Abstract
Mice infected with Trypanosoma cruzi, the agent of Chagas disease, rapidly develop anemia and thrombocytopenia. These effects are partially promoted by the parasite trans-sialidase (TS), which is shed in the blood and depletes sialic acid from the platelets, inducing accelerated platelet clearance and causing thrombocytopenia during the acute phase of disease. Here, we demonstrate that oral immunization of C57BL/6 mice with Phytomonas serpens, a phytoflagellate parasite that shares common antigens with T. cruzi but has no TS activity, reduces parasite burden and prevents thrombocytopenia and leukopenia. Immunization also reduces platelet loss after intraperitoneal injection of TS. In addition, passive transfer of immune sera raised in mice against P. serpens prevented platelet clearance. Thus, oral exposure to P. serpens attenuates the progression of thrombocytopenia induced by TS from T. cruzi. These findings are not only important for the understanding of the pathogenesis of T. cruzi infection but also for developing novel approaches of intervention in Chagas disease.
Introduction
The order Kinetoplastida is composed of flagellated unicellular organisms, some of which live in soil or aquatic environments, while others are parasites responsible for severe diseases in humans, animals, and plants [1], [2]. The combined number of people infected by Kinetoplastida pathogens is estimated to be over 20 million, resulting in various health problems and more than 100,000 deaths each year. With half a billion people at risk, mostly in tropical and subtropical areas, these parasites represent an important global health problem with associated significant economic burden [3]. Chagas disease is caused by Trypanosoma cruzi, a Kinetoplastid transmitted by the feces of blood-feeding triatomine insects [4]–[6]. The disease affects about 8 million people in Latin America, of whom 30–40% have or will develop neurologic manifestations [7], cardiomyopathy, and/or digestive megasyndromes [8]. The variability in disease outcome has been attributed to host responses and parasite heterogeneity [9].
T. cruzi infection in mice is associated with severe hematological changes, including thrombocytopenia [10], neutropenia followed by neutrophilia, and eosinophilia [11], which may contribute to mortality. Marcondes and collaborators [12] reported that acute T. cruzi infection is associated with anemia, thrombocytopenia, leukopenia, and bone marrow hypoplasia and that these alterations can be prevented by nifurtimox treatment. Similar hematological alterations have also been described in experimental African trypanosomiasis [13] and are common characteristics of human immunodeficiency virus infection [14] and malaria [15].
The mechanism responsible for hematological alterations observed in acute T. cruzi infection is not clearly understood. Our previous studies revealed that nitric oxide (NO) does not play a direct role in the development of anemia during T. cruzi infection, but contributes together with TNF-α to oxidative pre-hemolytic damage of erythrocytes in infected mice [16]. In addition, IFN-induced p47GTPase (LRG-47) influences T. cruzi control by simultaneously regulating macrophage microbicidal activity and hemopoietic function [17].
Sialic acid in the surface of T. cruzi plays an important role in the infectious process; however, T. cruzi is unable to synthesize sialic acid. Instead, the parasite expresses trans-sialidase (TS), which mediates transfer of sialic acid from host glycoconjugates to parasite mucins [18]–[20]. T. cruzi TS depletes platelets with sialic acid, increasing clearance and leading to thrombocytopenia during acute infection [21].
T. cruzi exhibit immunological cross-reactivity with other Kinetoplastida, including Leishmania spp. [22] and insect trypanosomatids that belong to the genera Crithidia, Herpetomonas, Leptomonas, and Blastocrithidia [23]. We showed that Phytomonas serpens, a tomato parasite also from the order Kinetoplastida, shares antigens with T. cruzi [24] but has no TS activity [25]. These antigens are recognized by human sera and induce nitric oxide-dependent protective immunity against experimental T. cruzi infection in susceptible BALB/c mice [24], [26], [27]. Phytomonas are etiologic agents of plant diseases found across southern Brazil, North and Central Africa, and several European countries. These trypanosomatids are found in plants of economic importance, including cashew, coffee, cassava, coconut, and oil palms, and infect edible fruits such as tomato, orange, guava, grape, and star fruit, [28], [29], [30]. The parasite is transmitted to plants by the bite of the coreid insect Phthia picta, as demonstrated by Jankevicius and collaborators using controlled cage experiments [31].
There is no information about how the protective immunity induced by P. serpens can modulate the biological activity of T. cruzi TS on thrombocytopenia and leukopenia in mice during acute T. cruzi infection. Here, we report that immunization with P. serpens prevented clearance of platelets and leukocytes from the circulation in T. cruzi-infected mice. Furthermore, antibodies raised by P. serpens immunization attenuated the thrombocytopenia induced by TS in vivo. Our results support the hypothesis that TS from T. cruzi is the causal factor of the hematological alterations observed early during infection and support the use of phytoflagellate trypanosomatids as a safer source of immunogenic agents for treatment and prevention of Chagas disease.
Materials and Methods
Ethics Statement
All animal procedures were performed in accordance with the guidelines of the Brazilian Code for the Use of Laboratory Animals: the protocols were approved by the Internal Scientific Commission and the Ethics in Animal Experimentation Committee of Londrina State University (Approval Number: CEEA-01.09).
Mice
Six- to 8-week-old C57BL/6 female and male mice were supplied by the Multi-Institutional Center for Biological Investigation, State University of Campinas, Brazil. Mice were maintained under standard conditions in the animal house of the Department of Pathological Sciences, Centre for Biological Sciences, State University of Londrina. Commercial rodent diet (Nuvilab-CR1, Nuvital, Campo Mourão, Brazil) and sterilized water were available ad libitum. Data analysis revealed no influence of sex on experimental outcomes.
Parasites
T. cruzi Y [32], a generous gift from Dr. Paulo Araújo, State University of Campinas, Brazil, was maintained by weekly intraperitoneal (i.p.) inoculation of Swiss mice with 2×105 trypomastigotes. To conduct our experiments, blood from previously inoculated Swiss mice was obtained by cardiac puncture with heparinized syringes.
P. serpens 15 T (see Figure S1) isolated from tomato fruit (Lycopersicum esculentum) [31] was cultured in GYPMI medium (glucose, yeast extract, peptone, and meat infusion) [24] at 28°C.
Immunization of Mice and Challenge with T. cruzi
For immunization of C57BL/6, living forms of P. serpens 15 T collected during log phase growth [31] were washed 3 times by centrifugation at 3000 g for 5 min in 15 mM PBS (phosphate-buffered saline, pH 7.2) and administered by gavage (per os). Each inoculum consisted of 2×108 living parasites/0.1 mL in 15 mM PBS, pH 7.2 given 4 times at 1-week intervals [24]. Seven days after the last oral immunization with P. serpens, C57BL/6 mice were infected i.p. with a non-lethal (102 or 5×103 cells/animal) or lethal (5×105 cells/animal) doses of trypomastigotes. Control mice received PBS alone.
Hematological Methods
Peripheral blood was collected from uninfected and infected mice by cardiac puncture under ether anesthesia and counted by standard methods [33]. Platelets were counted in peripheral blood collected in polypropylene tubes containing 3.8% (w/v) sodium citrate (citrate: blood ratio, 1∶9) [21]. All manipulations were carried out at room temperature. Platelets and leukocytes were counted manually with a Neubauer hemocytometer. All blood analysis and cell counts were performed 7, 12, or 21 days post-infection (p.i.).
Bone Marrow Cell Harvest
Bone marrow cells were harvested by flushing the femoral shafts with ice-cold PBS. The total number of megakaryocytes in cell suspensions from uninfected and infected mice (12 days p.i.) was determined by hemocytometer counting [12], [34].
