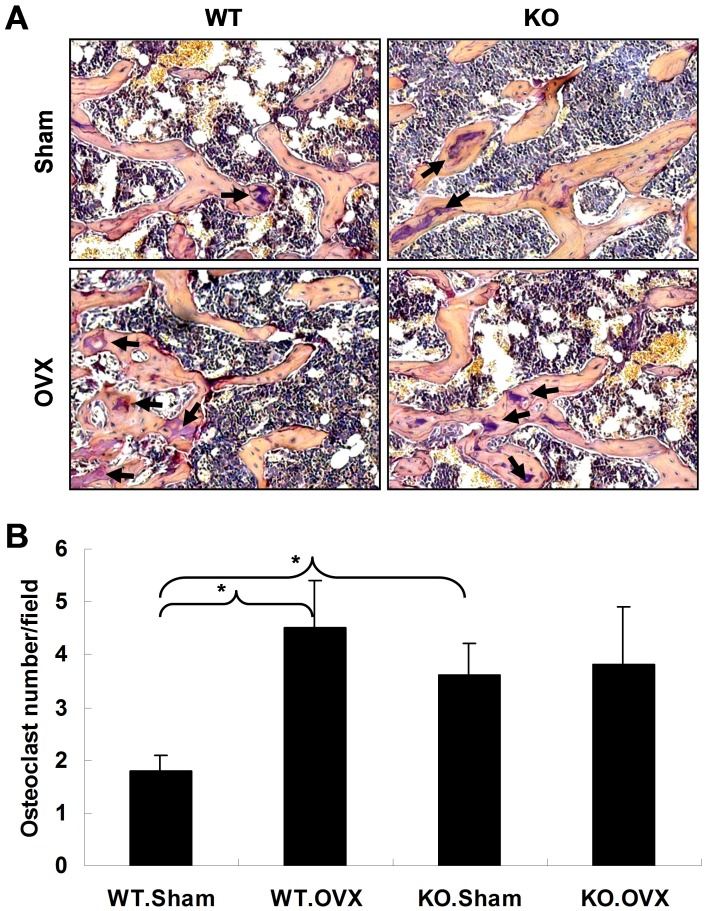
Osteoclasts in femora were visualized by tartrate-resistant acid phosphatase (TRAP) staining. As shown in figure 4A, 4B, OVX induced a significant increase in osteoclast number in the femora of WT mice when compared to that in the Sham mice. But there was no significant difference in osteoclast number between Sham and OVX mice deficient for APN. Furthermore, the number of osteoclast cells in KO mice with either sham or OVX was augmented in comparison to that of WT sham. These data suggest that APN deficiency may increase bone loss by increasing osteoclast cell number, and APN may be involved in mediating OVX-induced bone loss.
Figure 4. OVX surgery induces osteoclast formation.
(A) Femora sections were stained with tartrate resistant acid phosphatase (TRAP) for osteoclasts (arrows). Original magnification 200×. (B) Average number of osteoclasts on each section are shown. The results are expressed as mean ± SD (n = 3). *indicates p<0.05.
