Abstract
Background
Nitric oxide (NO) is protective for the cardiovascular system, and excessive NO exerts negative effects on the circulatory system. This study aimed to compare the effects of selective or non-selective NO synthase (NOS) inhibitors on blood flow perfusion of ischemia-reperfused myocardium.
Materials/Methods
Male mongrel dogs were randomly assigned to 4 groups: only ischemia-reperfusion (control), ischemia-reperfusion plus Nω-nitro-L-arginine methyl ester (NAME) treatment, ischemia-reperfusion plus aminoguanidine (AMD) treatment, and sham operation group. Myocardial contrast echocardiography (MCE) was performed. Blood samples were taken for measurement of NO. Background-subtracted peak videointensity (PVI) and PVI ratio in myocardium were measured.
Results
In the NAME-treated group, the PVI at 5 min reperfusion did not significantly differ from pre-LAD-occlusion, but declined to and retained at a level obviously lower than the pre-LAD-occlusion. In the AMD-treated group, the PVI at 5 min reperfusion was significantly higher than at pre-LAD-occlusion, and then restored to and remained at the pre-LAD-occlusion level. The changes of PVI ratios in the 3 groups were similar to PVI values. In the AMD-treated group, the curve width increased in the early reperfusion, but returned to the pre-LAD-occlusion level at 90 min reperfusion. The plasma NO concentration in the NAME-treated group greatly decreased and remained low during the whole period of reperfusion. In the AMD-treated group, there were only slight increases in NO concentrations during reperfusion.
Conclusions
NAME totally inhibited NO production and attenuated myocardial blood flow perfusion. Aminoguanidine significantly relieved the increase in NO production and alleviated the congestion of reperfused myocardium. Selective inhibitors of iNOS might be useful in the management of certain diseases associated with ischemia-reperfusion.
Keywords: myocardial ischemia, NAME, myocardial reperfusion, nitric oxide, nitric oxide synthase inhibitor
Background
Animal studies have demonstrated that the expression of inducible nitric oxide synthase (iNOS) was increased in ischemia-reperfused myocardium, resulting in increased production of nitric oxide (NO) and superoxide anions [1,2]. It is well known that NO is protective for the cardiovascular system and excessive NO exerts negative effects on the circulatory system. For instance, peroxynitrite anion formed from the NO and superoxide anion is a strongly cytotoxic substance and plays an important role in the occurrence of some forms of acute myocardial damage [3]. Therefore, myocardial ischemia-reperfusion injury might be alleviated by inhibiting excessive NO production. Nonetheless, it has been demonstrated that the left ventricular ejection fraction progressively declined in in vivo experiments when the non-selective NOS inhibitor Nω-nitro-L-arginine methyl ester (NAME) was administered [4]. The possible reason for this is that myocardial blood flow perfusion was impaired due to the inhibition of coronary arterial endothelial NOS (eNOS) by NAME.
We hypothesized that the selective iNOS inhibitor aminoguanidine (AMD) [5], in contrast to NAME, would alleviate the impairment of the myocardial blood flow perfusion through inhibition of iNOS-mediated NO. To test this hypothesis, we attempted to compare the effects between selective and non-selective NOS inhibitors on myocardial blood flow perfusion in an in vivo canine experimental model of myocardial ischemia-reperfusion.
Material and Methods
Animal model of myocardial ischemia-reperfusion
Male mongrel dogs weighing 13~18 kg were used in this study. The protocol was approved by the Experimental Animal Ethics Committee of Nanfang Hospital, Southern Medical University, Guangzhou, China, according to the guidelines for animal experiments established by the Chinese Association for Laboratory Animal Science.
Animals were randomly divided into 4 groups: only ischemia-reperfusion (control) group, ischemia-reperfusion plus NAME-treated group, ischemia-reperfusion plus AMD-treated group, and sham operation group. It was expected that 6 animals would successfully complete the experiment for each group. After the animal was anaesthetized using intravenous sodium pentobarbital at 35 mg/kg, trachea cannula was performed and linked to an animal respirator. Then a pigtail catheter was inserted into the right femoral artery for aortic and left ventricular pressure measurement. An expansion tube sheath was placed into the right femoral vein for infusion and ultrasound contrast injection. Thoracotomy was performed through the fifth intercostal space. The heart was elevated from the pericardial bed using a 4.0 silk suture. Another suture line was placed across the left anterior descending coronary artery (LAD) with a water sac laid on the surface of the heart. LAD ligation for 60 min was performed in the 3 treatment groups, followed by 120 min of reperfusion, and no ligation was performed in the sham operation group. In the NAME-treated group, the dogs received intravenous NAME at 10 mg/kg. Administration of one-third dosage of NAME started 10 min before LAD ligation, and continuously intravenous NAME of the remaining dosage initiated from 10 min before reperfusion to the end of 120 min reperfusion. In AMD-treated group, the animals received intravenous AMD at 100 mg/kg. Administration of one-third dosage AMD started 10 min before LAD ligation, and continuously intravenous AMD of the remaining dosage was initiated from 10 min before reperfusion to the end of 120 min reperfusion. Hemodynamic status and electrocardiogram were monitored during the whole experiment. After experiments, the dogs were killed using sodium pentobarbital.
