Abstract
Spatially heterogeneous distribution of interspecific competitors and intraspecific aggregation of offspring ramets may affect the growth and size structure of clonal plant populations, but these have been rarely studied. We conducted a greenhouse experiment in which we grew a population of eight offspring ramets (plants) of the stoloniferous clonal plant Hydrocotyle vulgaris aggregately or segregately in two homogeneous treatments with or without a competing grass Festuca elata and a heterogeneous treatment with a patchy distribution of the grass. In patchy grass treatments, H. vulgaris produced markedly more biomass, ramets and stolons in open patches (without grasses) than in grass patches, but displayed lower size variations as measured by coefficient of variation of biomass, ramets and stolons among the eight plants. In open areas, H. vulgaris produced statistically the same amounts of biomass and even more stolons and showed higher size variations in patchy grass treatments than in open (no grass) treatments. In grass areas, H. vulgaris grew much worse and displayed higher size variations in patchy grass treatments than in full grass treatments. Ramet aggregation decreased the growth of H. vulgaris in open treatments and in both open and grass patches in patchy grass treatments, but had little effect in full grass treatments. Ramet aggregation had little effect on size variations. Therefore, heterogeneous distribution of competitors can affect the growth and size structure of clonal plant populations, and ramet aggregation may decrease population growth when they grow in open environments or heterogeneous environments with a patchy distribution of interspecific competitors.
Introduction
In natural habitats, clonal plants can produce horizontal structures (stolons, rhizomes or roots) to connect individual ramets, thereby experiencing spatially heterogeneous environments with microsites of different qualities [1]–[3]. Such environments are often created by an uneven distribution of both abiotic and biotic factors [4]–[7]. In heterogeneous environments, clonal plants may exploit high quality microsites via producing more ramets and concentrating more shoot or root biomass in such microsites, or avoid low quality microsites by veering away from them [2], [8]–[11]. Such responses may affect not only the growth and size of individual plants, but also the productivity and structure of clonal plant populations [6], [9], [12]–[15].
Plant populations may vary greatly in their responses to environmental heterogeneity [16]–[23]. It is commonly found that plant populations under soil nutrient heterogeneous conditions can gain greater biomass and individual size than those under homogenous conditions [18], [22]. On the other hand, Casper and Cahill (1998) found that soil nutrient heterogeneity hardly affected populations of Abutilon theophrasti, and Hagiwara et al. (2010) found that heterogeneity in water availability decreased biomass of Perilla frutescens populations. So far, however, few studies have tested the effect of spatial heterogeneity created by neighbor plants (e.g. patchy distribution of plants) on the growth and size structure of clonal plant populations.
One effect of neighboring plants is to create spatial heterogeneity in soil nutrients [6], [24]–[26], which is known to affect the growth of clonal plants [18], [27]. Neighboring plants can also form spatial heterogeneity in other features. They can act as physical obstacles because their root systems may block the spread of belowground rhizomes and tubers of coexisting clonal plants and their aboveground shoot systems may affect the distributions of the shoots [24], [28]. Neighboring plants may also interfere and suppress the normal root growth of co-occurring clonal plants by releasing nonspecific diffusible root exudates [29]–[31].
Ramet aggregation vs. segregation is another factor likely to affect the growth and size structure of clonal plant populations. Dispersal of clonal offspring ramets is usually limited compared to seed dispersal [32]. Thus, offspring ramets are often locally aggregated, and more likely close to intraspecific rather than interspecific neighbors [32], [33]. However, the effect of intraspecific aggregation of offspring ramets on plant population dynamics is commonly related to the competitive ability of neighbors [34], [35]. When interspecific competition is weaker than intraspecific competition or when interspecific competition is absent, plants that produce offspring ramets with short dispersal may be seriously inhibited by intraspecific aggregation. In contrast, when interspecific competition is stronger than intraspecific competition, offspring ramets may benefit from intraspecific aggregation because aggregation may prevent them from being directly interfered by potential aggressive interspecific neighbors. Therefore, we hypothesize that, when confronted with interspecific competitors, clonal plants grow better and show less size variation when offspring ramets are aggregated than when they are segregated. To our knowledge, however, few studies have explicitly tested the effect of intraspecific aggregation of offspring ramets on the growth and size structure of clonal plant populations [36], [37], especially in environments with a patchy distribution of competitors.
We conducted a greenhouse experiment in which we grew a population of eight offspring ramets of the stoloniferous clonal plant Hydrocotyle vulgaris aggregately or segregately in two homogeneous environments with or without competing neighbors Festuca elata as well as in a heterogeneous environment with a patchy distribution of F. elata. We specifically asked: (1) Does heterogeneous distribution of interspecific competitors affect the growth and size structure of H. vulgaris? (2) Does intraspecific aggregation of offspring ramets affect the growth and size structure of H. vulgaris?
Materials and Methods
The Species
Hydrocotyle vulgaris L. (Araliaceae) is a perennial clonal herb and commonly occurs in bogs, valleys and dune grassland [38]. It can produce plagiotropic stems (i.e., stolons). Each node along the stolons has the potential to form a ramet that consists of a leaf and adventitious roots. The dispersal of H. vulgaris relies mainly on vegetative means rather than sexual reproduction [39]. In the field, H. vulgaris can produce extensive shoot systems and experience heterogeneous micro-environments created by either resource availability or aggregated distribution of neighboring plants [38], [40], [41]. H. vulgaris plants used in this experiment were collected from a wetland in the suburbs of Hangzhou, Zhejiang Province, China, and propagated vegetatively in a greenhouse at Forest Science Co. Ltd. of Beijing Forestry University. The sampling site did not belong to the part of any farms or national parks, so we did not need any relevant permissions/permits for collecting plant samples.