Monoclonal Antibody Anti-TS (mAb 39)
mAb 39 was selected from hybridomas prepared by fusion of spleen mice immunized with membrane fraction of T. cruzi trypomastigotes and p3U1 cells [36]. Antigen was prepared by three cycles of freeze-thawing trypomastigotes in detergent-free buffer and supernatant collection after centrifugation at 100,000 g. Positive clones were screened by immunoblotting of total trypomastigote lysates in SDS-PAGE sample buffer. Positive clones were further cloned by limiting dilution and injected i.p. into mice primed 24 h before with incomplete Freund Adjuvant. Ascitic fluids were collected. Alternatively, antibodies were purified by affinity chromatography with protein A Sepharose (GE) following standard procedures.
Trans-sialidase (TS) Production and Purification
Recombinant T. cruzi TS lacking the carboxy-terminal repeats was purified from Escherichia coli BL21 DE3 pLysS. The construct was ligated into the NdeI and BamHI sites of pET 14b (Novagen) using a fragment derived from pTS16 as described by Schenkman and collaborators [35]. Expression was induced with 0.1 mM IPTG for 20 h at 28°C. Cells collected by centrifugation, washed in 20 mM Tris-HCl pH 8, resuspended in the same buffer containing 0.1 M NaCl and a cocktail of protease inhibitors (EDTA-free, Roche), and lysed by 3 passages in a French Press. The extract was clarified by centrifugation at 10,000 g (30 min) and separated on a Ni2+-agarose column (Qiagen). Unbound material was washed with 100 volumes of 0.1 M NaCl, 50 mM sodium phosphate, pH 8 and 10 mL of the same buffer, but at pH 6, and eluted from the column in the same buffer containing 0.25 M imidazole. The eluted material was dialyzed against 20 mM Tris-HCl pH 8 and separated on a MonoQ column. Fractions containing purified enzyme, as judged by SDS-PAGE, were eluted between 0.15 and 0.2 M NaCl and stored at 4°C until use. Native TS was purified from LLC-MK2 culture supernatants by affinity chromatography on mAb 39 immobilized on CNBr-Sepharose, as described previously [36].
Mouse Immune Sera
Ten C57BL/6 male mice were treated with 2×108 living culture of P. serpens (15 T strain) by gavage 4 times at 7-day intervals. One week after the last inoculum, sera from immunized mice were collected and used as hyperimmune sera against P. serpens. All sera were tested by direct agglutination for P. serpens as described previously [24].
TS Administration in Mice
Animals were injected i.p. with 50 µg enzyme in 0.1 mL PBS for each experiment. We used a dose that is five times higher as used in reference [21] because it was injected intraperitoneally. Control mice received PBS alone. In 2 separate experiments, mice received a single dose (0.1 mL) of immune sera anti-P. serpens mixed with purified TS (50 µg) for 15 min at room temperature. Serum samples obtained from naïve mice were used as controls. Twenty-four hours after injection, platelets and leukocytes were counted.
Immunoblotting and ELISA