Deficiency of APN Enhances MSC Osteoblastic Differentiation and Consequent Extracellular Matrix Calcification
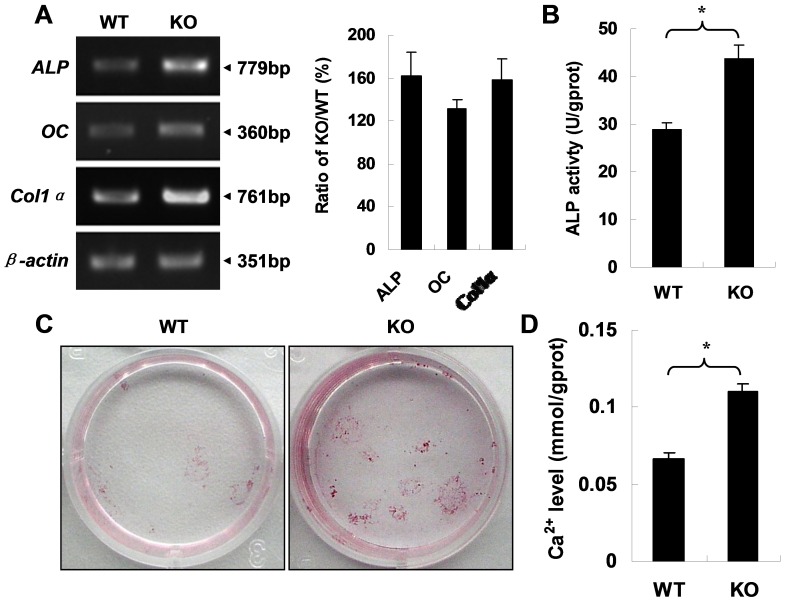
To confirm the effect of adponectin on osteogenic differentiation and mineralization, mouse bone marrow derived mesenchymal stem cells (MSCs) were isolated from APN KO mice and their WT littermates since it is well documented that MSCs can be directed towards the osteogenic lineage in vitro when they are treated with dexamethasone, β-glycerophosphate, and ascorbic acid [18]–[22]. MSCs were cultured for 8 days and induced toward osteoblastic differentiation for 3 days. Total RNA was extracted from the cultures. The mRNA expression of three differentiation-related genes as osteoblastic markers (ALP, osteocalcin and Col1α) was detected using RT-PCR, and ALP activity was evaluated. The results showed that mRNA expression (Figure 5A) and ALP activity (Figure 5B) were upregulated in the cell lysate from APN KO mice. Consistent with these data, extracellular matrix calcification was enhanced in MSCs from APN KO mice (Figure 5C, 5D). All these observations suggest that deletion of APN promotes MSC osteogenic differentiation and consequent extracellular matrix calcification.
Figure 5. Increased osteogenic differentiation and extracellular matrix calcification in MSCs from APN KO mice.
(A) RT-PCR shows that mRNA expression of the indicated genes (left). The respective ratio of band density (KO/WT, %) normalized with β-actin represents relative mRNA levels (right). A representative result of three independent experiments is shown. (B) Increased ALP activity in MSC lysate from APN KO mice. (C) Alizarin red S staining shows enhanced calcification ability of MSCs from APN KO mice (n = 3 for each). (D) Ca2+ content in cell lysate from APN KO mice is higher than that from WT mice (n = 3 for each). **indicates p<0.01.
APN Deficiency is Associated with Elevated MSC Proliferation and Expression of Runx2 and Osterix
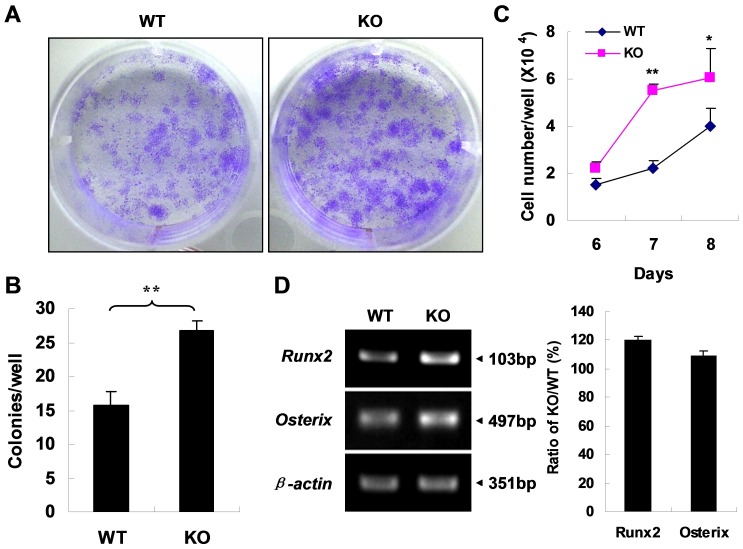
To further explore the mechanisms by which deficiency of APN promotes bone formation, the proliferation of MSCs from APN KO mice and WT littermates was evaluated. MSCs were isolated and cultured for 8 days followed by crystal violet staining (Figure 6A). The number of colonies was counted (Figure 6B). The data showed that MSCs deficient for APN showed higher proliferation than that of WT cells. To further confirm this result, the total number of MSCs at day 6–8 was counted by cytometry (Figure 6C). The result indicated that the number of APN KO MSCs increased faster than that of WT cells. These data suggest that ablation of self-produced adiponectin in vitro promotes proliferation of MSCs.