The Experiment
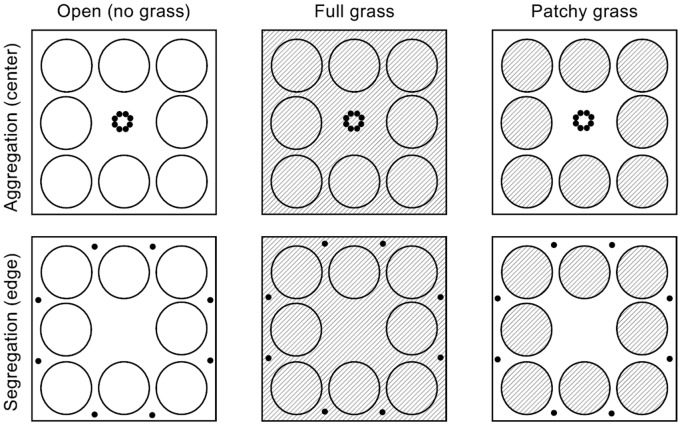
The experiment took a factorial design and had two factors: neighbor inference and ramet aggregation. There were three levels of neighbor interference (open - there was no grass in the square container, full grass - the grass Festuca elata was grown in the entire container, and patchy grass - F. elata was grown in eight regularly spaced circular patches within the container) and two levels of ramet aggregation (eight offspring ramets of H. vulgaris were initially planted in aggregation and were close to the center of the container, or in segregation and were closer to the inner borders of the container; Fig. 1). Each treatment had six replicate containers (40 cm long×40 cm wide×60 cm high) and thus there were 36 plastic containers. In each container, eight 0.2-cm-thick PVC tubes (10.5 cm in inner diameter×13 cm in height) were installed at regular, fixed positions, covering 43% surface area of the container (Fig. 1). Each container was filled to a depth of 15 cm with a 1∶1 (v:v) mixture of sand and peat-based substrate (Pindstrup Seeding; Pindstrup Mosebrug A/S, Denmark), plus 1 g L−1 slow-release fertilizer (15N-11P-13K-2Mg; Osmocote 301, Scotts, USA). The PVC tubes were installed in the way that their tops were 2 cm below the soil surface so that the tubes could not hamper the horizontal spread of stolons and ramets of H. vulgaris. The installation of the tubes ensured that roots of the grasses in the grass patches (tubes) could not spread into the open areas in the patchy grass treatment. The 60 cm high containers guaranteed that stolons and ramets of H. vulgaris could not grow out of the containers.
Figure 1. Schematic representation of the experimental design.
The experiment had three neighbor interference treatments [open - there was no grass in the square container, full grass - the grass Festuca elata (hatched) was grown in the entire container, and patchy grass - F. elata was grown in eight regularly spaced circular patches within the container] crossed with two ramet aggregation treatments [eight offspring ramets (black dots) of Hydrocotyle vulgaris were initially planted in aggregation (in the center of a container) or in segregation (closer to the edges)].
On 16 April 2012, in 12 containers the whole area were evenly sown with F. elata seeds at a density of 30 g cm−2 (full grass), in another 12 containers only the area in the eight PVC tubers were sown with F. elata seeds (patchy grass), and in the remaining 12 containers no area was sown with F. elata seeds (open). After 10 days, F. elata seedlings reached a height of about 10 cm. On 27 April, 288 ramets of H. vulgaris were selected. The initial petiole length was 8.87±0.36 (mean ± SE, n = 18) cm and dry mass was 30.61±3.34 mg. The ramets were divided into six groups according to petiole length and plant size, with 48 ramets in each group. In each group the 48 ramets were randomly assigned to the six treatments and each replicate container had eight ramets. In one ramet aggregation treatment, the eight ramets were regularly planted in the places close to the center of the container within a circular area of 4 cm in diameter, and in the other ramet aggregation treatment they were planted in eight positions 2 cm away from the container borders (Fig. 1).
The experiment was conducted from 27 April to 7 July 2012. The mean temperature and relative humidity (mean ± SE) during the experiment were 26.21±0.33°C and 59.02±1.46% (iButton DS1923, Maxim Integrated Products, USA). During the experiment, sufficient tap water was added to each container to keep the soil moist.
Measurements
At harvest, the new ramets produced by each initial plant (ramet) were interconnected by aboveground stolons, so we could harvest and measure the growth variables of the plants in each type of patches separately. For the homogeneous treatments (the open and full grass treatments), we counted number of ramets and number of stolons, measured total stolon length and weighed dry leaf, petiole, stolon and root mass of each H. vulgaris plant in each container. For the patchy grass treatments, each plant in a container was divided into three parts (initial plants that grew in the borders of the open and grass patches, offspring ramets that grew within the open patches, and offspring ramets that grew in the grass patches) and measured separately. All plant parts were oven-dried at 70°C for 72 h before weighing. Aboveground biomass (dry weight) of F. elata in the full grass treatments and the patchy grass treatments were also measured.
Data Analysis
We calculated total biomass per unit area, number of ramets per unit area, stolon length per unit area and number of stolons per unit area in each container for all treatments. For the patchy grass treatments, we also calculated such growth variables for the grass patches and open patches separately. To measure size variations, we calculated CV (i.e., standard deviation of the eight clones divided by the mean values of the eight clones) in each container for all treatments based on total biomass, number of ramets, stolon length and number of stolons of the eight plants that originated from the eight initial ramets of H. vulgaris [42]. For the patchy grass treatments, we calculated CV of H. vulgaris in the grass patches as standard deviation of the eight plants divided by the mean values of the eight plants. Similarly, we also calculated CV of H. vulgaris in the open patches as standard deviation of the eight plants in the open patches divided by the mean values of the eight plants in the open patches.
First, we used two-way ANOVA to test the effects of neighbor interference (open, full grass and patchy grass) and status of ramet aggregation (aggregation and segregation) on the measures of growth (biomass, number of ramets, stolon length, number of stolons) and size variation (CV of biomass, CV of ramet number, CV of stolon length and CV of stolon number) of H. vulgaris. Second, we used two-way ANOVA to test the effects of patchy distribution of grasses and status of ramet aggregation on all measures of H. vulgaris grown in the grass patches/areas, and in these analyses we included variables of the plants in the full grass treatment and the corresponding variables of the plant parts in the grass patches in the patchy grass treatment. Similarly, we used two-way ANOVA to test the effects of patchy distribution of grasses and status of ramet aggregation on all measures of H. vulgaris grown in the open patches/areas, and in these analyses we included variables of the plants in the open treatment and the corresponding variables of the plant parts in the open patches in the patchy grass treatment. Third, we employed repeated-measures ANOVA to test the effects of patch type (open patches and grass patches) and status of ramet aggregation on all measures, and in these analyses we only included the patchy grass treatments [43]. Patch type was treated as a repeated variable because the open and grass patches in each container were not independent from each other. By accident, we lost one replicate in the homogeneous grass treatment with the H. vulgaris ramets were initially planted segregately.
We also used two-way ANOVA to test the effects of the distribution type of F. elata (full grass and patchy grass) and ramet aggregation of H. vulgaris (aggregation and segregation) on aboveground biomass of F. elata. We further used Principal Component Analysis (PCA) to assess whether the eight response variables (biomass, number of ramets, stolon length, number of stolons, CV of biomass, CV of ramet number, CV of stolon length and CV of stolon number) of H. vulgaris were grouped by the correlations between the response variables either at the whole container level or at the patch level [44].
All analyses were conducted using SPSS 16.0 (SPSS, Chicago, IL, USA). The effects were considered significant if P<0.05.