Myocardial contrast echocardiography (MCE)
Using the Acuson SEQUOIA 512 ultrasound machine (Siemens AG, Munich, Germany) with 3.5-MHz frequency, the horizontal short-axis view map of the left ventricular papillary muscle was displayed by the transducer fixed into the water sac. The transducer was immobilized during the whole experiment and the quality of the image was maintained by adjusting signal gains. Then a second-harmonic imaging technique was applied for intravenous MCE. The trigger electrocardiographic (ECG) interval was up to 3 cardiac cycles. At each time, a bolus of 0.01 ml/kg microvesicle contrast octafluoropropane (C3F8)-exposed sonicated dextrose albumin (Department of Clinical Pharmacy, Nanfang Hospital, Guangzhou, China) was injected intravenously and ultrasound images were recorded for further analysis. MCE time-points included prior to LAD ligation, immediately before reperfusion, and at 5, 30, 60, 90, and 120 min reperfusion.
MCE image analysis
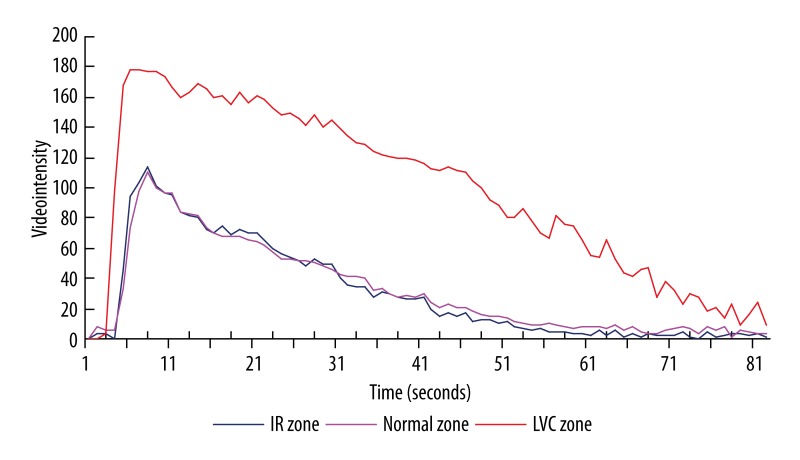
We used the TomTec Image Workstation to quantify the MCE image videointensity. The regions of interest were the anterior wall of the left ventricle but excluding the endocardium or epicardium membrane. The regions were then automatically analyzed by the program and finalized as a videointensity-time curve after background subtraction (Figure 1). Myocardial perfusion was indicated by peak videointensity (PVI) [6–8] and relative perfusion of the ischemia-reperfusioned district was represented by the ratio of PVI between ischemia-reperfusioned and intact myocardium zones (PVIR). MCE curve width was defined as the duration from the emergence of myocardial development to the myocardial videointensity of 10% PVI back from PVI.
Figure 1.
MCE videointensity-time curve (baseline). IR zone, ischemia-reperfusion myocardium zone; normal zone, non-ischemia myocardium zone; LVC zone, left ventricular chamber zone.
Determination of plasma NO concentration
At the individual MCE time-points, blood samples of 5 ml each time were taken from coronary venous sinus. Plasma separated by centrifugation was stored at −70°C until analysis. Nitrate and nitrite in plasma were measured by the nitrate reductase method (kits were purchased from Nanjing Juli Biomedical Engineering Institute, Nanjing, China) and stood for plasma concentrations of NO.
Statistics
We used SPSS 11.0 software package (Chicago, IL) for statistical analysis. Data are expressed as the mean value ± the standard deviation (SD) for continuous variables following a normal distribution, and one-way analysis of variance (ANOVA) was used for comparisons of them. Two-sided P values <0.05 are considered significant.