Figure 6. APN deficiency enhances MSC proliferation and osteoblastic differentiation.
(A) Crystal violet staining shows an increased number of CFU-F in MSCs from APN KO mice. (B) The graphs indicate the number of positive colonies/well by crystal violet staining. (C) MSC proliferation assay by cytometry shows increased proliferation of APN KO MSCs. *indicates p<0.05, **indicates p<0.01. (D) RT-PCR shows that up-regulation of two transcriptional factors Runx2 and Osterix in MSCs from APN KO mice. A representative result of three independent experiments is shown.
Moreover, total RNA was extracted from MSCs after 8 days of culture in ordinary medium. The RT-PCR results showed that two transcription factors, Runx2 and Osterix, that are critical for the process of osteoblastic differentiation were up-regulated in MSCs from APN KO mice (Figure 6D). Thus, our results indicate that a possible mechanism for APN deficiency induced protection of osteoporosis could be through increased proliferation of MSCs and expression of Runx2 and Osterix, thereby enhancing MSC osteoblastic differentiation and consequent extracellular matrix calcification.
Discussion
Clinical studies that report significant negative correlations between circulating APN and bone mass [7], [8], [23]–[28], suggest that APN may exert negative effects on bone mass and that these effects may be mediated by menopausal status. In the present study, we investigated the role of APN in bone formation in vivo and in vitro using an APN KO mouse model. To mimic the internal environment of postmenopausal women, we performed OVX surgery on APN KO female mice. In agreement with the literature, we found that APN deficiency protects against OVX induced reduction of BMD and biomechanical strength properties at skeletal sites. In support of these findings, in vitro data showed that APN deficiency increases MSCs osteogenic proliferation, differentiation and consequent extracellular matrix calcification.
Much evidence shows that obesity is strongly correlated with increased bone mass [29]–[33] and that reductions in body weight are related to bone loss [34]–[36]. Therefore, it would be possible that APN, an adipocyte-derived hormone, negatively associated with obesity, could have negative effects on bone. However, in the current study, we did not find significant differences in body weight between the APN KO and WT mice before or after OVX surgery, suggesting that APN deletion protects against bone loss via other pathways that have nothing to do with body weight. Lots of studies show that body weight of OVX mice increases several weeks after surgery as compared to their sham control [37], [38]. In agreement with these reports, our results show that body weight of OVX mice augments in both KO and WT mice.