Results
Effects of Neighbor Interference and Ramet Aggregation at the Whole Container Level
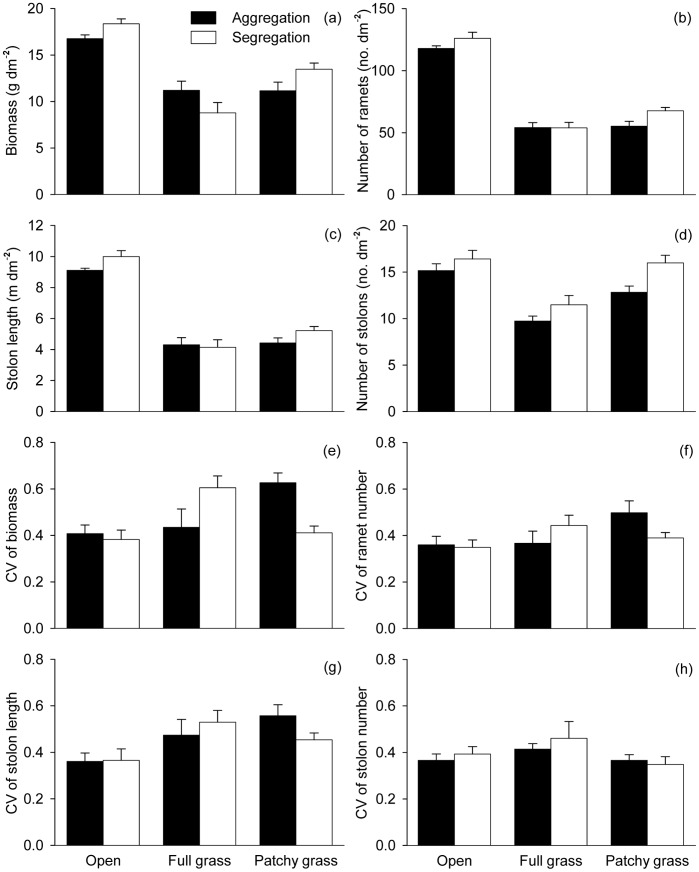
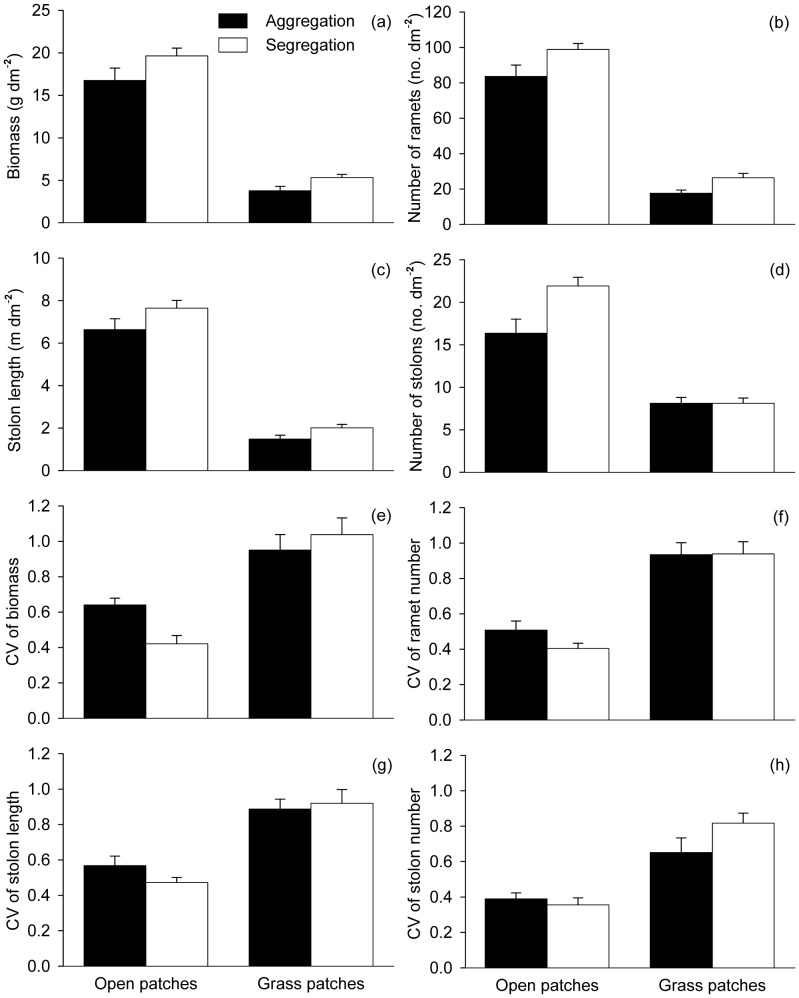
All growth measures (biomass, number of ramets, stolon length and number of stolons) of H. vulgaris were the largest in the open treatments, smallest in the full grass treatments, and intermediate in the patchy grass treatments (Table 1, Fig. 2a–d). H. vulgaris produced more ramets and stolons and tended to (P<0.1) produce longer stolons when the ramets were grown initially in segregation than when they were grown in aggregation (Table 1, Fig. 2b–d). H. vulgaris produced more biomass when offspring ramets were segregated than aggregated in the open and patchy grass treatments, but less in the full grass treatments (Table 1, Fig. 2a).
Table 1. ANOVAs results for effects neighbor interference (open vs. full grass vs. patchy grass) and status of ramet aggregation (aggregation vs. segregation) on the growth and size variation of Hydrocotyle vulgaris at the whole container level.
| Neighbor (N) | Aggregation (A) | N×A | ||||
| F 2,29 | P | F 1,29 | P | F 2,29 | P | |
| Biomass | 47.94 | <0.001 | 0.59 | 0.448 | 5.08 | 0.013 |
| Number of ramets | 198.52 | <0.001 | 4.89 | 0.035 | 1.44 | 0.254 |
| Stolon length | 140.05 | <0.001 | 3.15 | 0.087 | 1.35 | 0.276 |
| Number of stolons | 22.95 | <0.001 | 10.44 | 0.003 | 0.84 | 0.443 |
| CV of biomass | 4.32 | 0.023 | 0.35 | 0.560 | 7.56 | 0.002 |
| CV of ramet number | 2.42 | 0.107 | 0.17 | 0.680 | 2.44 | 0.105 |
| CV of stolon length | 5.71 | 0.008 | 0.14 | 0.713 | 1.41 | 0.261 |
| CV of stolon number | 2.52 | 0.098 | 0.39 | 0.536 | 0.40 | 0.672 |
Figure 2. Effects of neighbor interference (open vs. full grass vs. patchy grass) and ramet aggregation (aggregation vs. segregation) on the growth (a–d) and size inequality (e–h) of the Hydrocotyle vulgaris populations.
Error bars show +1 SE.
CV of biomass and stolon length were significantly higher in the full grass and the patchy grass treatments than in the open treatments, but did not differ between the full grass and patchy grass treatments (Table 1, Fig. 2e, g). CV of ramet number or stolon number was not affected by neighbor interference (Table 1, Fig. 2f, h). However, ramet aggregation affected none of the four size variation measures, except that there was a significant interaction effect on CV of biomass (Table 1, Fig. 2e–h).