Parasite protein lysates were analyzed by SDS-PAGE. Resolved proteins were electrophoretically transferred to nitrocellulose membranes (Hybond C, Amersham Biosciences, England) for western blotting. Membranes were blocked in 5% skim milk in PBS for 16 h at 4°C or 2 h at 22°C. After washing with PBS/0.2% Tween 20, membranes were incubated with diluted antibodies for 2 h at room temperature, then washed and incubated with peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibody (Sigma, 1∶10000) for 1 h. Detection was performed according to the manufacturer’s instructions. For ELISA, 96-well plates were coated by incubating 50 µL native TS at 10 µg/mL in 0.1 M sodium bicarbonate (pH 8.5) for 12 h at 4°C. The protein was removed and wells washed 3 times in PBS containing 0.05% Tween 20 and incubated in the same buffer with 2% BSA and 2.5% skim milk for 1 h at 25°C. Antibodies diluted in blocking solution were incubated 1 h at 25°C, washed 5 times with PBS, 0.05% Tween 20, and detected as described above using o-phenylenediamine and H2O2.
Statistical Analysis
Statistical analysis was conducted using ANOVA with the Bonferroni test. Comparisons between experimental groups were performed using Student’s t-test. Values are presented as mean ± SE. Differences were considered significant when p<0.05.
Results
T. cruzi-induced Transient Thrombocytopenia and Leukopenia
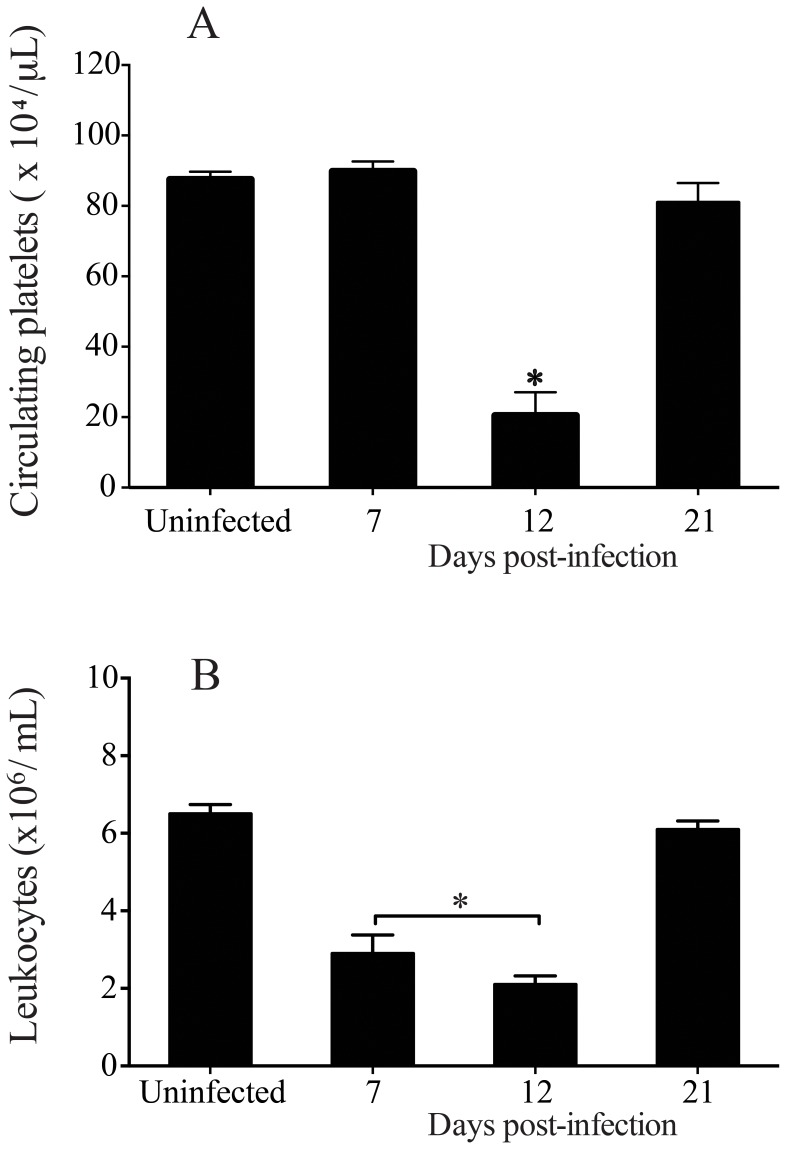
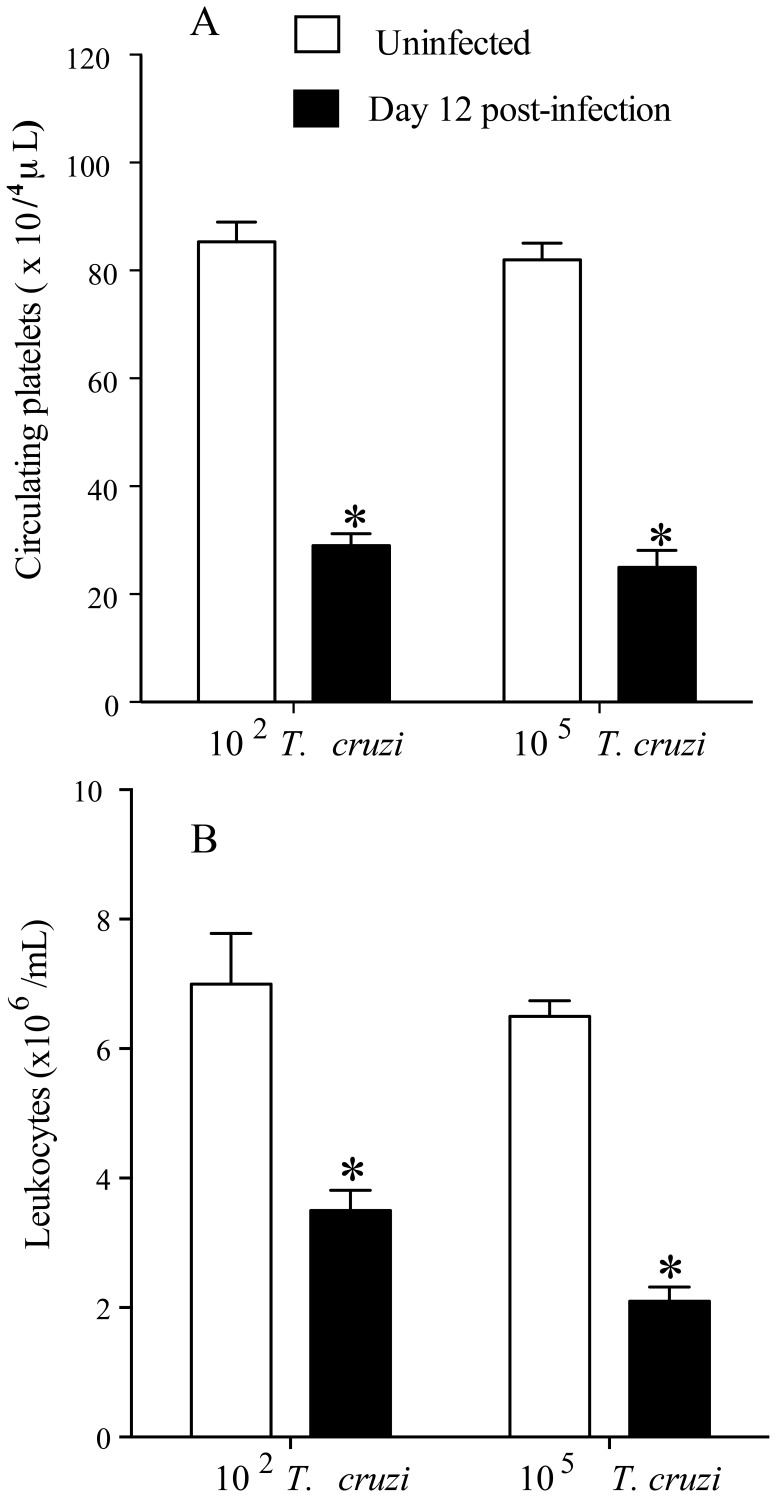
We initially confirmed that T. cruzi infection caused transient changes in platelets and leukocytes counts. As shown in figure 1, C57BL/6 mice infected with 5×103 blood T. cruzi trypomastigotes developed thrombocytopenia (Figure 1A) and leukopenia (Figure 1B) during the early stages of infection (day 12 p.i.), a transient effect that lasted as long as acute phase of T. cruzi infection remained. Platelet counts started to decrease after seven days of T. cruzi infection (day 8 p.i = 67.5±6.6×104/µL) and values returned to normal by day 35 p.i (103.3±11.7×104/µL) (data not shown). Interestingly, T. cruzi-infected mice developed thrombocytopenia (day 12 p.i) when using different inoculum of parasites (Figures 2 A and 2 B).
Figure 1. T. cruzi infection induces thrombocytopenia and leukopenia transients in the early of infection.
C5BL/6 mice were infected with 5×103 trypomastigotes (Y strain) via i.p. injection, monitored for the development of thrombocytopenia and leukopenia, and sacrificed at different time points post-T. cruzi infection. A: platelets and B: leukocytes were counts from peripheral blood from uninfected and infected mice. Values represent the mean ± standard error and are representative of three independent experiments, using 4–15 mice per group. Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. Asterisks indicates significant differences (p<0.05) when compared with control group (uninfected).
Figure 2. T. cruzi infection induces thrombocytopenia and leukopenia independent of the number of parasites used for infection.
Groups of C5BL/6 mice were infected with 102 or with 105 trypomastigotes (Y strain). A: platelets and B: leukocytes were counts from peripheral blood from uninfected and infected mice. All cell counts were performed 12 days p.i. Values represent the mean ± standard error and are representative of two independent experiments, using 6 mice per group. Comparisons between 2 experimental groups were performed using Student’s t-test. Asterisks indicate significant differences (p≤0.001) when compared with control group (uninfected).
