Abstract
Background
This study is aimed at evaluating the operation techniques and clinical significance of free flap transplantation combined with skin grafting and vacuum sealing drainage (VSD) in repairing severe traumatic extensive circumferential or semi-circumferential soft-tissue defects of the lower leg.
Material/Methods
Thirty patients with severe lower leg injuries were treated by free flap transplantation combined with skin grafting and VSD from January 2008 to June 2011. The size of the wounds ranged from 23×8 cm to 44×28 cm and all affected more 70% of the low leg circumferential area. Wounds were complicated by exposure, necrosis, or infection of deep tissues. The wounds were first debrided and covered by VSD. When the condition of the wound had improved (5 to 7 days later), free flaps were harvested to reconstruct damaged tissue and skin grafts and VSD was used to cover granulation tissues around the transplanted flap.
Results
Granulation tissues developed and the area requiring flap cover decreased in all 30 patients after debridement and VSD. In 28 of 30 cases, the transplanted flaps grew well without complication. Peripheral necrosis was observed in only 2 cases, which required a second debridement and skin graft. Ten wound areas covered by grafts were left with scattered peripheral wounds, which healed with the help of 1 more skin graft or dressing change. Morphological appearance and functional recovery were satisfactory in all 30 cases.
Conclusions
Initial debridement and the temporary VSD cover followed after several days by free flap transplantation combined with skin grafting and VSD protection is a reliable treatment regimen for traumatic large circumferential or sub-circumferential soft tissue wounds of the lower leg with deep tissue exposure.
Keywords: free flap transplantation, skin grafting, circumferential wounds, vacuum sealing drainage (VSD), microsurgical techniques
Background
Traumatic large circumferential or sub-circumferential soft-tissue injuries of the lower leg from high energy impacts are often complicated by extensive necrosis, severe contamination, damage to superficial veins, pressure-induced dermatorrhexis, seriously undermined blood supply and venous drainage, exposure, and, in severe cases, by deep tissue injuries to muscles, tendons, nerves, bones, and joints. In cases further complicated by fractures to other body parts or injury to major organs, patients are susceptible to traumatic or hemorrhagic shock and water-electrolyte imbalance. It is difficult to accurately judge the range and severity of the defect and to achieve primary repair with microsurgical techniques after debridement in the emergency department. Alternative solutions include frequent dressing changing and deferring microsurgical repair, which are rather intractable because wounds with exposed tissues, like tendons and bones, are susceptible to infection regardless of timely debridement and preventive antibiotic administration [1,2]. Conventional local flap grafting or single free flap transplantation may not cover wounds effectively or produce desired results due to insufficient donor site area, damaged host vessels or unsuitable donor blood vessels, and other complications [3]. Compound flaps with multiple anastomosed vessels are impractical because they require free flaps (or tissue flaps) harvested from several sites and anastomosis of several pairs of vessels. Moreover, harvesting from multiple donor sites compounds patient injury and elevates surgical risk [3–6]. Unsuccessful treatment may ultimately result in the need for limb amputation.
Early radical debridement and provisional vacuum sealing drainage (VSD) cover is a new approach for the protection of large wounds in the extremities that can prevent infection, stimulate granulation, and reduce the area requiring flap cover, thus improving the success of subsequent surgery, reducing postoperative complications, and promoting functional limb recovery [7–10]. Indeed, application of this technology is increasing for the treatment of skin defects, leading to improved healing of acute, subacute, and chronic wounds [11–14]. James et al compared vacuum-assisted closure (VAC) to conventional wrapping in a randomized study of patients with severe open fractures [2]. After thorough debridement in the emergency department, wounds of the experimental group were covered using a VAC system (Kinetic Concepts, Inc., San Antonio, TX), whereas wounds of the control group were treated by conventional sterile gauze wrapping. Wounds in the experimental group demonstrated significantly better granulation formation. Moreover, the number of patients with osteomyelitis and local infection after wound repair was significantly lower in the experimental group.
The purpose of the present study was to evaluate the clinical outcome of free flap transplantation combined with skin grafting and VSD for circumferential or sub-circumferential soft-tissue injuries below the knee. Temporary VSD cover minimized the required flap cover area and limited complications prior to reparative treatment. In the second stage, microsurgical techniques were applied to transplant free flap tissue to sites with exposed deep tissues and skin grafts for coverage. Due to the lack of intact nearby soft tissues for attachment, flaps on the lower legs are prone to slip or curl outward, leading to the aggregation of exudate and difficulty in flap implantation. Furthermore, conventional compression is not suitable for wounds with skin grafts. Therefore, VSD was employed again after reparative surgery. The use of VSD on skin grafts improved the survival of the free flap and skin graft.
Material and Methods
General information
In this prospective study, 30 patients (19 males and 11 females; 15 to 65 years of age; mean age of 36.8 years) were treated using a 2-step procedure of debridement-VSD and reparative microsurgery. The cause of lower leg injury included contusion by auto accident (20), mangling injury by motorcycle wheels (7), and leg impact by heavy loads (3). Wounds were characterized as fresh wounds (9) or infective necrotizing wounds (21). Wounds were all large traumatic circumferential or sub-circumferential soft-tissue defects that covered more than 70% of the lower leg surface. Total wound area ranged from 23×8 cm to 44×28 cm. Wounds were complicated by contamination, exposure, and structural damage or necrosis of tendon and (or) bone. In 16 patients, the wound was accompanied by tibia or fibula fracture and in 8 cases by fracture of other sites or injuries to other body parts.