Effects of Heterogeneity and Ramet Aggregation in Open Patches/areas
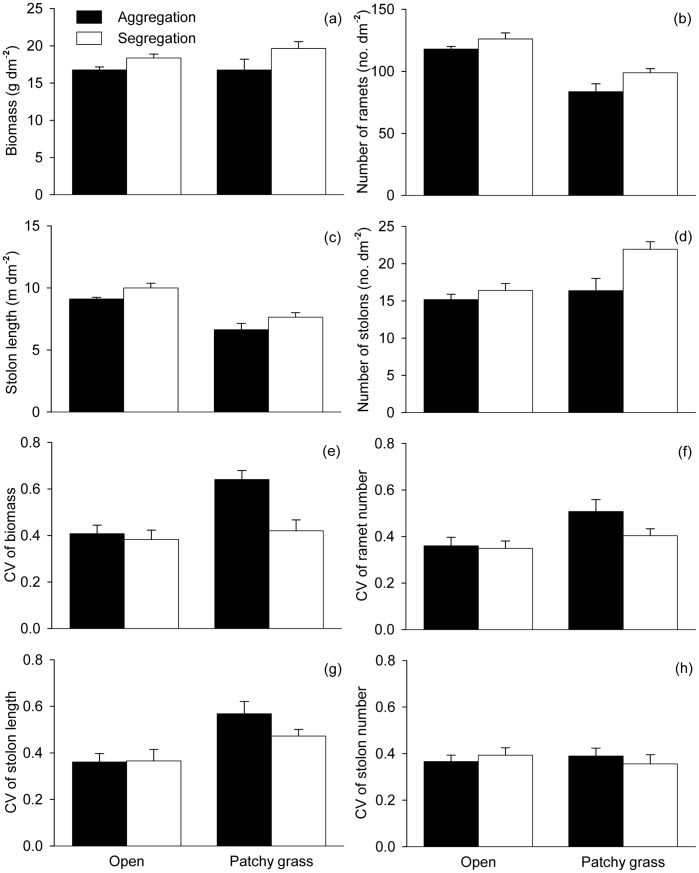
In the open areas, H. vulgaris produced more stolons in the patchy grass than in the open treatments (Table 2, Fig. 3d). However, compared with the open treatments, H. vulgaris produced statistically the same amounts of biomass and fewer ramets and shorter stolons in the patchy grass treatments (Table 2, Fig. 3a–c). All growth measures were greater when the ramets were grown segregately than aggregately (Table 2, Fig. 3a–d).
Table 2. ANOVAs results for effects of spatial heterogeneity (open vs. patchy grass) and status of ramet aggregation (aggregation vs. segregation) on the growth and size variation of Hydrocotyle vulgaris in the open patches/areas.
| Heterogeneity (H) | Aggregation (A) | H×A | ||||
| F 1,20 | P | F 1,20 | P | F 1,20 | P | |
| Biomass | 0.47 | 0.500 | 5.94 | 0.024 | 0.49 | 0.492 |
| Number of ramets | 47.00 | <0.001 | 6.81 | 0.017 | 0.60 | 0.448 |
| Stolon length | 41.97 | <0.001 | 6.49 | 0.019 | 0.03 | 0.874 |
| Number of stolons | 8.84 | 0.008 | 9.06 | 0.007 | 3.63 | 0.071 |
| CV of biomass | 10.92 | 0.004 | 8.96 | 0.007 | 5.64 | 0.028 |
| CV of ramet number | 6.90 | 0.016 | 2.20 | 0.153 | 1.43 | 0.246 |
| CV of stolon length | 13.48 | 0.002 | 1.14 | 0.299 | 1.35 | 0.258 |
| CV of stolon number | 0.04 | 0.848 | 0.01 | 0.916 | 0.83 | 0.372 |
Figure 3. Effects of spatial heterogeneity (open vs. patchy grass) and ramet aggregation (aggregation vs. segregation) on the growth (a–d) and size variation (e–h) of the Hydrocotyle vulgaris populations grown in the open areas.
Error bars show +1 SE.
In the open areas, H. vulgaris displayed higher CV of biomass, ramet number and stolon length in the patchy grass than in the open treatments (Table 2, Fig. 3e–g), and for CV of biomass such an effect of heterogeneity was larger when initial ramets were aggregated than segregated (Table 2, Fig. 3e). Ramet aggregation affected none of the size variation measures except CV of biomass (Table 2, Fig. 3e–h).
Effects of Heterogeneity and Ramet Aggregation in Grass Patches/areas
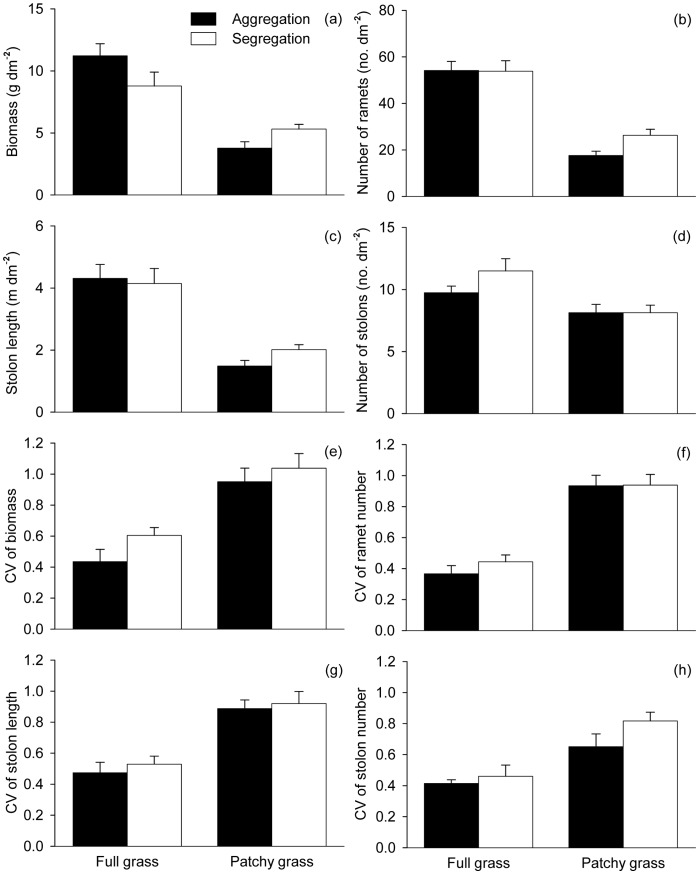
In the grass areas, all growth measures were greater in the full grass than in the patchy grass treatments (Table 3, Fig. 4a–d), and for biomass such an effect of spatial heterogeneity was larger when the ramets were initially in aggregation than in segregation (Table 3, Fig. 4a). Ramet aggregation did not affect number of ramets, stolon length or number of stolons (Table 3, Fig. 4b–d).