Oral Immunization with P. serpens Prevented Reduction of Platelets and Leukocytes
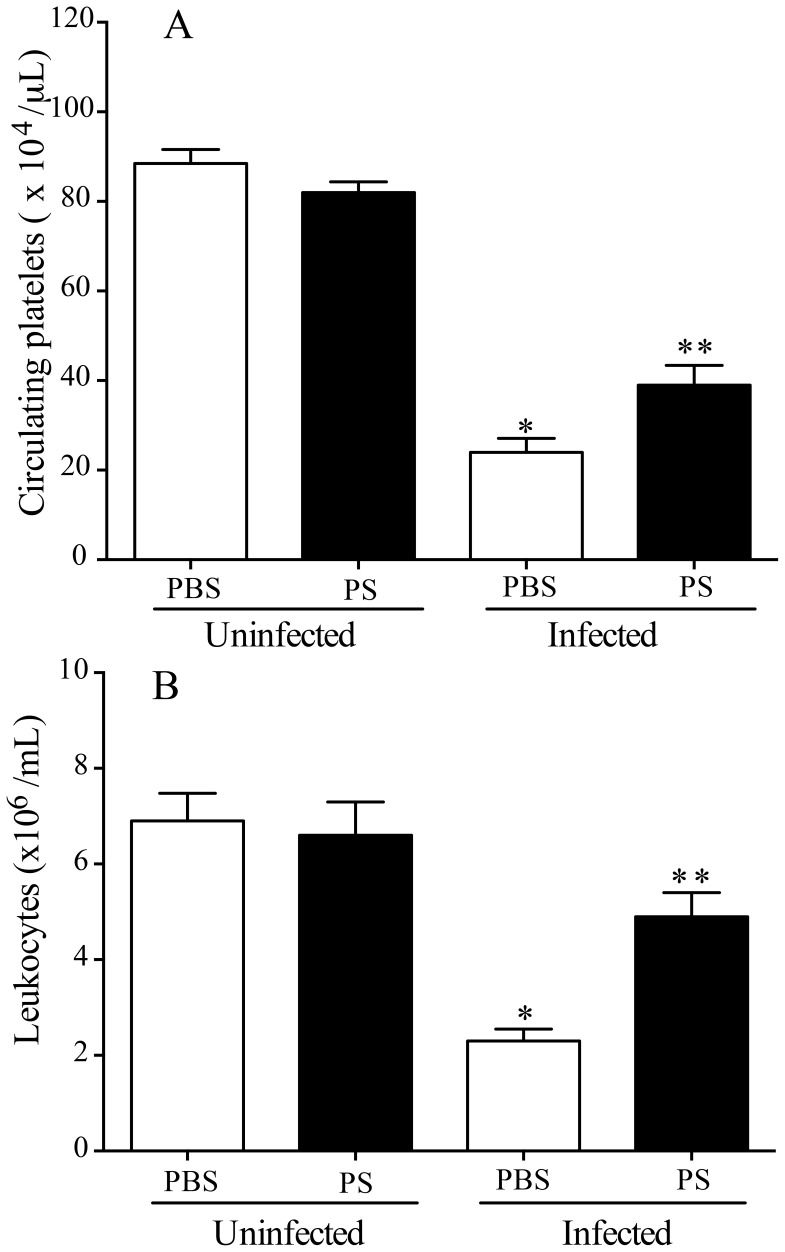
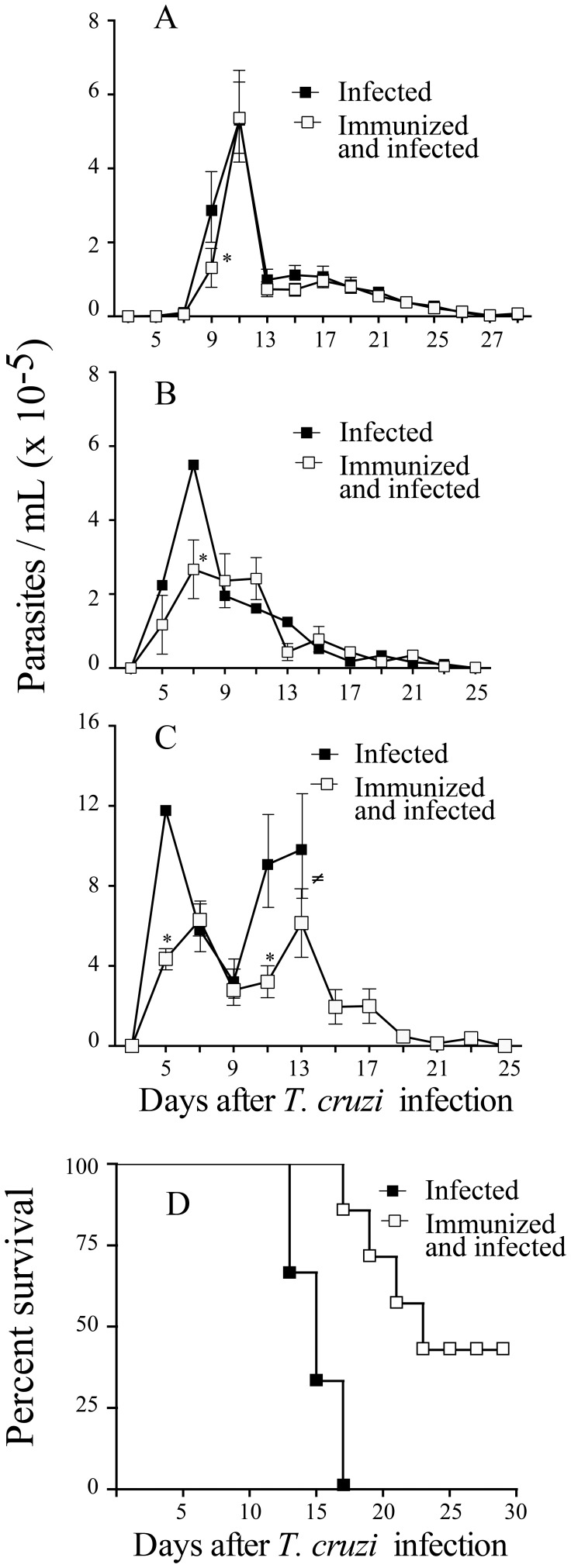
Next, we tested whether immunization with P. serpens prevent reduction in vivo of platelets and leukocytes in the host infected with T. cruzi. For this, C57BL/6 mice previously immunized were infected with trypomastigote forms of T. cruzi (105 cells/mouse, lethal dose). Twelve days after infection, immunized mice displayed a reduced decrease in platelets and leukocytes counts (Figures 3 A and 3 B, p = 0.001) when compared with control mice. Thus, platelets and leukocytes in infected mice appear to be sensitive to oral exposure to P. serpens. Moreover, the immunization reduced the parasitemia upon a T. cruzi challenge (Figure 4, p<0.05). This reduction occurs in the early of infection and protected mice to lethal dose of parasites (Figure 4C and Figure 4D, p<0.05).
Figure 3. Oral exposure to P. serpens attenuates thrombocytopenia and leukopenia induced by T. cruzi infection.
C57BL/6 mice received by gavage 2×108 living P. serpens parasites four times at weekly intervals and an i.p. challenge 1 week later with 105 blood trypomastigotes by i.p. route. Whole blood samples were collected on day 12 p.i. A: Platelets counts and B: Leukocytes counts. Values represent the mean ± standard error and are representative of three independent experiments, using 8–12 mice per group. Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. Asterisks indicate significant differences (p<0.001) between infected and uninfected controls. Double asterisks indicate significant differences (p<0.05) between infected mice given PBS (phosphate-buffered saline, pH 7.2) or immunized with P. serpens (PS) prior to infection with T. cruzi.
Figure 4. Oral exposure to P. serpens decreases parasitemia and mortality in response to T. cruzi infection.
C57BL/6 mice were immunized with P. serpens (2×108 living parasites per 0.1 mL PBS administered by gavage) four times with one-week intervals. Seven days after the last immunization mice were infected with Y strain of T. cruzi, A: 102, B: 5×103 and C: 105 trypomastigote forms, respectively. Parasitemia were assessed over 30 days post infection. D: Survival of immunized mice and infected with 105 (lethal dose). Data are represented as mean ± standard error represented of at last 10 mice per group. Asterisks indicates significant differences (p<0.05). (≠) indicates that all animals died.
TS Reduces Platelet Blood Counts
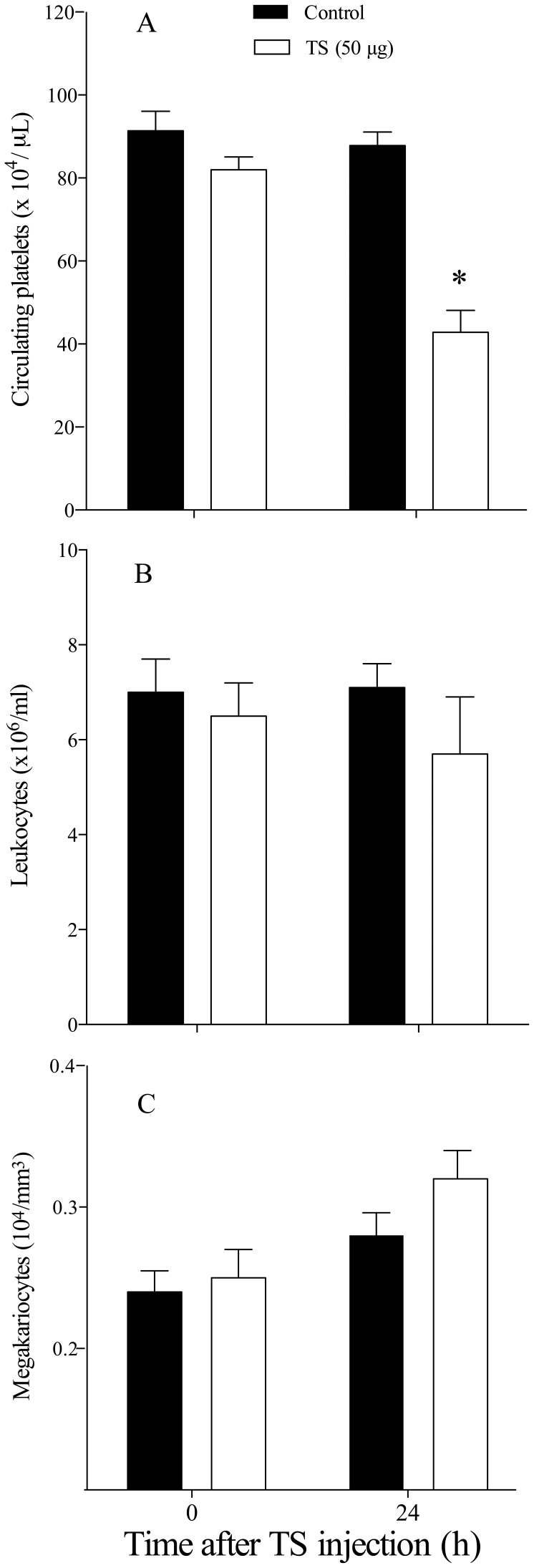
When TS (50 µg) was injected intraperitonially in mice (same route of T. cruzi infection), a strong reduction (around 50%) in the normal platelet count was observed 24 h after the enzyme injection (Figure 5 A, p<0.05). No effect was observed when TS was heat-inactivated (see Figure S2). We did not observe changes in the leukocyte (Figure 5B) and megakaryocytes (Figure 5C) counts of mice inoculated with TS.