Previous studies have shown that the development of alkaline phosphatase-positive cells is inhibited in preosteoblast cultures by APN [5]. In agreement with this notion, Luo XH et al. found that APN inhibited ALP activity, osteocalcin secretion, Runx2 protein expression, and the formation of mineralized nodules [39]. The present study clarifies a direct effect of APN on osteoblast cells. Specifically, we have demonstrated the effect of APN on MSC proliferation, differentiation and mineralization. The bone-forming activity of osteoblasts is important in determining bone mass [40], [41]. Our study reveals that the mRNA expression and protein activity of ALP from lumbar and femora are significantly increased in APN KO mice over those in WT mice. These findings are further confirmed by the effect of APN on the osteoblastogenesis of MSCs through an autocrine pathway, supported by a significant increase in proliferation, differentiation and mineralization activity of MSCs of APN KO mice as compared with that of WT mice. These results are similar to those reported in the literatures [5], [39]. Many osteoblast differentiation transcription factors have been identified through human genetic studies as well as through mouse genetics and molecular studies [42]. Among those, Runx2 and Osterix are required for bone formation [43]. Runx2, a member of the Runt domain family of transcription factors, has been shown to be the earliest and most powerful molecular determinant of osteoblast differentiation. Osterix is a Runx2-dependent osteoblast-specific transcription factor that is specifically expressed in osteoblasts of all skeletal elements [43]. Inactivation of Osterix in mice results in perinatal lethality owing to a complete absence of bone formation [43]. In the current study, the elevation of Osterix and Runx2 expression could explain, at least in part, the mechanism of APN deficiency effects on bone formation.
Maintenance of bone mass and integrity requires a tight balance between resorption by osteoclasts and formation by osteoblasts. Our results indicate that there is a much higher ALP activity, as well as a larger amount of osteoclasts in KO mice, compared with WT mice. This might explain why there is no significant difference in BMD between KO and WT mice without surgery. When performed surgery, osteoclasts in femora increased remarkably in WT.OVX mice, compared with that of WT.Sham mice. However, no increase has been found in KO mice when performed OVX surgery. The increased osteoclasts in WT.OVX mice may lead to enhanced bone resorption which results in significant loss of bone mass. While in APN KO mice, the increased osteogenesis might compensate the enhanced bone resorption induced by OVX.
Collectively, the data from our study indicate that APN deficiency can protect against OVX induced osteoporosis in mice. In this study, we have shown that APN deficiency has positive effect on both bone formation and resorption, reflecting a complex role of adiponectin in regulating bone metabolism. This could be the reason, at least in part, why no obvious bone phenotype was observed in several adiponectin knock-out mouse lines and adiponectin overexpressing transgenic mouse lines [5], [12]. In fact,the role of APN on BMD regulation remains controversial in the previous studies including clinical association, animal models, and in vitro studies [3]–[12]. Although we didn’t evaluate the MSC osteoclastic differentiation and proliferation in the absence of APN in vitro, we found that APN deficiency may increase bone loss through promoting osteoclastogenesis evidenced by increased osteoclast cell number in vivo. This finding is similar to previous reports [5], [44]–[46]. Since we showed that APN deficiency inhibits OVX-induced bone loss and promotes osteoclastogenesis, it is very possible that increased osteoblastic activity exerts an important role in the process of bone remodeling. Therefore, we focused our research on bone formation and found APN deficiency increases osteoblastic activity by enhancing MSC osteoblastic differentiation and consequent extracellular matrix calcification.
We have shown that APN deficiency exerts positive effect on both osteoblast and osteoclast activity in the process of OVX-induced osteoporosis in mice. It seems that APN plays a role in regulating bone metabolism through more complicated mechanism. Actually, more and more evidences suggest that the molecular adipokine-bone interactions should be considered in the context of different pathology status or diseases such as obesity and metabolic syndromes. Besides adiponectin, various biologically active molecules, such as estrogen, resistin, leptin, insulin, preptin and some proinflammatory cytokines, not only affect energy homeostasis, but are also involved in bone metabolism and, thus, contribute to the complex cross-talk between fat mass and bone [44]. This would be helpful for better understanding the contradictory effect of adiponectin on bone metabolism reported by different studies. In this study, we found that adiponectin deficiency is of advantage to regulating the balance between bone formation and bone resorption after OVX surgery and protects against OVX-induced osteoporosis in mice. This suggests that targeting APN could be therapeutically beneficial for patients with reduced bone mass, especially for postmenopausal women’s osteoporosis. Although APN shows a complex biology, reduced APN is also associated to greater cardiometabolic risk.