Table 3. ANOVAs results for effects of spatial heterogeneity (full grass vs. patchy grass) and status of ramet aggregation (aggregation vs. segregation) on the growth and size variation of Hydrocotyle vulgaris in the grass patches/areas.
| Heterogeneity (H) | Aggregation (A) | H×A | ||||
| F 1,19 | P | F 1,19 | P | F 1,19 | P | |
| Biomass | 49.10 | <0.001 | 0.32 | 0.577 | 6.50 | 0.020 |
| Number of ramets | 95.68 | <0.001 | 1.67 | 0.212 | 1.88 | 0.186 |
| Stolon length | 53.37 | <0.001 | 0.28 | 0.601 | 1.04 | 0.321 |
| Number of stolons | 12.85 | 0.002 | 1.59 | 0.222 | 1.59 | 0.222 |
| CV of biomass | 33.10 | <0.001 | 2.42 | 0.136 | 0.25 | 0.620 |
| CV of ramet number | 76.41 | <0.001 | 0.45 | 0.508 | 0.35 | 0.564 |
| CV of stolon length | 37.64 | <0.001 | 0.45 | 0.510 | 0.03 | 0.861 |
| CV of stolon number | 22.65 | <0.001 | 2.91 | 0.104 | 0.92 | 0.351 |
Figure 4. Effects of spatial heterogeneity (full grass vs. patchy grass) and ramet aggregation (aggregation vs. segregation) on the growth (a–d) and size variation (e–h) of the Hydrocotyle vulgaris populations grown in the grass areas.
Error bars show +1 SE.
In the grass areas, all measures of size variation were lower in the full grass than in the patchy grass treatments, but none was affected by ramet aggregation (Table 3, Fig. 4e–h).
Effects of Patch Type and Ramet Aggregation in the Patchy Grass Treatments
In the patchy grass treatments, all growth measures were greater in the open patches than in the grass patches (Table 4, Fig. 5a–d). Compared with aggregation, initial ramet segregation significantly increased number of ramets and stolons and tended to (P<0.1) increase biomass and stolon length (Table 4, Fig. 5a–d).
Table 4. Repeated measure ANOVA results for effects of patch type (open patches vs. grass patches) and status of ramet aggregation (aggregation vs. segregation) on the growth and size variation of Hydrocotyle vulgaris.
| Patch type (T) | Aggregation (A) | T×A | ||||
| F 1,10 | P | F 1,10 | P | F 1,10 | P | |
| Biomass | 310.74 | <0.001 | 4.53 | 0.059 | 0.76 | 0.403 |
| Number of ramets | 403.25 | <0.001 | 7.34 | 0.022 | 0.88 | 0.369 |
| Stolon length | 365.20 | <0.001 | 4.04 | 0.072 | 0.73 | 0.413 |
| Number of stolons | 86.21 | <0.001 | 8.94 | 0.014 | 5.49 | 0.041 |
| CV of biomass | 38.52 | <0.001 | 0.97 | 0.349 | 4.21 | 0.067 |
| CV of ramet number | 58.67 | <0.001 | 0.98 | 0.346 | 0.75 | 0.406 |
| CV of stolon length | 36.78 | <0.001 | 1.55 | 0.242 | 2.82 | 0.124 |
| CV of stolon number | 49.30 | <0.001 | 0.29 | 0.602 | 1.37 | 0.269 |
Figure 5. Effects of patch type (open patches vs. grass patches) and ramet aggregation (aggregation vs. segregation) on the growth (a–d) and size variation (e–h) of the Hydrocotyle vulgaris populations.
Error bars show +1 SE.
All measures of size variation were lower in the open patches than in the grass patches, but none was affected by ramet aggregation (Table 4, Fig. 5e–h).
The Response Pattern of H. vulgaris to Patch Type and Ramet Aggregation
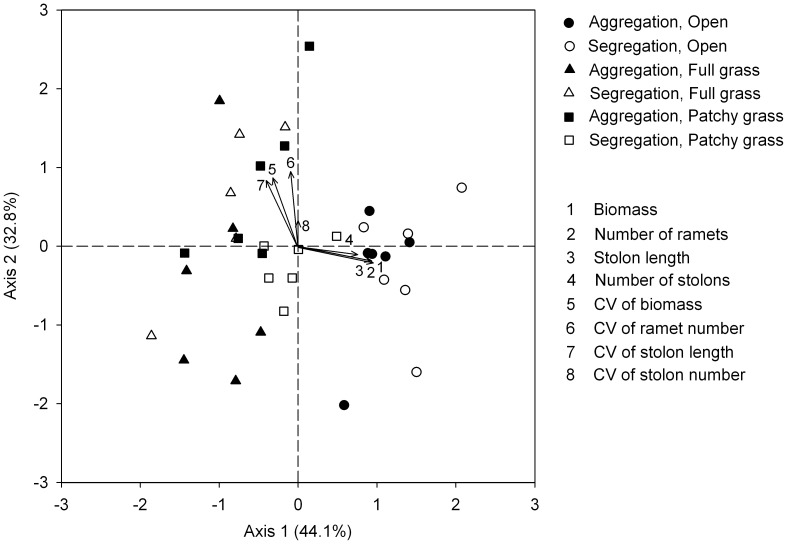
At the whole container level, the first PCA axis was identified as an axis of the growth of the population, accounting for 44.1% of the total variance, and PCA axis 2 was identified as an axis of the size structure of the population, accounting for 32.8% of the total variance. The open treatments were located at the higher end of PCA axis 1 and clearly separated from the other treatments (Fig. 6).
Figure 6. Scatter plot from Principal Component Analysis of the Hydrocotyle vulgaris populations at the whole container level.
Axis 1 and 2 explained 44.1% and 32.8% of the total variance, respectively. Response variable scores (arrows) are given.
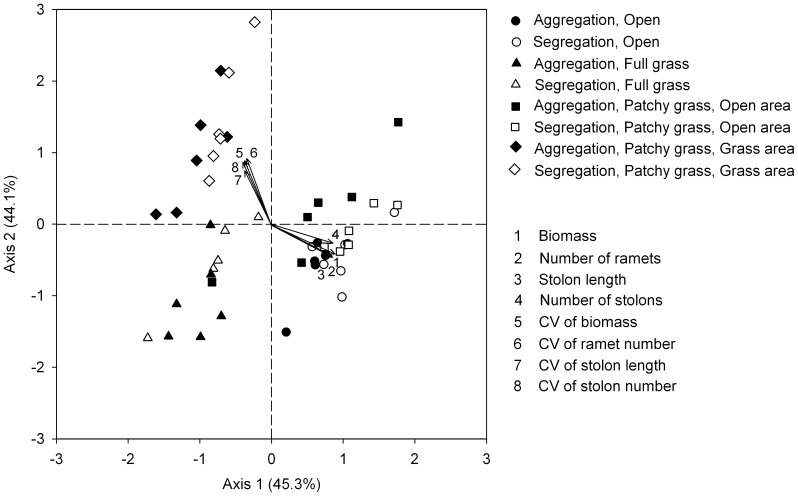
At the patch level, PCA axis 1 explained 45.3% of the total variation and was related with the growth of the population, and PCA axis 2 explained 44.1% of the total variation and was related with the size structure of the population (Fig. 7). The open treatments and the corresponding open areas in the grass patchy treatments were located at the higher end of PCA axis 1, while the full grass treatments and the corresponding grass areas in the grass patchy treatments were located at the lower end of PCA axis 1. The open areas in the grass patchy treatments were located at the higher end of PCA axis 2, and the full grass treatments were at the lower end of PCA axis 2. These results generally agreed with results from univariate ANOVAs.