Figure 5. TS reduces blood platelets counts in naïve C57BL/6 mice.
Mice were inoculated i.p with 50 µg of recombinant TS. A: platelet, B: leukocyte and C: megakariocyte counts were determined 24 h later. Values represent the mean ± standard error and are representative of two independent experiments; using 7–15 mice per group Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. Asterisks indicate significant differences (p<0.05).
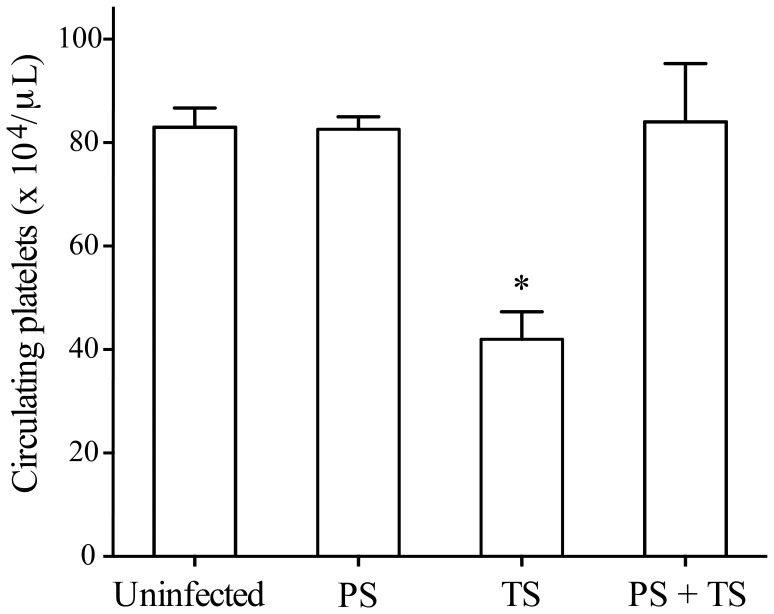
Oral Exposure to P. serpens Reduces Thrombocytopenia Induced by TS
To test whether oral immunization with P. serpens was able to reduce the effects of TS on platelets, the enzyme was directly injected in immunized animals. As shown in Figure 6, the immunization prevented the effects of TS on platelets in mice. Furthermore, the counts of megakaryocytes in the bone marrow were essentially the same in all groups (Figure S3, p>0.05).
Figure 6. Oral exposure to P. serpens restored the thrombocytopenia induced by TS.
C57BL/6 Mice received by gavage 2×108 living P. serpens parasites four times at weekly intervals and an i.p. challenge 1 week the mice were inoculated i.p with 50 µg of recombinant TS. Values represent the mean ± standard error and are representative of two independent experiments, using 7–10 mice per group. Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. PBS (phosphate-buffered saline, pH 7.2) and PS (immunized with P. serpens). Asterisks indicate significant differences (p≤0.001) when compared with control group (uninfected).
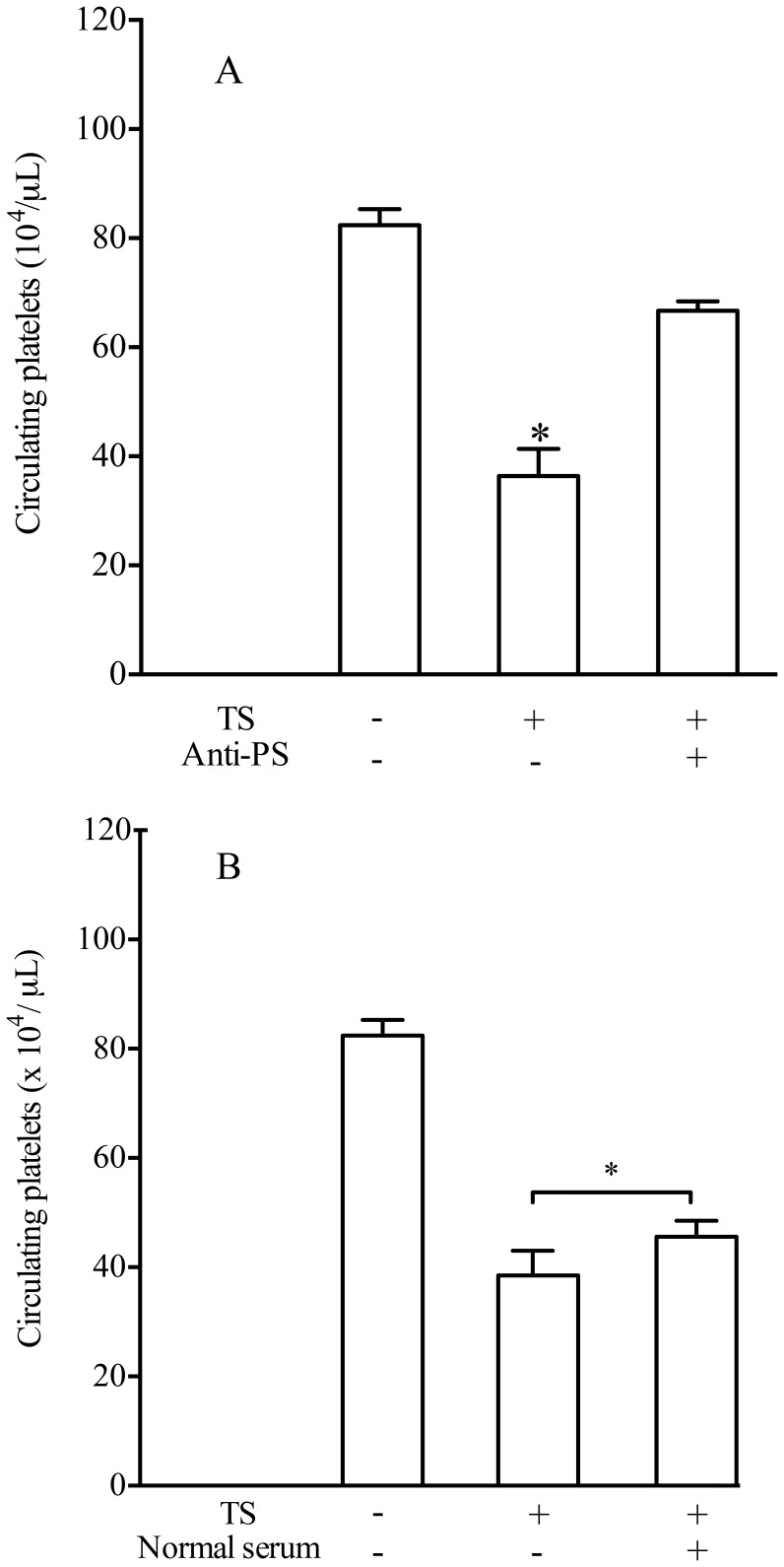
Hyperimmune Sera Against P. serpens Attenuates the Effects of TS on Platelet Counts
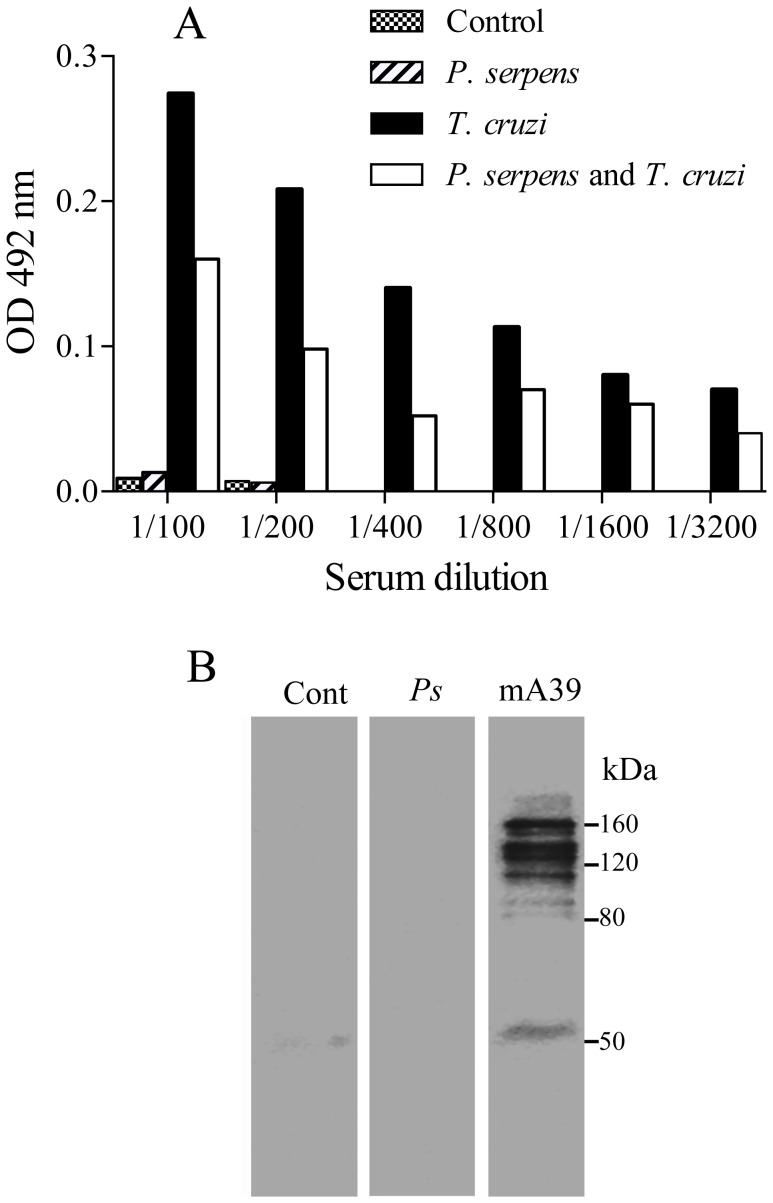
Incubation of sera from mice immunized with P. serpens with TS inhibited its thrombocytopenic effect on platelets (Figure 7 A, p<0.05). Moreover, serum samples obtained from naïve mice do not inhibit the action of TS in vivo (Figure 7 B, p>0.05). By means of immunoblotting analyses we showed that antibodies present in the serum of P. serpens-immunized mice recognized polypeptides in the cellular extract of P. serpens. However, antibodies from mice immunized with P. serpens did not recognize the purified native TS, which was recognized by the monoclonal antibody anti-TS (mAb 39) in Western blots (Figure 8). Indeed, no effect of anti-P. serpens sera in the enzymatic activity was found using sialyllactose as substrates (data not shown).
Figure 7. Sera of P. serpens-immunized mice inhibit the activity of TS on platelets.
The mice were injected i.p. with 50 µg of TS in 0.1 ml previously mixed with immune serum A or with normal serum B. A third group of mice received only PBS instead of TS. Platelet counts were determined 24 h later. Values represent the mean ± standard error and are representative of two independent experiments, using 4–7 mice per group. Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. Asterisks indicate significant differences (p<0.05) of samples compared with PBS.
Figure 8. Mice immunized with P. serpens lack antibodies to TS.
A. ELISA of plates coated with recombinant TS using control mice serum or sera immunized with P. serpens, followed by infection with T. cruzi. B. Immunoblotting showing the polypeptides recognized by hyperimmune sera anti-P. serpens detected in the whole cellular extract from P. serpens. Alternatively, the TS was also revealed using anti-TS antibody (mAb 39). Number on the left indicate the apparent molecular mass of protein standards expressed in kDa.