Funding Statement
This work is partially supported by the grants from the National Natural Science Foundation of China (31000986, 30900156, 81071444, URL: http://www.nsfc.gov.cn/Portal0/default152.htm), Shanghai Science and Technology Commission (09JC1410300, URL: http://www.stcsm.gov.cn/structure/index.htm), the Health Bureau of Shanghai (2008053, URL: http://wsj.sh.gov.cn/), and by grants from the National Natural Science Foundation of Shanghai (10ZR1417400, URL: http://www.stcsm.gov.cn/structure/index.htm). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, et al. (1996) cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem Biophys Res Commun 221: 286–289. [DOI] [PubMed] [Google Scholar]
- 2. Berner HS, Lyngstadaas SP, Spahr A, Monjo M, Thommesen L, et al. (2004) Adiponectin and its receptors are expressed in bone-forming cells. Bone 35: 842–849. [DOI] [PubMed] [Google Scholar]
- 3. Luo XH, Guo LJ, Yuan LQ, Xie H, Zhou HD, et al. (2005) Adiponectin stimulates human osteoblasts proliferation and differentiation via the MAPK signaling pathway. Exp Cell Res 309: 99–109. [DOI] [PubMed] [Google Scholar]
- 4. Luo XH, Guo LJ, Xie H, Yuan LQ, Wu XP, et al. (2006) Adiponectin stimulates RANKL and inhibits OPG expression in human osteoblasts through the MAPK signaling pathway. J Bone Miner Res 21: 1648–1656. [DOI] [PubMed] [Google Scholar]
- 5. Shinoda Y, Yamaguchi M, Ogata N, Akune T, Kubota N, et al. (2006) Regulation of bone formation by adiponectin through autocrine/paracrine and endocrine pathways. J Cell Biochem 99: 196–208. [DOI] [PubMed] [Google Scholar]
- 6. Oshima K, Nampei A, Matsuda M, Iwaki M, Fukuhara A, et al. (2005) Adiponectin increases bone mass by suppressing osteoclast and activating osteoblast. Biochem Biophys Res Commun 331: 520–526. [DOI] [PubMed] [Google Scholar]
- 7. Jürimäe J, Jürimäe T (2007) Plasma adiponectin concentration in healthy preand postmenopausal women: relationship with body composition, bone mineral, and metabolic variables. Am J Physiol Endocrinol Metab 293: E42–E47. [DOI] [PubMed] [Google Scholar]
- 8. Richards JB, Valdes AM, Burling K, Perks UC, Spector TD (2007) Serum adiponectin and bone mineral density in women. J Clin Endocrinol Metab 92: 1517–1523. [DOI] [PubMed] [Google Scholar]
- 9. Ealey KN, Kaludjerovic J, Archer MC, Ward WE (2008) Adiponectin is a negative regulator of bone mineral and bone strength in growing mice. Exp Biol Med (Maywood) 233: 1546–1553. [DOI] [PubMed] [Google Scholar]
- 10. Williams GA, Wang Y, Callon KE, Watson M, Lin JM, et al. (2009) In vitro and in vivo effects of adiponectin on bone. Endocrinology 150: 3603–3610. [DOI] [PubMed] [Google Scholar]
- 11. Magni P, Dozio E, Galliera E, Ruscica M, Corsi MM (2010) Molecular Aspects of Adipokine-Bone Interactions. Current Molecular Medicine 10: 522–532. [DOI] [PubMed] [Google Scholar]
- 12. Ruscica M, Steffani L, Magni P (2012) Adiponectin interactions in bone and cartilage biology and disease. Vitamins and Hormones 90: 321–339. [DOI] [PubMed] [Google Scholar]
- 13. Bacchetta J, Boutroy S, Guebre-Egziabher F, Juillard L, Drai J, et al. (2009) The relationship between adipokines, osteocalcin and bone quality in chronic kidney disease. Nephrol Dial Transplant 24: 3120–3125. [DOI] [PubMed] [Google Scholar]