Figure 7. Scatter plot from Principal Component Analysis of the Hydrocotyle vulgaris populations at the patch level.
Axis 1 and 2 explained 45.3% and 44.1% of the total variance, respectively. Response variable scores (arrows) are given.
The Growth of F. elata
Aboveground biomass of F. elata was significantly affected by distribution type (F 1,19 = 322.22; P<0.001), but not ramet aggregation of H. vulgaris (F 1,19<0.01; P = 0.969) or the interaction effect of ramet aggregation by distribution type (F 1,19 = 0.13; P = 0.721). Aboveground biomass of F. elata in the patchy grass treatments (5.96±0.18 g dm−2; mean ± SE) was significantly higher than in the full grass treatments (1.16±0.19 g dm−2; mean ± SE).
Discussion
The growth of the H. vulgaris plants was severely inhibited when they were grown with the grass F. elata (Fig. 6), suggesting that the presence of F. elata caused intense interspecific competition likely for light [45] and physical space [28] and possibly also for soil nutrients [24]. Moreover, the presence of interspecific competitors increased size variations as indicated by increased CV of biomass and stolon length in the full grass and patchy grass treatments as compared with those in the open treatments (Table 1, Fig. 2e, g). These results also mean that the individual plants of the H. vulgaris populations were suppressed unequally by interspecific interference of F. elata, possibly due to the difference in the time to produce offspring ramets between individuals. Plants that produced offspring ramets earlier could act promptly (e.g., enhancing petiole length to increase light acquisition at the early development) and thus capture more light before the interspecific suppression became serious, while plants that produced offspring ramets later would be shaded and suppressed by either F. elata or intraspecific neighbors [40]. Thus, interspecific competition also altered population structure of H. vulgaris [46], [47].
Effects of Heterogeneous Distribution of Competitors
In environments with a patchy distribution of grasses, H. vulgaris grew much worse in the grass patches than in the open patches (Table 4, Figs. 5 and 7). Moreover, H. vulgaris produced much less biomass and fewer ramets in the grass patches in the patchy grass treatments than in the full grass treatments (Table 3, Fig. 4). These results suggest that, when grown in environments with a patchy distribution of grasses, H. vulgaris reduced the chance to grow into the grass patches. Such a response may be a passive process, because the aggregation of F. elata in the patchy grass treatments could produce more aboveground biomass than in the full grass treatments. Thus, the suppression of H. vulgaris by F. elata was more severe in the patchy grass treatments than in the full grass treatments. The results also indicate that connections to ramets in the open patches could not contribute much to the growth and clonal reproduction of the ramets in the grass patches [48], [49].
On the other hand, the patchy grass environments might also have triggered active foraging responses of H. vulgaris to minimize the interspecific competition [24], [28], [49]. This argument is supported further by the fact that in the patchy grass treatment H. vulgaris markedly decreased stolon branching in the grass patches, and tended to expand their stolons along the edge of grass patches rather than across the patches (personal observations). Similar foraging responses were reported in at least three previous studies [24], [28], [50], and these active foraging responses are thought to be adaptive for clonal plants to improve the space-use efficiency in heterogeneous environments with a patchy distribution of neighbor plants [51].
H. vulgaris produced statistically the same amounts of biomass and even increased stolon production in the open areas in the patchy grass treatments than in the open treatments. These results suggest that connections to ramets in the grass patches could not cause significant costs to the growth of the ramets in the open patches [48], [52], [53]. The likely reason is that resource transportation to the ramets in the grass patches was rather low or even did not exist [48], [49], as shown also by the fact that connections to the ramets in the open patches did not increase the growth of the ramets growing in the grass patches.
In environments with a patchy distribution of grasses, H. vulgaris showed markedly higher size variations in the grass patches than in the open patches (Figs. 5e–h and 7). Moreover, H. vulgaris had higher size variations in both open and grass patches in the patchy grass treatment than in the corresponding areas in the open and full grass treatments (Tables 2 and 3, Figs. 3e–g and 4e–h). These results provide the first evidence that spatial heterogeneity in distribution of interspecific competitors can markedly alter size structure of clonal plant populations. One obvious consequence of spatial heterogeneity in competitor distribution is to cause variations in the difficulty of resource acquisition in different types of patches [9], [22], [54]. Physical space is also considered as a resource for plants [55]–[57]. Therefore, another possible consequence is that spatial heterogeneity in competitor distribution generates variations in the availability of physical space and thus variations in the difficulty for plants to find available space [55]–[57]. Such variations further resulted in the changes in the size structure of the clonal plant populations.
Effects of Aggregation of Offspring Ramets
Compared with aggregation, initial ramet segregation significantly increased biomass, ramet and stolon production of H. vulgaris in the open treatments (Tables 1, Fig. 2a–d), suggesting that ramet aggregation negatively affects the growth of clonal plant populations when they grow in an open environment or spread into open patches. Similarly, Lenssen et al. (2005) found that ramet aggregation reduced the competitive ability of Agrostis stolonifera. The likely reason is that, when interspecific competition was relative weak or absent, aggregation of offspring ramets aggravated either self-competition between different ramets within the same clone or intraspecific competition between different clones by increasing the overlapping zones of influence [58], [59].
Ramet aggregation had little effect on the growth of H. vulgaris in the full grass treatments (Table 1, Fig. 2a–d), suggesting that ramet aggregation cannot benefit clonal plants when they grow with a homogeneous distribution of interspecific neighbors [34], [35]. These results seem not to support the view that intraspecific aggregation can change the influence frequency of inter- vs. intraspecific encounters and thus contribute to species coexistence [36], [60]–[62]. However, because there is only one seed-sowing density treatment of F. elata in the experiment, we are not sure whether the benefit of intraspecific aggregation is density dependent and whether it will become more important when the seed-sowing density is lower [34], [35]. Further studies that compare the effect of intraspecific aggregation under different seed-sowing density treatments may help us understand the potential mechanism.
Ramet aggregation in the patchy grass treatments had even negative effects on the growth of H. vulgaris at the patch and the container levels (Tables 1–4, Figs. 2a–d, 3a–d, 4a–d and 5a–d). When intraspecific competitor F. elata was restricted in fixed regions in the patchy grass treatments, H. vulgaris could easily occupy the open areas and maintain the long-term control of local resources by producing new ramets, thereby resulting in the strong spatial segregation between different species [34], [35]. Under such circumstances, the effect of ramet aggregation in the open areas seems to determine the performance of H. vulgaris in the grass area and even at the whole container level.