Treatment
Treatment was divided into 2 stages. In the first stage, the wound was debrided and covered by VSD for 5 to 7 days. Debridement and VSD was performed again if necessary. After 5 to 7 days, granulation tissues developed and the flap area required was reduced. In the second stage, reparative free flap transplantation combined with skin grafting and VSD cover was performed. The VSD cover was applied for 7 to 9 days.
Thorough debridement and broad-spectrum antibiotics were administered at presentation. For wounds with local tissue necrosis and infection, debridement included elimination of ischemic and irreparably damaged tissues, as well as removal of foreign substances, opening of dead space, and eradication of sequestra and sclerotic bone with a sharp osteotome until blood oozed to the bone surface. External fixation or simple limited internal fixation was selected to reconstruct bone based on patient age and the location and type of fracture. After debridement, VSD sponge was tailored to veil the wounds thoroughly, leaving no dead space, following documented methods [15]. The vacuum on large circumferential wounds to the lower leg should be kept at between –20 and –40 kPa (150~300 mmHg) because excessive vacuum pressure may restrict the blood supply and lead to hypoproteinemia from over-exudation.
In the second stage, surgeons removed the VSD, performed debridement again, and repaired deep tissue injuries if possible. Appropriate free flaps and skin grafts were selected. In this study, flaps included anterolateral femoral flaps (13 cases), thoraco-umbilical flaps (10 cases), latissimus dorsi flaps (6 cases), and a lateral thoracic flap (1 case). A few other cases were treated with combined local flap transfer and free flap transplantation, double free flap transplantation, and cross-leg bridge flap transplantation (but were not included in this study). Abdominal hypodermal vascular net flaps or split-thickness skin grafts from other sites were needed if suturing was not feasible at the donor sites after flap extraction. Remaining wounds surrounding transplanted flaps were covered with split-thickness skin from the scalp, lower limbs, chest, or abdomen. Skin grafts were veiled by VSD, sponges were sutured to the flap fringe, and semi-permeable membrane was attached within 2 cm of the flap fringe, creating an airtight wrap.
Postoperative management
Anti-inflammatory, anti-convulsive, and anti-coagulation medications were routinely prescribed. Dressing change was not required for repaired sites, and unobstructed vacuum drainage was assured to prevent compression over the flap. Sedatives and analgesics were administered to prevent vasospasm caused by various stimuli. Nutritional support, supportive care, and management of complications were provided as necessary. The VSD on the skin grafts was removed 7 to 9 days after the operation. If wound surface and bone fixation conditions permitted, early postoperative active and passive rehabilitation exercises were advocated.