Discussion
During the experimental infection of mice with T. cruzi, several hematological abnormal parameters, including marked thrombocytopenia and leukopenia, are observed [11], [13], [16], [17], [37]. These alterations are transient [10] and can be prevented by trypanocidal drugs [12], but there is still no suitable molecular explanation for this effect. By using a model of infection with a T. cruzi Y strain in C57BL/6 and BALB/c mice, which are prototype hosts for the study of resistance and anemia in murine Chagas disease [34], we could obtain in C57BL/6 mice (resistant type) a more severe anemia compared to Swiss (susceptible mice) [37]. Ours results demonstrate for the first time, that the previous immunization with P. serpens, a tomato parasite, prevented the clearance of platelets and leukocytes from circulation in T. cruzi-infected mice. In addition, we found that a single i.p. injection (same route of infection with T. cruzi) of TS into mice reduced the platelet count by 50%, 24 h after TS injection. As described previously, TS has the ability to disseminate systemically within the host: the blood and bone marrow are the main sites where the enzyme may act to reduce the platelet count [21]. More important, the immunization with P. serpens reverted the effects of TS on platelets in mice. We did not observe changes in the leukocyte and megakaryocytes counts from mice inoculated with TS and previously immunized. These data could indicate that TS does not cause deleterious effects on the bone marrow and confirms the studies of Tribulatti and co-workers [21].
T. cruzi is unable to synthesize sialic acids de novo [38], but circumvents this limitation by expressing the enzyme TS, which is able to directly transfer α(2, 3)-linked sialyl residues among the glycoproteins or glycolipids [20]. TS is anchored to the membrane by glycosylphosphatidylinositol and is shed into the surrounding environment, and it is detected in the blood of infected animals and human patients during the acute stage of the infection [39]–[41]. In the C-terminus, the enzyme has tandem repetitive amino acid units that allow it to persist in blood for at least 3 days [42], allowing it to induce pathological disorders even far from the infectious foci or to act on the blood cells [39]. Previous reports indicated that TS from T. cruzi alters the platelet surface sialic acid content, acting as a neuraminidase [39], which induces accelerated clearance of the platelets leading to the thrombocytopenia observed during acute Chagas disease [21]. Also, immunization with culture forms of insect trypanosomatids has been shown to induce partial protection against lethal T. cruzi inoculations [43]. In fact, we previously demonstrated that BALB/c mice immunized with P. serpens and later challenged with a lethal inoculum of T. cruzi trypomastigote forms show a significant decrease in parasitemia and mortality [24]. As described previously, P. serpens is highly immunogenic in mice and rabbits [24] and the sera from patients with Chagas disease present a strong reactivity to P. serpens antigens [26], [44].