Therefore, spatial distribution of offspring ramets is an important factor to determine the establishment of clonal plant populations when they are introduced in an open environment or an environment with a patchy distribution of interspecific competitors, but may not be so when they invade into a closed community with a homogeneous distribution of competitors. However, we found that ramet aggregation had little effect on size variations (Tables 2–>4, Figs. 3f–h, 4e–h and 5e–h). Thus, there is no evidence that ramet aggregation can affect size structure of clonal plant populations.
Conclusions
Both spatially heterogeneous distribution of interspecific competitors and intraspecific aggregation of offspring ramets can greatly affect the growth of clonal plant populations, and heterogeneous distribution of competitors can also alter population size structure. However, due to the limited area of the experimental container, we cannot completely rule out the potential edge effect caused by initial ramet positions (e.g., the ramets planted segregately were located also more closely to the edges of the containers). In further studies, therefore, larger containers should be used so that an extra buffer can be added to reduce the potential confounding effects of edges [36], [47].
Acknowledgments
We thank Qian Zhang, Lin Huang, Wei Xue and Dong-Hong Zhao for help with the experiment.
Funding Statement
This research was supported by the Specific Programs in Graduate Science and Technology Innovation of Beijing Forestry University (BLYJ201104), the Fundamental Research Funds for the Central Universities (TD-JC-2013-1) and the Program for New Century Excellent Talents in University (NECT-10-0234). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. de Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ (2009) A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant, Cell and Environment 32: 704–712. [DOI] [PubMed] [Google Scholar]
- 2. Cahill JF Jr, McNickle GG (2011) The behavioral ecology of nutrient foraging by plants. Annual Review of Ecology, Evolution, and Systematics 42: 289–311. [Google Scholar]
- 3. Hutchings MJ, Wijesinghe DK (2008) Performance of a clonal species in patchy environments: effects of environmental context on yield at local and whole-plant scales. Evolutionary Ecology 22: 313–324. [Google Scholar]
- 4. Alpert P, Mooney HA (1996) Resource heterogeneity generated by shrubs and topography on coastal sand dunes. Vegetatio 122: 83–93. [Google Scholar]
- 5.Caldwell MM, Pearcy RW (1994) Exploitation of environmental heterogeneity by plants: ecophysiological processes above-and belowground. New York: Academic Press.
- 6. Huber-Sannwald E, Jackson RB (2001) Heterogeneous soil-resource distribution and plant responses from individual-plant growth to ecosystem functioning. Process in Botany 62: 451–476. [Google Scholar]
- 7. Li P-X, Wang N, He W-M, Krüsi BO, Gao S-Q, et al. (2008) Fertile islands under Artemisia ordosica in inland dunes of northern China: effects of habitats and plant developmental stages. Journal of Arid Environments 72: 953–963. [Google Scholar]
- 8. de Kroon H, Hutchings MJ (1995) Morphological plasticity in clonal plants: the foraging concept reconsidered. Journal of Ecology 83: 143–152. [Google Scholar]
- 9. Hutchings MJ, John EA, Wijesinghe DK (2003) Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology 84: 2322–2334. [Google Scholar]
- 10. de Kroon H, Huber H, Stuefer JF, van Groenendael J (2005) A modular concept of phenotypic plasticity in plants. New Phytologist 166: 73–82. [DOI] [PubMed] [Google Scholar]
- 11. Hutchings MJ, John EA (2004) The effects of environmental heterogeneity on root growth and root/shoot partitioning. Annals of Botany 94: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu C-Y (2010) Schooler SS, Van Klinken RD (2010) Effects of clonal integration and light availability on the growth and physiology of two invasive herbs. Journal of Ecology 98: 833–844. [Google Scholar]
- 13. Chu Y, Yu F-H, Dong M. 2006. Clonal plasticity in response to reciprocal patchiness of light and nutrients in the stoloniferous herb Glechoma longituba L. Journal of Integrative Plant Biology 48: 400–408. [Google Scholar]
- 14. Roiloa SR, Alpert P, Tharayil N, Hancock G, Bhowmik PC (2007) Greater capacity for division of labour in clones of Fragaria chiloensis from patchier habitats. Journal of Ecology 95: 397–405. [Google Scholar]
- 15. Chen J-S, Lei N-F, Dong M (2010) Clonal integration improves the tolerance of Carex praeclara to sand burial by compensatory response. Acta Oecologica 36: 23–28. [Google Scholar]
- 16. Casper BB, Cahill Jr JF (1996) Limited effects of soil nutrient heterogeneity on populations of Abutilon theophrasti (Malvaceae). American Journal of Botany 83: 333–341. [PubMed] [Google Scholar]
- 17. Casper BB, Cahill Jr JF (1998) Population-level responses to nutrient heterogeneity and density by Abutilon theophrasti (Malvaceae): an experimental neighborhood approach. American Journal of Botany 85: 1680–1687. [PubMed] [Google Scholar]
- 18. Zhou J, Dong B-C, Alpert P, Li H-L, Zhang M-X, et al. (2012) Effects of soil nutrient heterogeneity on intraspecific competition in the invasive, clonal plant Alternanthera philoxeroides . Annals of Botany 109: 813–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang P, Lei J-P, Li M-H, Yu F-H (2012) Spatial heterogeneity in light supply affects intraspecific competition of a stoloniferous clonal plant. PloS One 7: e39105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hagiwara Y, Kachi N, Suzuki J-I (2010) Effects of temporal heterogeneity of water supply on the growth of Perilla frutescens depend on plant density. Annals of Botany 106: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hagiwara Y, Kachi N, Suzuki J-I (2012) Effects of temporal heterogeneity of water supply and nutrient levels on plant biomass growth depend on the plant’s relative size within its population. Ecological Research 27: 1079–1086. [Google Scholar]
- 22. Day KJ, Hutchings MJ, John EA (2003) The effects of spatial pattern of nutrient supply on the early stages of growth in plant populations. Journal of Ecology 91: 305–315. [Google Scholar]
- 23. Day KJ, Hutchings MJ, John EA (2003) The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. Journal of Ecology 91: 541–553. [Google Scholar]
- 24. Huber-Sannwald E, Pyke DA, Caldwell MM, Durham S (1998) Effects of nutrient patches and root systems on the clonal plasticity of a rhizomatous grass. Ecology 79: 2267–2280. [Google Scholar]
- 25.Stark JM (1994) Causes of soil nutrient heterogeneity at different scales. In: Caldwell MM, Pearcy RW, editors. Exploitation of environmental heterogeneity by plants. San Diego: Academic Press. pp.255–284.