Results
After emergency debridement and VSD, granulation tissue developed and infection was effectively controlled. In all 30 cases, the area requiring flap cover was reduced by this first stage of treatment. After the second debridement, free flap transplantation combined with skin grafts and VSD were applied to repair massive wounds. In 28 cases (90%), transplanted flaps grew well, without complications. In 2 cases, necrosis was observed at the margins of the flap and further debridement and skin grafting was required for complete wound healing in these cases. Twenty cases had primary repair on all VSD-covered sites and 10 had dispersed remaining wounds due to unsuccessful flap coverage. Of these 10 cases, 6 healed following another skin graft and 4 healed after proactive dressing changes. During the follow-up period of 8 to 26 months (mean: 12.8 months), the flaps grew well without infection or sinus formation. Limb appearance and recovery of function were satisfactory (refer to the appendix for typical cases: Figures 1 and 2).
Figure 1A–H.
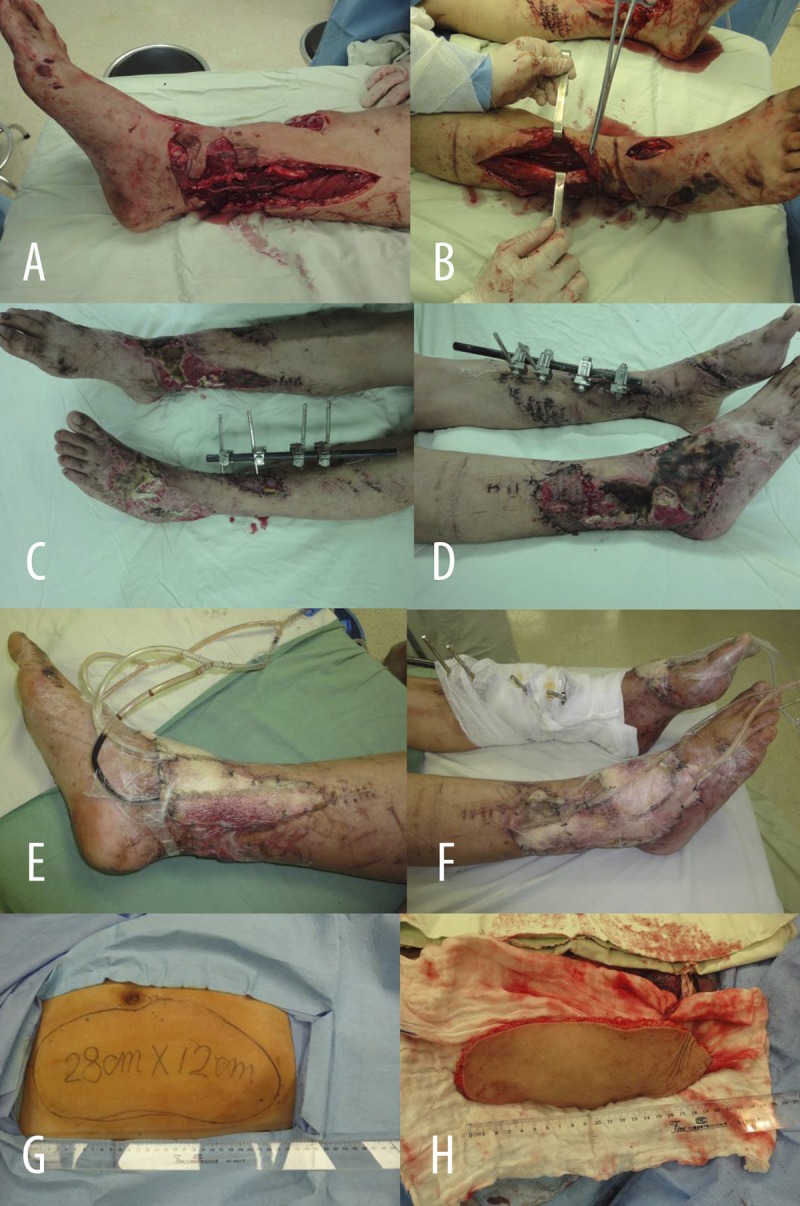
Typical Case 1: Male, 43 years of age, pain, bleed and deformity in bilateral legs for 12 hours because of accident injury. (A, B) Crush injury of subcircular soft tissue in the right lower leg and ankle, and bruise and spasmodism in the posterior and anterior tibial vascular (A – medial view; B – anterolateral view). (C, D) Necrosis and defect of soft tissue in the right lower leg and ankle, reveal of deep tissue 7 days late (C – medial view; D – lateral view). (E, F) Debridement and VSD treatment on subcircular soft tissue in the right lower leg and ankle (E – medial view; F – lateral view). (G, H) Designed (G) and incised (H) thoraco-umbilical flap from thorax and abdomen.
Figure 2A–H.
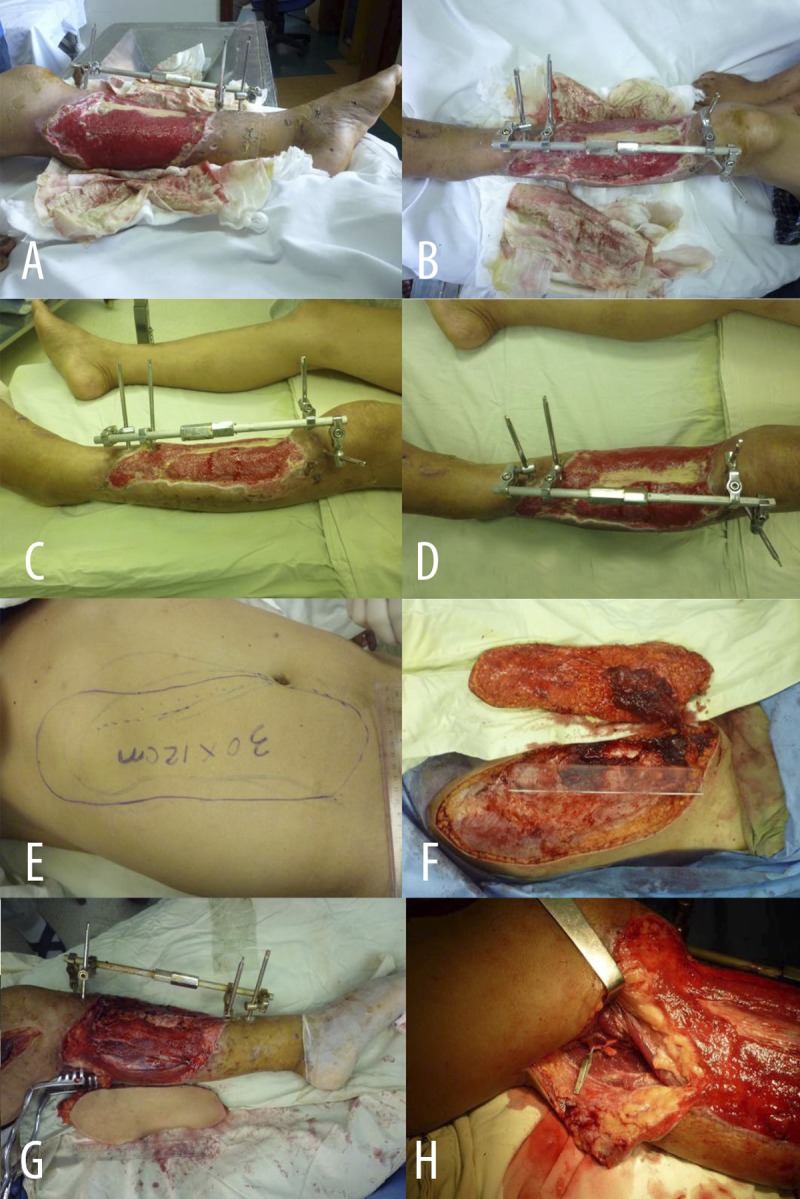
Typical Case 2: Male, 48 years of age, referred from other hospital with subcircular soft tissue defect and pyogenic infection in left lower leg after external fixation of left tibia fracture induced by mechanical wring 3 weeks ago. (A, B) Subcircular soft tissue defect and pyogenic infection in left lower leg (A – medial view; B – lateral view). (C, D) 7d after the first debridement and VSD treatment on subcircular soft tissue defect on left lower leg. Granulation tissues were fresh, but tibia was partially bare. (C – lateral view; D – front view). (E, F) Designed (E) and incised (F) thoraco-umbilical flap from thorax and abdomen. (G, H) Repaired lower leg defect with thoraco-umbilical flap (G), which was nourished by medial head of the sural artery (H).