Results
Ischemia-reperfusion dog model and hemodynamic observations
Six dogs were used in both the AMD-treated and sham groups with no deaths and 8 dogs were killed in both the control and NAME-treated groups with 2 deaths for each, thus 6 dogs completed the experiment in each group. All the 4 deaths were due to ventricular fibrillation in the early period of reperfusion. None of the animals showed other complications except that visible ventricular premature beats during LAD ligation was noticed.
During LAD ligation, no contrast filling in focal ventricular wall was found using MCE with wall akinesis and thinning in two-dimensional ultrasound images. After release from LAD ligation, adequate contrast filling was observed in the ventricular wall zone in which there was no contrast filling during LAD ligation. Although the wall motion improved with reperfusion, a complete recovery of the function was not noted at the end of the 120-min reperfusion. The model was accepted as reliable because no myocardial infarction was observed in myocardial 2,3,5-triphenyltetrazolium chloride (TTC) staining.
Hemodynamics monitoring displayed reduced heart rate during reperfusion and elevated mean aortic pressure prior to and during reperfusion in the NAME-treated group and no significant changes in other groups. Left ventricular diastolic pressure markedly rose before and in early reperfusion in the control and NAME-treated groups and restored to their pre-ligation levels by 60- and 90-min reperfusion, respectively. In the AMD-treated group, left ventricular diastolic pressure went up only prior to reperfusion.
Changes in MCE videointensity
In the control group, PVI of reperfused myocardium markedly increased in the early reperfusion (within 30-min reperfusion) and thereafter returned to the pre-ligation level (Table 1). In the NAME-treated group, PVI was only slightly higher than the pre-ligation level at 5 min reperfusion (P>0.05), but was significantly lower than the pre-ligation level from 30 to 120 min reperfusion. In the AMD-treated group, PVI was significantly higher than the pre-ligation level only at 5 min reperfusion, and was insignificantly different from the pre-ligation level at other reperfusion time-points. A comparison of PVI at each corresponding reperfusion time-point among groups showed that PVI in the control group was higher than in the AMD-treated group, which was higher than in the NAME-treated group but without statistical significance.
Table 1.
MCE PVI of ischemia-reperfused myocardium (grayscale units, mean ±SD, n=6 for each group).
| Treatment | Preligation | Reperfusion time (min) | ||||
|---|---|---|---|---|---|---|
| 5 | 30 | 60 | 90 | 120 | ||
| Control | 103±20 | 126±26** | 115±23* | 108±22 | 102±19 | 102±21 |
| NAME | 103±21 | 111±26 | 91±20* | 85±23* | 85±20* | 80±21* |
| AMD | 103±19 | 114±22** | 107±20 | 105±20 | 102±18 | 100±18 |
| Sham | 101±23 | 97±19 | 104±27 | 107±20 | 98±17 | 100±23 |
| F value | 0.015 | 1.870 | 1.251 | 1.797 | 1.312 | 1.847 |
| P value | 0.997 | 0.167 | 0.318 | 0.180 | 0.298 | 0.171 |
Compared with preligation, * P<0.05, ** P<0.01. MCE – myocardial contrast echocardiography; PVI – peak videointensity; NAME – Nω-nitro-L-arginine methyl ester; AMD – aminoguanidine.
The change of PVIR in all groups was similar to that of PVI (Table 2). A comparison among all groups showed that PVIR was remarkably lower than the control group at 60 min reperfusion in the NAME-treated group and at 30 min reperfusion in the AMD-treated group. Furthermore, PVIRs in the NAME-treated group were significantly lower than in the AMD-treated group from 30 to 120 min reperfusion.
Table 2.
MCE PVIR (mean ±SD, n=6 for each group).
| Treatment | Preligation | Reperfusion time (min) | ||||
|---|---|---|---|---|---|---|
| 5 | 30 | 60 | 90 | 120 | ||
| Control | 1.02±0.04 | 1.30±0.08** | 1.27±0.05** | 1.11±0.04* | 1.02±0.04 | 0.98±0.02 |
| NAME | 1.01±0.05 | 1.09±0.09## | 0.92±0.06*,## | 0.88±0.07*,## | 0.89±0.10* | 0.85±0.09* |
| AMD | 1.02±0.07 | 1.12±0.10**,## | 1.05±0.08*,##,+ | 1.04±0.08+ | 1.02±0.07+ | 1.01±0.06+ |
| Sham | 1.02±0.04 | 1.01±0.04 | 1.02±0.05 | 1.01±0.05 | 1.01±0.03 | 1.02±0.05 |
| F value | 0.033 | 20.811 | 46.634 | 22.484 | 6.320 | 10.304 |
| P value | 0.992 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 |
Compared with preligation, * P<0.05, ** P<0.01; NAME and AMD groups compared with control group, #P<0.05, ##P<0.01; NAME group compared with AMD group, +P<0.05, ++P<0.01. MCE – myocardial contrast echocardiography; PVI – peak videointensity; PVIR – ratio of PVI between stunned and intact myocardium zone; NAME – Nω-nitro-L-arginine methyl ester; AMD – aminoguanidine.