- 26. Richards CL, Walls RL, Bailey JP, Parameswaran R, George T, et al. (2008) Plasticity in salt tolerance traits allows for invasion of novel habitat by Japanese knotweed S.L. (Fallopia japonica and F.× bohemica, Polygonaceae). American Journal of Botany 95: 931–942. [DOI] [PubMed] [Google Scholar]
- 27. Birch CPD, Hutchings MJ (1994) Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea . Journal of Ecology 82: 653–664. [Google Scholar]
- 28. Evans JP, Cain ML (1995) A spatially explicit test of foraging behavior in a clonal plant. Ecology 76: 1147–1155. [Google Scholar]
- 29. Mahall BE, Callaway RM (1991) Root communication among desert shrubs. Proceedings of the National Academy of Sciences 88: 874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annual Review of Plant Biology 57: 233–266. [DOI] [PubMed] [Google Scholar]
- 31.de Kroon H, Visser EJW (2003) Root ecology. Berlin: Springer-Verlag.
- 32. Zobel M, Moora M, Herben T (2010) Clonal mobility and its implications for spatio-temporal patterns of plant communities: what do we need to know next? Oikos 119: 802–806. [Google Scholar]
- 33. Winkler E, Fischer M (2001) The role of vegetative spread and seed dispersal for optimal life histories of clonal plants: a simulation study. Evolutionary Ecology 15: 281–301. [Google Scholar]
- 34. Bolker BM, Pacala SW (1999) Spatial moment equations for plant competition: understanding spatial strategies and the advantages of short dispersal. The American Naturalist 153: 575–602. [DOI] [PubMed] [Google Scholar]
- 35. Bolker BM, Pacala SW, Neuhauser C (2003) Spatial dynamics in model plant communities: what do we really know? The American Naturalist 162: 135–148. [DOI] [PubMed] [Google Scholar]
- 36. Lenssen JPM, Hershock C, Speek T, During HJ, de Kroon H (2005) Experimental ramet aggregation in the clonal plant Agrostis stolonifera reduces its competitive ability. Ecology 86: 1358–1365. [Google Scholar]
- 37. Gough L, Goldberg DE, Hershock C, Pauliukonis N, Petru M (2001) Investigating the community consequences of competition among clonal plants. Evolutionary Ecology 15: 547–563. [Google Scholar]
- 38. Dong M (1995) Morphological responses to local light conditions in clonal herbs from contrasting habitats, and their modification due to physiological integration. Oecologia 101: 282–288. [DOI] [PubMed] [Google Scholar]
- 39. Hua M-L, Cheng J-M, Ying W-Y, Dong Q-D, Chu C-Y (2011) Study on invasion risk of Hydrocotyle vulgaris as an alien species in wetlands. Journal of Zhejiang University (Agric and Life Sci) 37: 425–431 (in Chinese with English abstract).. [Google Scholar]
- 40. Leeflang L, During HJ, Werger MJA (1998) The role of petioles in light acquisition by Hydrocotyle vulgaris L. in a vertical light gradient. Oecologia 117: 235–238. [DOI] [PubMed] [Google Scholar]
- 41. Gibson DJ (1988) The relationship of sheep grazing and soil heterogeneity to plant spatial patterns in dune grassland. Journal of Ecology 76: 233–252. [Google Scholar]
- 42. Weiner J, Thomas SC (1986) Size variability and competition in plant monocultures. Oikos 47: 211–222. [Google Scholar]
- 43.von Ende C (2001) Repeated-measures analysis: growth and other time-dependent measures. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. New York: Oxford University Press. pp.134–157.
- 44.Quinn GGP, Keough MJ (2002) Experimental Design and Data Analysis for Biologists. New York: Cambridge University Press. pp.443–458.
- 45. Price EAC, Hutchings MJ (1996) The effects of competition on growth and form in Glechoma hederacea . Oikos 75: 279–290. [Google Scholar]
- 46. Weiner J (1985) Size hierarchies in experimental populations of annual plants. Ecology 66: 743–752. [Google Scholar]
- 47. Fransen B, de Kroon H, Berendse F (2001) Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology 82: 2534–2546. [Google Scholar]
- 48. Stuefer JF, Hutchings MJ (1994) Environmental heterogeneity and clonal growth: a study of the capacity for reciprocal translocation in Glechoma hederacea L. Oecologia. 100: 302–308. [DOI] [PubMed] [Google Scholar]
- 49. Guo W, Song Y-B, Yu F-H (2011) Heterogeneous light supply affects growth and biomass allocation of the understory fern Diplopterygium glaucum at high patch contrast. PloS One 6: e27998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Novoplansky A, Cohen D, Sachs T (1990) How Portulaca seedlings avoid their neighbours. Oecologia 82: 490–493. [DOI] [PubMed] [Google Scholar]
- 51. Novoplansky A (2009) Picking battles wisely: plant behaviour under competition. Plant, Cell and Environment 32: 726–741. [DOI] [PubMed] [Google Scholar]
- 52. Liu H-D, Yu F-H, He W-M, Chu Y, Dong M (2009) Clonal integration improves compensatory growth in heavily grazed ramet populations of two inland-dune grasses. Flora 204: 298–305. [Google Scholar]
- 53. He W-M, Alpert P, Yu F-H, Zhang L-L, Dong M (2011) Reciprocal and coincident patchiness of multiple resources differentially affect benefits of clonal integration in two perennial plants. Journal of Ecology 99: 1202–1210. [Google Scholar]
- 54. Facelli E, Facelli JM (2002) Soil phosphorus heterogeneity and mycorrhizal symbiosis regulate plant intra-specific competition and size distribution. Oecologia 133: 54–61. [DOI] [PubMed] [Google Scholar]
- 55. Dimitrakopoulos PG, Schmid B (2004) Biodiversity effects increase linearly with biotope space. Ecology Letters 7: 574–583. [Google Scholar]
- 56. McConnaughay K, Bazzaz F (1991) Is physical space a soil resource? Ecology 72: 94–103. [Google Scholar]
- 57. Von Felten S, Schmid B (2008) Complementarity among species in horizontal versus vertical rooting space. Journal of Plant Ecology 1: 33–41. [Google Scholar]
- 58. Ross M, Harper JL (1972) Occupation of biological space during seedling establishment. Journal of Ecology 60: 77–88. [Google Scholar]
- 59.Begon M, Townsend CR, Harper JL (2006) Ecology: from individuals to ecosystems. Oxford: Blackwell Publishing.
- 60.Tilman D, Kareiva P (1997) Spatial Ecology: The role of space in population dynamics and interspecific interactions. Princeton: Princeton University Press.
- 61. Monzeglio U, Stoll P (2005) Spatial patterns and species performances in experimental plant communities. Oecologia 145: 619–628. [DOI] [PubMed] [Google Scholar]
- 62. Stoll P, Prati D (2001) Intraspecific aggregation alters competitive interactions in experimental plant communities. Ecology 82: 319–327. [Google Scholar]