The results showing that hyperimmune sera against P. serpens attenuated the effect of TS on platelets led us to ask whether this inhibition was due to the presence of specific antibodies directed against the enzymatic domain of TS since P. serpens shares common antigens with T. cruzi [26], [45]. However, the serum from mice immunized with P. serpens do not recognize TS when tested by Western blot or ELISA. Moreover, the monoclonal antibody anti-TS (mAb 39) [36] do not recognize antigens in P. serpens. In addition, no effect of sera in the enzymatic activity was found using sialyllactose as substrates, while inhibit of platelet desialylation was previously observed. These results suggest that the effect of P. serpens immunization could be done through recognizing or changing the substrate in vivo. Alternatively, the inhibition could be consequence of changes in immune responses, as upon T. cruzi infection TS elicits the formation of antibodies that inhibit its activity (also called neutralizing and anti-catalytic domain), and non-inhibitory antibodies (anti-lectin-like, anti-SAPA, and anti-several epitopes on the proteins), also called cross-reacting determinants [20]. Because of the cross reactive determinants shared by TS with T. cruzi other molecules, v.g. SAPA, mainly situated on the non-catalytic portion of the TS, it is conceivable that these highly conserved sequences are present also in P. serpens. Indeed, sialidases are highly conserved in insect trypanosomatids and also in some bacteria [46]. Therefore, antibodies to P. serpens cross-reactive to the non-catalytic portion of TS could neutralize the injected TS, which would prevent its activity on the platelets. However, the frequency and of such antibodies in the polyclonal P. serpens immune serum could be low and prevent their direct detection. We confirmed the presence of anti-TS antibodies on day 12 after infection, which might contribute to decrease the thrombocytopenia. For example, mice infected with T. cruzi produce antibodies that are able to neutralize TS activity only if mice survive the acute phase of infection [40]. Accordingly, the sera from chronic Chagasic patients and rodents infected with T. cruzi can inhibit TS by recognizing its amino-terminal and catalytic domain [41]. Another possibility is that hyperimmune P. serpens sera affect TS clearance preventing platelet desialylation. Moreover, the reduction in the clearance of platelets from circulation in T. cruzi-infected mice and previously immunized with P. serpens, can be partly explained if we consider a wide distribution of the carbohydrate epitopes galactosyl α(1–3) galactose in P. serpens, as described by Gazzinelli and collaborators [47]. In fact, potent inhibitors of T. cruzi propagation in vitro and in vivo IN humans are antibodies directed against TS or the α-galactosyl residues of trypanosomal mucins [48]. Therefore, antibodies to carbohydrate epitopes present in the sera of animals immunized with P. serpens could promote a decrease of activity of TS on platelets.
In conclusion, our observations demonstrate an effect of P. serpens immunization of the action of TS on platelets during T. cruzi infection. Further elucidation of the mechanism by which the P. serpens affect TS can provide new tools to understand the progression of Chagas disease.
Supporting Information
Tomatoes ( Lycopersicum esculentum ) infected with Phytomonas serpens . A and B . (A) Tomatoes infected. (B) Living culture flagellates forms of P. serpens (original magnification 400 X). Arrows indicate local infection on the fruit.
(TIF)
Heat-inactivated TS does not modify the life span of platelets. C57BL/6 mice were inoculated i.p. with 50 µg of recombinant TS heat-inactivated. Platelets counts were determined 24 h later. Values represent the mean ± standard error and are representative of two independent experiments; using 4 mice per group Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. Asterisks indicate significant differences (p<0.05) when compared with control group (PBS).
(TIF)
Oral exposure to P. serpens does not induce alterations in megakariocyte counts. The mice received by gavage 2×108 living P. serpens parasites four times at weekly intervals and an i.p. 1 week later the mice were inoculated i.p. with 50 µg of recombinant TS. Megakariocyte counts were determined 24 h later. Values represent the mean ± standard error and are representative of two independent experiments, using 12 mice per group. Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. PBS (phosphate-buffered saline, pH 7.2) and PS (immunized with P. serpens).
(TIF)
Acknowledgments
The authors thank Adernal dos Santos, Ediel Clementino da Costa, Mr Jesus A. Vargas, Maria Isabel Lovo Martins and Irene Maria da Silva for excellent technical assistance.
Funding Statement
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Conselho Nacional de Desenvolvimento Científico (CNPq), and Fundação Araucária. Rosiane V. da Silva was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) fellowship. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McGhee RB, Cosgrove WB (1980) Biology and physiology of the lower Trypanosomatidae. Microbiological Reviews 44: 140–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vickerman K (1994) The evolutionary expansion of the trypanosomatid flagellates. International Journal for Parasitology 24: 1317–1331. [DOI] [PubMed] [Google Scholar]
- 3. Stuart K, Brun R, Croft S, Fairlamb A, Gurtler RE, et al. (2008) Kinetoplastids: related protozoan pathogens, different diseases. The Journal of Clinical Investigation 118: 1301–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moncayo A, Ortiz Yanine MI (2006) An update on Chagas disease (human American trypanosomiasis). Annals of Tropical Medicine and Parasitology 100: 663–677. [DOI] [PubMed] [Google Scholar]
- 5. Toso A, Vial F, Galanti N (2011) Oral transmission of Chagas’ disease. Revista Medica de Chile 139: 258–266. [PubMed] [Google Scholar]
- 6. Shikanai-Yasuda MA, Carvalho NB (2012) Oral Transmission of Chagas Disease. Clinical Infectious Diseases 54: 845–852. [DOI] [PubMed] [Google Scholar]
- 7. Py M (2011) Neurologic Manifestations of Chagas Disease. Current Neurology and Neuroscience Reports 11: 536–542. [DOI] [PubMed] [Google Scholar]
- 8. Rassi A Jr, Rassi A, Marin-Neto JA (2010) Chagas disease. Lancet 375: 1388–1402. [DOI] [PubMed] [Google Scholar]
- 9. Junqueira C, Caetano B, Bartholomeu DC, Melo MB, Ropert C, et al. (2010) The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Reviews in Molecular Medicine 12: e29. [DOI] [PubMed] [Google Scholar]
- 10. Cardoso JE, Brener Z (1980) Hematological changes in mice experimentally infected with Trypanosoma cruzi . Memorias do Instituto Oswaldo Cruz 75: 97–104. [DOI] [PubMed] [Google Scholar]
- 11. Repka D, Rangel HA, Atta AM, Gavino VA, Piedrabuena AE (1985) Experimental Chagas’ disease in mice infected with one LD50 of parasite. Revista Brasileira de Biologia 45: 309–316. [PubMed] [Google Scholar]
- 12. Marcondes MC, Borelli P, Yoshida N, Russo M (2000) Acute Trypanosoma cruzi infection is associated with anemia, thrombocytopenia, leukopenia, and bone marrow hypoplasia: reversal by nifurtimox treatment. Microbes and Infection 2: 347–352. [DOI] [PubMed] [Google Scholar]