Changes in MCE curve width
MCE curve width during reperfusion in the control group increased markedly and restored slowly, but not to the pre-ligation level by 120 min reperfusion (Table 3). In the NAME-treated group, MCE curve width was greater than in the control group and no restoration was observed. In the AMD-treated group, MCE curve width increased in the early reperfusion, but restored rapidly and reached the pre-ligation level by 90 min reperfusion.
Table 3.
MCE curve width of ischemia-reperfused myocardium (seconds, mean ±SD, n=6 for each group).
| Treatment | Preligation | Reperfusion time (min) | ||||
|---|---|---|---|---|---|---|
| 5 | 30 | 60 | 90 | 120 | ||
| Control | 50±11 | 60±11* | 65±10** | 63±11** | 64±10** | 61±10** |
| NAME | 51±13 | 69±15** | 75±11** | 69±12** | 70±10** | 68±11** |
| AMD | 51±11 | 61±11** | 58±12** | 53±11* | 51±10+ | 50±11 |
| Sham | 51±13 | 52±13 | 49±12 | 51±11 | 53±14 | 50±11 |
| F value | 0.007 | 1.807 | 4.630 | 4.256 | 4.845 | 4.296 |
| P value | 0.999 | 0.178 | 0.013 | 0.018 | 0.011 | 0.017 |
Compared with preligation, * P<0.05, ** P<0.01; NAME group compared with AMD group, +P<0.05, ++P<0.01. MCE – myocardial contrast echocardiography; NAME – Nω-nitro-L-arginine methyl ester; AMD – aminoguanidine.
Changes in coronary sinus plasma NO concentrations
Plasma NO concentrations in the control group were increased during reperfusion and did not returned to the pre-ligation level by 120 min reperfusion (Table 4). The NO concentrations in the NAME-treated group greatly decreased and remained low during the whole period of reperfusion and notably lower than in the control group. In the AMD-treated group, there were slight increases in NO concentrations during reperfusion, but the differences versus their pre-ligation levels were not statistically significant. In the sham group, no significant changes in NO levels were observed during the whole experiment (Tables 1–4).
Table 4.
Coronary sinus plasma NO concentrations (μmol/L, mean ±SD, n=6 for each group).
| Treatment | Preligation | Pre-reperfusion | Reperfusion time (min) | ||||
|---|---|---|---|---|---|---|---|
| 5 | 30 | 60 | 90 | 120 | |||
| Control | 25.9±14.1 | 23.4±12.6 | 39.2±13.3** | 49.0±15.7** | 46.8±16.5** | 42.9±14.7** | 36.1±15.7** |
| NAME | 27.9±16.2 | 20.6±10.9 | 18.3±10.3* | 15.8±11.9*,# | 14.5±10.1*,# | 15.9±11.5*,# | 15.7±11.5* |
| AMD | 26.6±16.0 | 26.5±8.8 | 27.3±9.1 | 31.4±11.5 | 32.5±8.9+ | 30.3±12.7 | 30.9±12.5 |
| Sham | 27.3±16.3 | 24.9±13.1 | 28.6±14.5 | 29.7±17.0 | 28.8±15.5 | 25.0±13.2 | 25.6±12.7 |
| F value | 0.020 | 0.293 | 3.221 | 5.495 | 6.156 | 4.427 | 2.833 |
| P value | 0.996 | 0.830 | 0.045 | 0.006 | 0.004 | 0.015 | 0.064 |
Compared with preligation, * P<0.05, ** P<0.01; NAME and AMD groups compared with control group, #P<0.05, ##P<0.01; NAME group compared with AMD group, +P<0.05, ++P<0.01. NO – nitric oxide; NAME – Nω-nitro-L-arginine methyl ester; AMD – aminoguanidine.
Discussion
The role of NO in the ischemia-reperfused heart is unclear. The main findings of the present study are that NO production by ischemia-reperfused myocardium increased, that the non-selective NOS inhibitor NAME caused insufficiency of myocardial blood flow perfusion, and that the selective iNOS inhibitor aminoguanidine could retain a normal level of myocardial blood perfusion.