Discussion
Two-stage repair is now regarded as a more reliable treatment for large soft tissue defects of the lower leg because both the wound condition and general patient condition are usually complicated on presentation. However, exactly when to perform deferred surgical restoration is still debated [7,8,16]. Vacuum sealing drainage facilitates deferred restoration by providing optimal protection for fresh wounds. It can effectively control infection, stimulate granulation growth, and optimize wound condition for later repair. Microsurgical techniques can then be used in the second stage to repair defects. The coverage by VSD after primary debridement reduces exposure of deep tissues and decreases the size of the flap area required for repair so that parts of the wound can heal using only skin grafts, simplifying the surgical procedure. In addition, it has been reported that some wounds with minimal uncovered deep tissues can be repaired with skin grafting only after temporary coverage by VSD [17,18].
Vacuum sealing drainage is already in widespread use for wound restoration in orthopedic departments [2,7,8,13,16,19–23]. Andreas et al demonstrated the efficacy of VSD for deferred restoration in 43 cases of severe open limb fracture [7]. A study on the effects of negative pressure on blood flow at the wound periphery in a porcine model (using different blood flow measurement techniques, including thermodiffusion, transcutaneous, and invasive laser Doppler velocimetry) reported that both increases and decreases in blood flow can be seen in the periwound tissue depending on the distance from the wound edge (0.5, 1.0, and 2.5 cm) and the pressure level, which may accelerate wound healing due to the combination of hypoperfusion and hyperperfusion caused by negative pressure wound therapy [23]. After debridement and VSD covering, granulation tissues on the wound surface developed well in all 30 cases treated in our study, decreasing the required flap area, providing a better soft-tissue bed for free flap and skin graft transplantation, and generally improving both the success of the second-stage operation and postsurgical outcome. In particular, in all 21 cases with infection and necrosis in deep tissues, infection was well controlled by 1 to 3 debridement-VSD treatment cycles. Moreover, deferred microsurgical repair provided patients with ample time to receive comprehensive management, which made subsequent reparative surgery less challenging. Deferral also provided doctors with enough time to design an optimal procedure based on the patient’s individual situation. In addition, it provided the patient’s family sufficient time to consult and consider, thus avoided medical disputes [15].
Vacuum sealing drainage has been used to temporarily cover wounds after debridement [15,16,24–26] and to cover sites with well formed granulation tissue prior to skin grafting [27–29]. However, the advantages of VSD prior to and following free flap transplantation combined with skin grafting have not been examined in detail. Following flap transplantation, we applied skin grafts to the surrounding granulation tissues, sutured VSD sponge to the flap fringes, and attached semi-permeable membranes within 2 cm of the flap fringe to seal the grafts and prevent slippage of the flap. Vacuum sealing drainage eliminated exudate and effusion around the transplanted flap, which kept the flap fringe in place, alleviated swelling, facilitated capillary development into the flap, and prevented congestion of flap veins. For those cases with severe osteomyelitis, when the wound involved the ankle, or if the wound released voluminous exudate, we would place the drainage tube encapsulated by lamellar sponge under the flap and away from the vascular pedicle so that secretions were drawn out from below the flap, thus further aiding flap implantation.
Vacuum sealing drainage is superior to conventional wrap and compression after skin grafting in many ways. The continuous suction leads to a tighter and smoother attachment between grafts and granulation tissues on the surface of the wound, resulting in improved appearance. Furthermore, conventional wrapping stretches the flap fringe and VSD helps secure the flap and facilitate survival of flap fringe. Negative pressure efficiently clears exudates and secretion in all directions and reduces the risk of infection because these fluids act as media for bacteria growth and obstacles for capillary growth. In sum, VSD accelerates detumescence and promotes flap survival [27–29]. However, care must be taken to assure that the skin grafts are sufficiently permeable to allow for sufficient negative pressure drainage.
Though now widely used in trauma medicine, VSD has several limitations [21,30]. It does not directly increase blood supply, so degeneration and necrosis of bones, tendons, and nerves are unavoidable in cases with prolonged ischemia or irreparable circulatory damage. Continued vacuum sealing drainage cannot be used in cases with high ischemic risk or the possibility of anaerobic infection or for patients with coagulation abnormalities [31]. In wounds with evident infection or an indistinct soft-tissue necrosis range (e.g., cases with severe muscle injury), a newer VSD model with both perfusion and drainage systems may avoid the frequent drainage tube obstruction observed with the conventional VSD. Chong et al. [32] demonstrated a design that maintains negative wound pressure during hyperbaric oxygen therapy without causing additional pain and with continued extraction of exudate to allow the dressing to remain undisturbed. This device could be useful for the treatment of wounds that have anaerobic infection.