- 14. Ren WH, LI XH, Wang F (2006) Generation of Adiponectin Gene Knock-out and LacZ Gene Knock-in Mouse Model. Prog Bbiochem Biophys 33: 846–853. [Google Scholar]
- 15. Shu RZ, Zhang F, Wang F, Feng DC, Li XH, et al. (2009) Adiponectin deficiency impairs liver regeneration through attenuating STAT3 phosphorylation in mice. Laboratory Investigation 89: 1043–1052. [DOI] [PubMed] [Google Scholar]
- 16. Hankenson KD, Bain SD, Kyriakides TR, Smith EA, Goldstein SA, et al. (2000) Increased marrow-derived osteoprogenitorcells and endosteal bone formation in mice lacking thrombospondin 2. J Bone Miner Res 15: 851–862. [DOI] [PubMed] [Google Scholar]
- 17. Yoshimura H, Muneta T, Nimura A, Yokoyama A, Koga H, et al. (2007) Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res 327: 449–462. [DOI] [PubMed] [Google Scholar]
- 18. Caplan AI, Bruder SP (2001) Mesenchymal stem cells: building blocks for molecular medicine in the 21st century. Trends Mol Med 7: 259–264. [DOI] [PubMed] [Google Scholar]
- 19. Caplan AI (1991) Mesenchymal stem cells. J Orthop Res 9: 641–650. [DOI] [PubMed] [Google Scholar]
- 20. Khan SN, Cammisa FP Jr, Sandhu HS, Diwan AD, Girardi FP, et al. (2005) The biology of bone grafting. J Am Acad Orthop Surg 13: 77–86. [PubMed] [Google Scholar]
- 21. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284: 143–147. [DOI] [PubMed] [Google Scholar]
- 22. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP (1997) Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem 64: 295–312. [PubMed] [Google Scholar]
- 23. Zhang H, Xie H, Zhao Q, Xie GQ, Wu XP, et al. (2010) Relationships between serum adiponectin, apelin, leptin, resistin, visfatin levels and bone mineral density, and bone biochemical markers in post-menopausal Chinese women. J Endocrinol Invest 33: 707–711. [DOI] [PubMed] [Google Scholar]
- 24. Lei X, Peng X, Wu N, Hu M, Sun Z (2009) Serum adiponectin, leptin level, and bone mineral density in postmenopausal women. Zhong Nan Da Xue Xue Bao Yi Xue Ban 34: 559–562. [PubMed] [Google Scholar]
- 25. Jürimäe J, Rembel K, Jürimäe T, Rehand M (2005) Adiponectin is associated with bone mineral density in perimenopausal women. Horm Metab Res 37: 297–302. [DOI] [PubMed] [Google Scholar]
- 26. Lenchik L, Register TC, Hsu FC, Lohman K, Nicklas BJ, et al. (2003) Adiponectin as a novel determinant of bone mineral density and visceral fat. Bone 33: 646–651. [DOI] [PubMed] [Google Scholar]
- 27. Jürimäe J, Jürimäe T (2007) Adiponectin is a predictor of bone mineral density in middle-aged premenopausal women. Osteoporos Int 18: 1253–1259. [DOI] [PubMed] [Google Scholar]
- 28. Frost M, Abrahamsen B, Nielsen TL, Frystyk J, Flyvbjerg A, et al. (2007) Adiponectin and peak bone mass in men: a cross-sectional, population-based study. Calcif Tissue Int 87: 36–43. [DOI] [PubMed] [Google Scholar]
- 29. Taaffe DR, Cauley JA, Danielson M, Nevitt MC, Lang TF, et al. (2001) Race and sex effects on the association between muscle strength, soft tissue, and bone mineral density in healthy elders: the Health, Aging, and Body Composition Study. J Bone Miner Res 16: 1343–1352. [DOI] [PubMed] [Google Scholar]
- 30. Douchi T, Yamamoto S, Kuwahata R, Oki T, Yamasaki H, et al. (2000) Effect of non-weight-bearing body fat on bone mineral density before and after menopause. Obstet Gynecol 96: 13–17. [DOI] [PubMed] [Google Scholar]