When ischemic myocardium was reperfused, the endogenous cytokine network was activated. These cytokines could stimulate multiple cells in myocardium expressing iNOS and then persistently produce a large quantity of NO [1,2]. Recent studies have found that the blood flow perfusion of ischemia-reperfused myocardium did not decline, and instead even congested [9]. This phenomenon was considered as responsive congestion to reperfusion following ischemia; excessive NO production may contribute to this. The present study showed that coronary sinus plasma NO levels in an in vivo canine model of ischemia-reperfusion notably increased and that the blood perfusion of ischemia-reperfused myocardium also markedly increased. These findings further confirmed the increase of NO production and responsive congestion in ischemia-reperfused myocardium. Overproduction of NO is detrimental to the cardiovascular system. Peroxynitrite anion, a strongly cytotoxic agent formed by the reaction of NO with superoxide anion, can directly damage cardiac myocytes [3]. In addition, the increase of MCE curve width during reperfusion suggests intramyocardial microcirculatory stagnation. As such, we speculate that the use of NOS inhibitors could alleviate myocardial ischemia-reperfusion injury through restraining excessive production of NO.
NAME is a type of non-selective NOS inhibitor that inhibits both eNOS and iNOS. Accordingly, it can completely restrain NO production in vivo. Nonetheless, concomitant inhibition of coronary arterial eNOS will impair myocardial blood perfusion and then aggravate myocardial injury. Studies have established that NO produced through the eNOS route during ischemia-reperfusion is protective to myocardium [10,11]. It was reported that the use of NAME brought about progressive decline of left ventricular ejection fraction [4]. The present study shows that NAME totally inhibited the NO production of ischemia-reperfused myocardium and remarkably reduced the myocardial blood flow perfusion to a lower than normal level that resulted in myocardial re-ischemia, which shows that NAME could cause myocardial hypoperfusion. Administration of NAME brought about further increase of widened MCE curve width during reperfusion, suggesting the aggravation of intramyocardial microcirculatory stagnation. Therefore, non-selective NOS inhibitors administrated in the settings of ischemia-reperfusion might be deleterious.
Aminoguanidine is a selective iNOS inhibitor that restrains only iNOS and not eNOS. Cottart et al. [10] reported that selective iNOS inhibitors had no harmful effects on ischemia-reperfused liver. Wildhirt et al. [12] found that cardiac dysfunction developed accompanied with increased myocardial iNOS activity, whereas aminoguanidine significantly inhibited iNOS activity and improved left ventricular function in a rabbit model of myocardial stunning. The present study shows that aminoguanidine inhibited excessive NO production in ischemia-reperfused myocardium and then reduced only the degree of myocardial blood hyperperfusion and kept normal blood flow perfusion. Furthermore, it relieved intramyocardial microcirculatory stagnation and accelerated its restoration to a normal level. These findings suggest that because it did not inhibited eNOS, aminoguanidine overcame the disadvantages of NAME, alleviating myocardial blood perfusion impairment and microcirculatory stagnation aggravation. Selective iNOS inhibitor aminoguanidine, therefore, may be beneficial if used in the settings of ischemia-reperfusion.
MCE was an indirect methodology in evaluating myocardial blood perfusion. It can also display the spatial distribution of myocardial blood perfusion, measure myocardial blood flow and blood volume, and evaluate coronary blood flow reserve and endothelial function [13–15]. Compared with single-photon emission computed tomography imaging, MCE provides a similar image of blood flow [6]. Using MCE, the PVI was closely related to the myocardial blood flow measured by radio-microspheres [7] or ultrasonic flowmeter [8]. Porter et al. [16] reported a linear relationship between the coronary blood flow and the myocardial videointensity generated by the intravenous injection of fluoride carbon contrast agent. The sensitivity and reliability of MCE for evaluating myocardial blood perfusion and coronary microcirculation have significantly improved with the improvement of acoustic contrast agents, the application of a second harmonic imaging technology, and the advance in analytical methods for MCE images. MCE is now considered one of the most effective methods for evaluation of myocardial microvascular dysfunction.
Conclusions
Administration of NAME totally inhibited NO production and attenuated myocardial blood flow perfusion and aminoguanidine significantly relieved the increase in NO production and alleviated the congestion of reperfused myocardium in a dog model. Selective inhibitors of iNOS may be useful in the management of certain diseases associated with ischemia-reperfusion.