Excellent flap coverage is the key to the recovery of limb function, so the free flap should be optimized to cover all exposed deep tissues (bones, muscles, tendons, and nerves) while leaving enough room for muscle and tendon movement. At the same time, the free flap should be taken from large covert sites with little mobility. In clinical practice, anterolateral femoral flaps, thoraco-umbilical flaps, latissimus dorsi flaps, and lateral thoracic flaps are common choices and each has its own merits and disadvantages [33–35]. To restore sensation, in particular sensation of the sole, a neurocutaneous anterolateral femoral (perforator) flap is preferred. For circumferential defects with severe infection and deep tissue damage, flaps with better antibiotic capacity are preferred, including anterolateral femoral flaps and latissimus dorsi flaps. For large wounds, thoraco-umbilical flaps and latissimus dorsi (perforator) flaps are favored. The condition of the recipient vessel is also essential to the success of the operation. In the preparation stage, Doppler flowmetry or CT angiography (with digital subtraction enhancement if necessary) is required to reveal injury or obstruction of recipient vessels and variation of graft vessels [36]. Individualized anastomosis procedures must to planned based on the condition of the recipient vessels. When the recipient site lacks appropriate vessels, vein grafting or T-shape microvascular anastomosis can be performed [37,38]. When a recipient vessel for anastomosis is not available, a cross-leg bridge flap is the only solution [39] (but was not employed in this study group). Bridge flap transplantation requires long-term fixation of both lower limbs and so makes daily life and care difficult. Major posterior tibial vessels on the unaffected side can be repaired during pedicle division, although this method is not routine. With large wounds, complete homeostasis is requisite during the operation to prevent hematoma formation beneath the flap because this increases tensile stress on the flap. At the same time, suture tension between the flap and VSD should be controlled to prevent circulatory block and necrosis of the flap fringe. Split thick skin grafting is preferred for wounds with fresh granulation tissues but without exposed bones or tendons around the flap after transplantation. Skin grafts can be taken from the scalp, thigh, thorax, back, or abdomen according to required size. After surgery, movement of the affected limb should be constrained to prevent flap slippage and ensure successful implantation of the free flap and skin grafts.