- 31. Crepaldi G, Romanato G, Tonin P, Maggi S (2007) Osteoporosis and body composition. J Endocrinol Invest 30: 42–47. [PubMed] [Google Scholar]
- 32. Ijuin M, Douchi T, Matsuo T, Yamamoto S, Uto H, et al. (2002) Difference in the effects of body composition on bone mineral density between pre- and postmenopausal women. Maturitas 43: 239–244. [DOI] [PubMed] [Google Scholar]
- 33. Radak TL (2004) Caloric restriction and calcium’s effect on bone metabolism and body composition in overweight and obese premenopausal women. Nutr Rev 62: 468–481. [DOI] [PubMed] [Google Scholar]
- 34. Chao D, Espeland MA, Farmer D, Register TC, Lenchik L, et al. (2000) Effect of voluntary weight loss on bone mineral density in older overweight women. J Am Geriatr Soc 48: 753–759. [DOI] [PubMed] [Google Scholar]
- 35. Park HA, Lee JS, Kuller LH, Cauley JA (2007) Effects of weight control during the menopausal transition on bone mineral density. J Clin Endocrinol Metab 92: 3809–3815. [DOI] [PubMed] [Google Scholar]
- 36. Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, et al. (2005) Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res 20: 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jun AY, Kim HJ, Park KK, Son KH, Lee DH, et al. (2012) Extract of Magnoliae Flos inhibits ovariectomy-induced osteoporosis by blocking osteoclastogenesis and reducing osteoclast-mediated bone resorption. Fitoterapia 83: 1523–1531. [DOI] [PubMed] [Google Scholar]
- 38. Rendina E, Lim YF, Marlow D, Wang Y, Clarke SL, et al. (2012) Dietary supplementation with dried plum prevents ovariectomy-induced bone loss while modulating the immune response in C57BL/6J mice. J Nutr Biochm 23: 60–68. [DOI] [PubMed] [Google Scholar]
- 39. Luo XH, Zhao LL, Yuan LQ, Wang M, Xie H, et al. (2009) Development of arterial calcification in adiponectin-deficient mice: adiponectin regulates arterial calcification. J Bone Miner Res 24: 1461–1468. [DOI] [PubMed] [Google Scholar]
- 40. Buckwalter JA, Cooper RR (1987) Bone structure and function. Instr Course Lect 36: 27–48. [PubMed] [Google Scholar]
- 41. T Katagiri, N Takahashi (2002) Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis 8: 147–159. [DOI] [PubMed] [Google Scholar]
- 42. Karsenty G (2008) Transcriptional Control of Skeletogenesis. Annu Rev Genomics Hum Genet 9: 183–196. [DOI] [PubMed] [Google Scholar]
- 43. Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, et al. (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108: 17–29. [DOI] [PubMed] [Google Scholar]
- 44. Tu Q, Zhang J, Dong LQ, Saunders E, Luo E, et al. (2011) Adiponectin inhibits osteoclastogenesis and bone resorption via APPL1-mediated suppression of Akt1. J Biol Chem 286: 12542–12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yamaguchi N, Kukita T, Li YJ, Martinez Argueta JG, Saito T, et al. (2007) Adiponectin inhibits osteoclast formation stimulated by lipopolysaccharide from Actinobacillus actinomycetemcomitans. FEMS Immunol Med Microbiol 49: 28–34. [DOI] [PubMed] [Google Scholar]
- 46. Yamaguchi N, Kukita T, Li YJ, Kamio N, Fukumoto S, et al. (2008) Adiponectin inhibits induction of TNF-alpha/RANKL-stimulated NFATc1 via the AMPK signaling. FEBS Lett 582: 451–456. [DOI] [PubMed] [Google Scholar]