Footnotes
Source of support: The study was funded by the Guangdong Provincial Planning Project for Science and Technology (Grant number: 2010B031600017
References
- 1.Kudej RK, Zhang XP, Ghaleh B, et al. Enhanced cAMP-induced nitric oxide-dependent coronary dilation during myocardial stunning in conscious pigs. Am J Physiol Heart Circ Physiol. 2000;279:H2967–74. doi: 10.1152/ajpheart.2000.279.6.H2967. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Bissing JW, Xu L, et al. Nitric oxide synthase inhibitors decrease coronary sinus-free radical concentration and ameliorate myocardial stunning in an ischemia-reperfusion model. J Am Coll Cardiol. 2001;38:546–54. doi: 10.1016/s0735-1097(01)01400-0. [DOI] [PubMed] [Google Scholar]
- 3.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oyama J, Shimokawa H, Momii H, et al. Role of nitric oxide and peroxynitrite in the cytokine-induced sustained myocardial dysfunction in dogs in vivo. J Clin Invest. 1998;101:2207–14. doi: 10.1172/JCI986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misko TP, Moore WM, Kasten TP, et al. Selective inhibition of the inducible nitric oxide synthase by aminoguanidine. Eur J Pharmacol. 1993;233:119–25. doi: 10.1016/0014-2999(93)90357-n. [DOI] [PubMed] [Google Scholar]
- 6.Hickman M, Janardhanan R, Dwivedi G, et al. Clinical significance of perfusion techniques utilising different physiological mechanisms to detect myocardial viability: a comparative study with myocardial contrast echocardiography and single photon emission computed tomography. Int J Cardiol. 2007;114:139–40. doi: 10.1016/j.ijcard.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 7.Galiuto L, DeMaria AN, May-Newman K, et al. Evaluation of dynamic changes in microvascular flow during ischemia-reperfusion by myocardial contrast echocardiography. J Am Coll Cardiol. 1998;32:1096–101. doi: 10.1016/s0735-1097(98)00349-0. [DOI] [PubMed] [Google Scholar]
- 8.Porter TR, Xie F, Kricsfeld A, Kilzer K. Noninvasive identification of acute myocardial ischemia and reperfusion with contrast ultrasound using intravenous perfluoropropane-exposed sonicated dextrose albumin. J Am Coll Cardiol. 1995;26:33–40. doi: 10.1016/0735-1097(95)00132-j. [DOI] [PubMed] [Google Scholar]
- 9.Mori E, Haramaki N, Ikeda H, Imaizumi T. Intra-coronary administration of L-arginine aggravates myocardial stunning through production of peroxynitrite in dogs. Cardiovasc Res. 1998;40:113–23. doi: 10.1016/s0008-6363(98)00146-1. [DOI] [PubMed] [Google Scholar]
- 10.Cottart CH, Do L, Blanc MC, et al. Hepatoprotective effect of endogenous nitric oxide during ischemia-reperfusion in the rat. Hepatology. 1999;29:809–13. doi: 10.1002/hep.510290317. [DOI] [PubMed] [Google Scholar]
- 11.Jones SP, Greer JJ, Kakkar AK, et al. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–82. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 12.Wildhirt SM, Schulze C, Conrad N, et al. Aminoguanidine inhibits inducible NOS and reverses cardiac dysfunction late after ischemia and reperfusion – implications for iNOS-mediated myocardial stunning. Thorac Cardiovasc Surg. 1999;47:137–43. doi: 10.1055/s-2007-1013128. [DOI] [PubMed] [Google Scholar]
- 13.Kaul S. Assessment of coronary microcirculation with myocardial contrast echocardiography: current and future clinical applications. Br Heart J. 1995;73:490–95. doi: 10.1136/hrt.73.6.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rakhit DJ, Becher H, Monaghan M, et al. The clinical applications of myocardial contrast echocardiography. Eur J Echocardiogr. 2007;8:S24–29. doi: 10.1016/j.euje.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Ward RP, Lang RM. Myocardial contrast echocardiography in acute coronary syndromes. Curr Opin Cardiol. 2002;17:455–63. doi: 10.1097/00001573-200209000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Porter TR, Xie F, Kilzer K. Intravenous perfluoropropane-exposed sonicated dextrose albumin produces myocardial ultrasound contrast that correlates with coronary blood flow. J Am Soc Echocardiogr. 1995;8:710–18. doi: 10.1016/s0894-7317(05)80386-4. [DOI] [PubMed] [Google Scholar]