Cocnlusions
Excellent clinical outcome can be achieved in the treatment of severe traumatic circumferential or sub-circumferential soft-tissue defects of the lower leg using a 2-stage treatment regimen of debridement-VSD and deferred reparative microsurgery using free flaps, skin grafting, and VSD. Initial debridement and temporary covering with VSD can promote granulation growth, reduce wound size, and prevent infection and obstruction of nearby vessels. In the second stage, free flaps and skin grafts are combined to cover the whole wound, and VSD is utilized again to cover skin grafts to prevent flap slippage, aid in removal of exudate, protect against postoperative infection, and aid in restoration of circulation.
Figure 1I-Q.
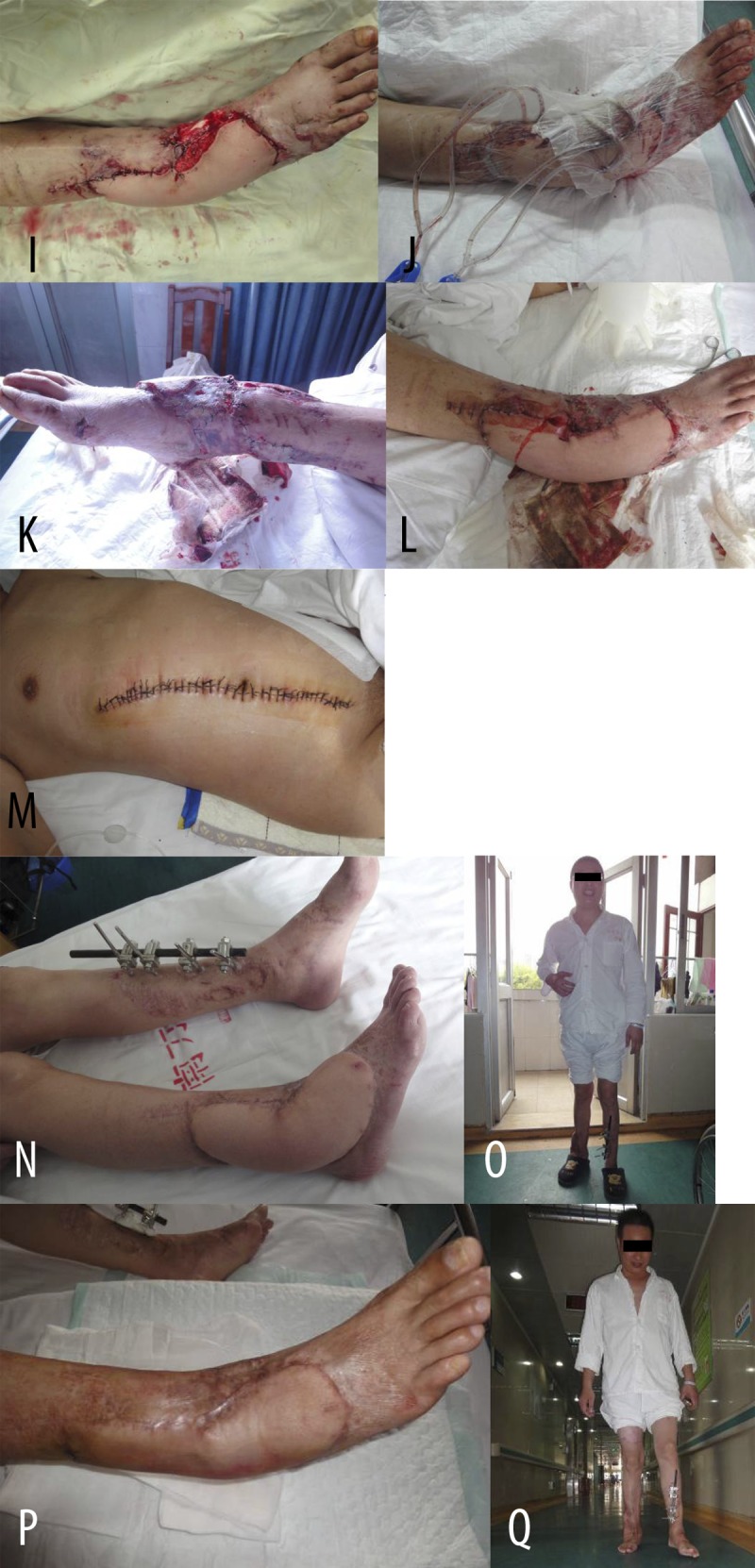
(I, J) Reconstruction the wound with deep tissue exposure in the right lower leg and ankle using thoraco-umbilical flap (I). Covered remnant wounds around the flap with skin grafts; covered recipient site with VSD (J). (K, L) The free flap and skin grafts survived well when VSD was removaled 8 days later. (K – medial view; L – lateral view). (M) Direct suture in the donor site after thoraco-umbilical flap was cut. (N, O) Wound healing and weight-bearing in both legs, only slightly bloated in the flap, 6 months after operation. (P, Q) Good appearance and texture in flap after the operation of reshaping (P). Normal weight-bearing walking (Q).
Figure 2I–O.
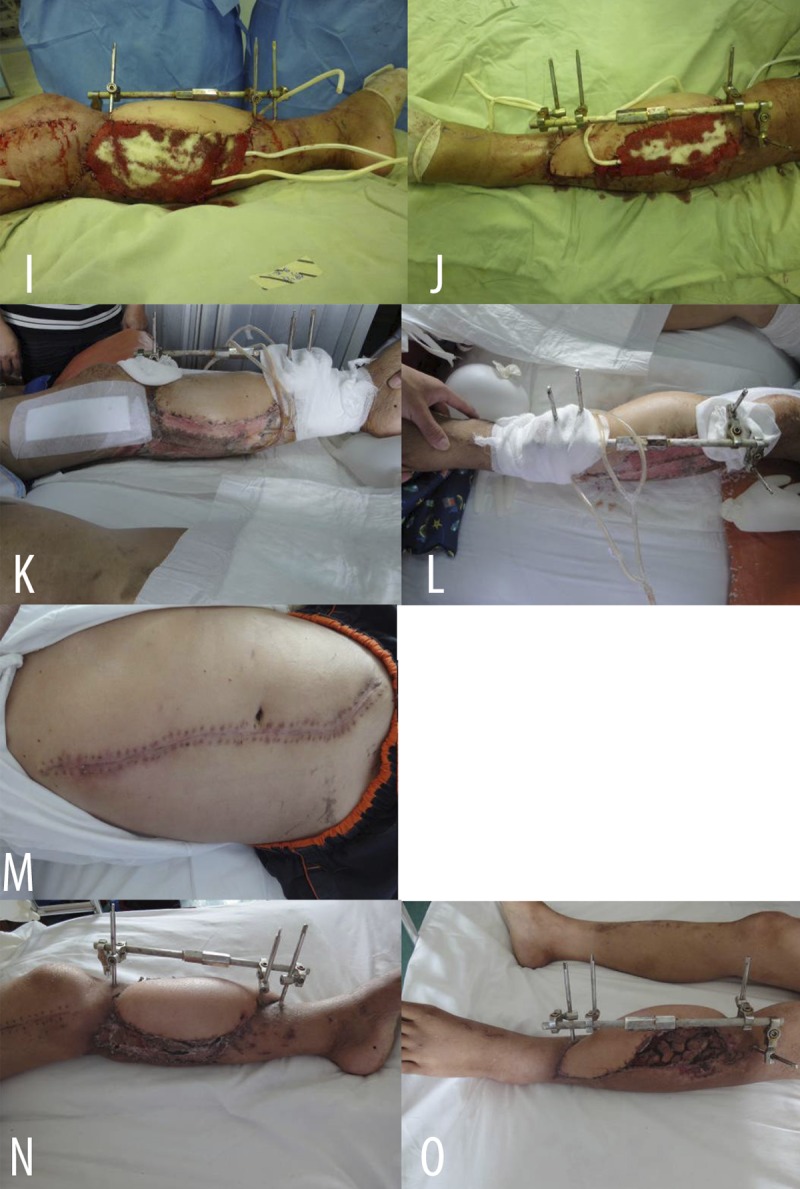
(I, J) Covered remnant wounds around the flap with skin grafts; covered recipient site with VSD (I – medial view; J – lateral view). (K, L) 5d after operation (K – medial view; L – anterolateral view). (M) 2 weeks after operation. Donor site of thoraco-umbilical flap healed. (N, O) 2 weeks after operation. Flap and skin grafts successfully grew, infection was well controlled and subcircular wound achieved primary repair (N – medial view; O – lateral view).
Footnotes
Source of support: This work was supported by the “Natural Science Foundation of Guangdong Province” (Approval number S2012010009434) and “Special Project on the Integration of Industry, Education and Research of Guangdong Province” (Approval number 2012B091100462) in China
References
- 1.Moues CM, van den Bemd GJ, Heule F, Hovius SE. Comparing conventional gauze therapy to vacuum-assisted closure wound therapy: a prospective randomised trial. J Plast Reconstr Aesthet Surg. 2007;60:672–81. doi: 10.1016/j.bjps.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 2.Stannard JP, Volgas DA, Stewart R, et al. Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma. 2009;23:552–57. doi: 10.1097/BOT.0b013e3181a2e2b6. [DOI] [PubMed] [Google Scholar]
- 3.Wong CH, Ong YS, Wei FC. The anterolateral thigh – Vastus lateralis conjoint flap for complex defects of the lower limb. J Plast Reconstr Aesthet Surg. 2011;65:235–39. doi: 10.1016/j.bjps.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 4.Maghari A, Forootan KS, Fathi M, Manafi A. Free transfer of expanded parascapular, latissimus dorsi, and expander “capsule” flap for coverage of large lower-extremity soft-tissue defect. Plast Reconstr Surg. 2000;106:402–5. doi: 10.1097/00006534-200008000-00024. [DOI] [PubMed] [Google Scholar]
- 5.Randon C, Jacobs B, De Ryck F, et al. A 15-year experience with combined vascular reconstruction and free flap transfer for limb-salvage. Eur J Vasc Endovasc Surg. 2009;38:338–45. doi: 10.1016/j.ejvs.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 6.Tham C, Tan BK, Hong SW, et al. Salvage of the massively traumatized lower extremity with sequential free flaps. Plast Reconstr Surg. 2004;113:1746–50. doi: 10.1097/01.prs.0000117373.67460.2a. [DOI] [PubMed] [Google Scholar]
- 7.Steiert AE, Gohritz A, Schreiber TC, et al. Delayed flap coverage of open extremity fractures after previous vacuum-assisted closure (VAC) therapy – worse or worth? J Plast Reconstr Aesthet Surg. 2009;62:675–83. doi: 10.1016/j.bjps.2007.09.041. [DOI] [PubMed] [Google Scholar]
- 8.Hou Z, Irgit K, Strohecker KA, et al. Delayed Flap Reconstruction With Vacuum-Assisted Closure Management of the Open IIIB Tibial Fracture. J Trauma. 2011;71:1705–8. doi: 10.1097/TA.0b013e31822e2823. [DOI] [PubMed] [Google Scholar]
- 9.Suissa D, Danino A, Nikolis A. Negative-pressure therapy versus standard wound care: a meta-analysis of randomized trials. Plast Reconstr Surg. 2011;128:498e–503e. doi: 10.1097/PRS.0b013e31822b675c. [DOI] [PubMed] [Google Scholar]
- 10.Runkel N, Krug E, Berg L, et al. Evidence-based recommendations for the use of Negative Pressure Wound Therapy in traumatic wounds and reconstructive surgery: steps towards an international consensus. Injury. 2011;42:S1–12. doi: 10.1016/S0020-1383(11)00041-6. [DOI] [PubMed] [Google Scholar]
- 11.Thoner B, Fleischmann W, Moch D. Wound treatment by vacuum sealing. Krankenpfl J. 1998;36:78–82. [PubMed] [Google Scholar]
- 12.Vuerstaek JD, Vainas T, Wuite J, et al. State-of-the-art treatment of chronic leg ulcers: A randomized controlled trial comparing vacuum-assisted closure (V.A.C.) with modern wound dressings. J Vasc Surg. 2006;44:1029–37. doi: 10.1016/j.jvs.2006.07.030. discussion 38. [DOI] [PubMed] [Google Scholar]
- 13.Kanakaris NK, Thanasas C, Keramaris N, et al. The efficacy of negative pressure wound therapy in the management of lower extremity trauma: review of clinical evidence. Injury. 2007;38(Suppl 5):S9–18. doi: 10.1016/j.injury.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 14.Saziye K, Mustafa C, Ilker U, Afksendyios K. Comparison of vacuum-assisted closure device and conservative treatment for fasciotomy wound healing in ischaemia-reperfusion syndrome: preliminary results. Int Wound J. 2011;8:229–36. doi: 10.1111/j.1742-481X.2011.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li RG, Yu B, Wang G, et al. Sequential therapy of vacuum sealing drainage and free-flap transplantation for children with extensive soft-tissue defects below the knee in the extremities. Injury. 2012;43:822–28. doi: 10.1016/j.injury.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Liu DS, Sofiadellis F, Ashton M, et al. Early soft tissue coverage and negative pressure wound therapy optimises patient outcomes in lower limb trauma. Injury. 2012;43:772–78. doi: 10.1016/j.injury.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Tang J, Guo WC, Yu L, Zhao SH. Clinical efficacy of artificial skin combined with vacuum sealing drainage in treating large-area skin defects. Chin J Traumatol. 2011;13:289–92. [PubMed] [Google Scholar]
- 18.Wada A, Ferreira MC, Tuma P, Junior, Arrunategui G. Experience with local negative pressure (vacuum method) in the treatment of complex wounds. Sao Paulo Med J. 2006;124:150–53. doi: 10.1590/S1516-31802006000300008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herscovici D, Jr, Sanders RW, Scaduto JM, et al. Vacuum-assisted wound closure (VAC therapy) for the management of patients with high-energy soft tissue injuries. J Orthop Trauma. 2003;17:683–88. doi: 10.1097/00005131-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Damiani G, Pinnarelli L, Sommella L, et al. Vacuum-assisted closure therapy for patients with infected sternal wounds: a meta-analysis of current evidence. J Plast Reconstr Aesthet Surg. 2011;64:1119–23. doi: 10.1016/j.bjps.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 21.Lambert KV, Hayes P, McCarthy M. Vacuum assisted closure: a review of development and current applications. Eur J Vasc Endovasc Surg. 2005;29:219–26. doi: 10.1016/j.ejvs.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Moues CM, Heule F, Hovius SE. A review of topical negative pressure therapy in wound healing: sufficient evidence? Am J Surg. 2011;201:544–56. doi: 10.1016/j.amjsurg.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Borgquist O, Anesater E, Hedstrom E, et al. Measurements of wound edge microvascular blood flow during negative pressure wound therapy using thermodiffusion and transcutaneous and invasive laser Doppler velocimetry. Wound Repair Regen. 2011;19:727–33. doi: 10.1111/j.1524-475X.2011.00741.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan M, Daly D, Stemkowski S. Early intervention of negative pressure wound therapy using Vacuum-Assisted Closure in trauma patients: impact on hospital length of stay and cost. Adv Skin Wound Care. 2009;22:128–32. doi: 10.1097/01.ASW.0000305451.71811.d5. [DOI] [PubMed] [Google Scholar]
- 25.Hunter JE, Teot L, Horch R, Banwell PE. Evidence-based medicine: vacuum-assisted closure in wound care management. Int Wound J. 2007;4:256–69. doi: 10.1111/j.1742-481X.2007.00361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schintler MV. Negative pressure therapy: theory and practice. Diabetes Metab Res Rev. 2012;28(Suppl 1):72–77. doi: 10.1002/dmrr.2243. [DOI] [PubMed] [Google Scholar]
- 27.Petkar KS, Dhanraj P, Kingsly PM, et al. A prospective randomized controlled trial comparing negative pressure dressing and conventional dressing methods on split-thickness skin grafts in burned patients. Burns. 2011;37:925–29. doi: 10.1016/j.burns.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Blume PA, Key JJ, Thakor P, et al. Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: comparing V.A.C.(R) therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J. 2010;7:480–87. doi: 10.1111/j.1742-481X.2010.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saaiq M, Hameed Ud D, Khan MI, Chaudhery SM. Vacuum-assisted closure therapy as a pretreatment for split thickness skin grafts. J Coll Physicians Surg Pak. 2010;20:675–79. [PubMed] [Google Scholar]
- 30.Toporcer T, Radonak J. Vacuum assisted wound closure – overview of lesson and applications. Cas Lek Cesk. 2006;145:702–7. discussion 07. [PubMed] [Google Scholar]
- 31.Chester DL, Waters R. Adverse alteration of wound flora with topical negative-pressure therapy: a case report. Br J Plast Surg. 2002;55:510–11. doi: 10.1054/bjps.2002.3890. [DOI] [PubMed] [Google Scholar]
- 32.Chong SJ, Kwan TM, Weihao L, et al. Maintenance of negative-pressure wound therapy while undergoing hyperbaric oxygen therapy. Diving Hyperb Med. 2011;41:147–50. [PubMed] [Google Scholar]
- 33.Yazar S, Lin CH. Selection of recipient vessel in traumatic lower extremity. J Reconstr Microsurg. 2012;28:199–204. doi: 10.1055/s-0032-1306366. [DOI] [PubMed] [Google Scholar]
- 34.Korompilias AV, Lykissas MG, Vekris MD, et al. Microsurgery for lower extremity injuries. Injury. 2008;39(Suppl 3):S103–8. doi: 10.1016/j.injury.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Sekido M, Yamamoto Y, Furukawa H, Sugihara T. Change of weight-bearing pattern before and after plantar reconstruction with free anterolateral thigh flap. Microsurgery. 2004;24:289–92. doi: 10.1002/micr.20022. [DOI] [PubMed] [Google Scholar]
- 36.Tan O, Yuce I, Kantarci M, Algan S. Evaluation of lower-limb arteries with multidetector computed tomography angiography prior to free flap surgery: a radioanatomic study. J Reconstr Microsurg. 2010;27:199–206. doi: 10.1055/s-0030-1270538. [DOI] [PubMed] [Google Scholar]
- 37.Kim JT, Kim CY, Kim YH. T-anastomosis in microsurgical free flap reconstruction: an overview of clinical applications. J Plast Reconstr Aesthet Surg. 2008;61:1157–63. doi: 10.1016/j.bjps.2008.03.048. [DOI] [PubMed] [Google Scholar]
- 38.Kawamura K, Yajima H, Kobata Y, et al. Anatomy of Y-shaped configurations in the subscapular arterial system and clinical application to harvesting flow-through flaps. Plast Reconstr Surg. 2005;116:1082–89. doi: 10.1097/01.prs.0000178791.85118.ca. [DOI] [PubMed] [Google Scholar]
- 39.Ren GH, Li JW, Li RG, et al. Treatment of large circular soft tissue defect in lower extremities with a combination of bridge flaps and free skin graft covered by vacuum sealing drainage. Zhonghua Wai Ke Za Zhi. 2012;50:39–44. [PubMed] [Google Scholar]