- 13. Ikede BO, Lule M, Terry RJ (1977) Anaemia in trypanosomiasis: mechanisms of erythrocyte destruction in mice infected with Trypanosoma congolense or T. brucei . Acta Tropica 34: 53–60. [PubMed] [Google Scholar]
- 14. Claster S (2002) Biology of anemia, differential diagnosis, and treatment options in human immunodeficiency virus infection. The Journal of Infectious Diseases 185 Suppl 2S105–109. [DOI] [PubMed] [Google Scholar]
- 15. Paul RE, Brey PT (2003) Malaria parasites and red blood cells: from anaemia to transmission. Molecules and Cells 15: 139–149. [PubMed] [Google Scholar]
- 16. Malvezi AD, Cecchini R, de Souza F, Tadokoro CE, Rizzo LV, et al. (2004) Involvement of nitric oxide (NO) and TNF-alpha in the oxidative stress associated with anemia in experimental Trypanosoma cruzi infection. FEMS Immunology and Medical Microbiology 41: 69–77. [DOI] [PubMed] [Google Scholar]
- 17. Santiago HC, Feng CG, Bafica A, Roffe E, Arantes RM, et al. (2005) Mice deficient in LRG-47 display enhanced susceptibility to Trypanosoma cruzi infection associated with defective hemopoiesis and intracellular control of parasite growth. Journal of Immunology 175: 8165–8172. [DOI] [PubMed] [Google Scholar]
- 18. Giorgi ME, Ratier L, Agusti R, Frasch AC, de Lederkremer RM (2010) Synthesis of PEGylated lactose analogs for inhibition studies on T. cruzi trans-sialidase. Glycoconjugate Journal 27: 549–559. [DOI] [PubMed] [Google Scholar]
- 19. Schauer R, Kamerling JP (2011) The Chemistry and Biology of Trypanosomal trans-Sialidases: Virulence Factors in Chagas Disease and Sleeping Sickness. Chembiochem: European Journal of Chemical Biology 2246: 2246–2764. [DOI] [PubMed] [Google Scholar]
- 20. Dc-Rubin SS, Schenkman S (2012) Trypanosoma cruzi trans-sialidase as a multifunctional enzyme in Chagas’ disease. Cellular Microbiology 14: 1522–1530. [DOI] [PubMed] [Google Scholar]
- 21. Tribulatti MV, Mucci J, Van Rooijen N, Leguizamon MS, Campetella O (2005) The trans-sialidase from Trypanosoma cruzi induces thrombocytopenia during acute Chagas’ disease by reducing the platelet sialic acid contents. Infection and Immunity 73: 201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goncalves CC, Reiche EM, De Abreu Filho BA, Silveira TG, Felizardo TC, et al. (2002) Evaluation of antigens from various Leishmania species in a Western blot for diagnosis of American tegumentary leishmaniasis. The American Journal of Tropical Medicine and Hygiene 66: 91–102. [DOI] [PubMed] [Google Scholar]
- 23. Lopes JD, Caulada Z, Barbieri CL, Camargo EP (1981) Cross-reactivity between Trypanosoma cruzi and insect trypanosomatids as a basis for the diagnosis of Chagas’ disease. The American Journal of Tropical Medicine and Hygiene 30: 1183–1188. [DOI] [PubMed] [Google Scholar]
- 24. Bregano JW, Picao RC, Graca VK, Menolli RA, Itow Jankevicius S, et al. (2003) Phytomonas serpens, a tomato parasite, shares antigens with Trypanosoma cruzi that are recognized by human sera and induce protective immunity in mice. FEMS Immunology and Medical Microbiology 39: 257–264. [DOI] [PubMed] [Google Scholar]
- 25. Medina-Acosta E, Franco AMR, Jansen AM, Sampol M, Nev‚s N, et al. (1994) Trans-sialidase and sialidase activities discriminate between morphologically indistinguishable trypanosomatids. European Journal of Biochemistry 225: 333–339. [DOI] [PubMed] [Google Scholar]
- 26. Graca-de Souza VK, Monteiro-Goes V, Manque P, Souza TA, Correa PR, et al. (2010) Sera of chagasic patients react with antigens from the tomato parasite Phytomonas serpens . Biological Research 43: 233–241. [PubMed] [Google Scholar]
- 27. Pinge-Filho P, Peron JP, de Moura TR, Menolli RA, Graca VK, et al. (2005) Protective immunity against Trypanosoma cruzi provided by oral immunization with Phytomonas serpens: role of nitric oxide. Immunology Letters 96: 283–290. [DOI] [PubMed] [Google Scholar]
- 28. Dollet M (1984) Plant-Diseases Caused by Flagellate Protozoa (Phytomonas). Annual Review of Phytopathology 22: 115–132. [Google Scholar]
- 29. Camargo EP, Kastelein P, Roitman I (1990) Trypanosomatid Parasites of Plants (Phytomonas). Parasitology Today 6: 22–25. [DOI] [PubMed] [Google Scholar]
- 30. Camargo EP (1999) Phytomonas and other trypanosomatid parasites of plants and fruit. Advances in Parasitology 42: 29–112. [DOI] [PubMed] [Google Scholar]
- 31. Jankevicius JV, Jankevicius SI, Campaner M, Conchon I, Maeda LA, et al. (1989) Life-Cycle and Culturing of Phytomonas-serpens (Gibbs), a Trypanosomatid Parasite of Tomatoes. Journal of Protozoology 36: 265–271. [Google Scholar]
- 32. Silva LHP, Nussenzweig V (1953) Sobre uma cepa de Trypanosoma cruzi altamente virulenta para o camundongo branco. Folia Clinica Biologica 20: 191–203. [Google Scholar]
- 33.Dacie JV, Lewis SM (1995) Practical haematology: Churchill Livingstone. [Google Scholar]
- 34. Hideko Tatakihara VL, Cecchini R, Borges CL, Malvezi AD, Graca-de Souza VK, et al. (2008) Effects of cyclooxygenase inhibitors on parasite burden, anemia and oxidative stress in murine Trypanosoma cruzi infection. FEMS Immunology and Medical Microbiology 52: 47–58. [DOI] [PubMed] [Google Scholar]
- 35. Schenkman S, Chaves LB, Pontes de Carvalho L, Eichinger D (1994) A proteolytic fragment of Trypanosoma cruzi trans-sialidase lacking the carboxy-terminal domain is active, monomeric and generates antibodies that inhibit enzymatic activity. Journal of Biological Chemistry 269: 7970–7975. [PubMed] [Google Scholar]
- 36. Schenkman S, Pontes de Carvalho L, Nussenzweig V (1992) Trypanosoma cruzi trans-sialidase and neuraminidase activities can be mediated by the same enzymes. The Journal of Experimental Medicine 175: 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Estevam M, Appoloni CR, Malvezi AD, Tatakihara VL, Panis C, et al. (2012) Trypanosoma cruzi: in vivo evaluation of iron in skin employing X-ray fluorescence (XRF) in mouse strains that differ in their susceptibility to infection. FEMS Immunology and Medical Microbiology 64: 334–342. [DOI] [PubMed] [Google Scholar]
- 38. Frasch AC (2000) Functional diversity in the trans-sialidase and mucin families in Trypanosoma cruzi . Parasitology Today 16: 282–286. [DOI] [PubMed] [Google Scholar]
- 39. de Titto EH, Araujo FG (1988) Serum neuraminidase activity and hematological alterations in acute human Chagas’ diseases. Clinical Immunology and Immunopathology 46: 157–161. [DOI] [PubMed] [Google Scholar]
- 40. Leguizamon MS, Campetella O, Russomando G, Almiron M, Guillen I, et al. (1994) Antibodies inhibiting Trypanosoma cruzi trans-sialidase activity in sera from human infections. Journal of Infectious Diseases 170: 1570–1574. [DOI] [PubMed] [Google Scholar]
- 41. Pereira-Chioccola VL, Schenkman S, Kloetzel J (1994) Sera from chronic Chagasic patients and animals infected with Trypanosoma cruzi inhibit trans-sialidase by recognizing its catalytic domain. Infection and Immunity 62: 2973–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buscaglia CA, Alfonso J, Campetella O, Frasch AC (1999) Tandem amino acid repeats rrom Trypanosoma cruzi shed antigens increase the half-life of proteins in blood. Blood 93: 2025–2032. [PubMed] [Google Scholar]
- 43. Souza Mdo C, Reis AP, Da Silva WD, Brener Z (1974) Mechanism of acquired immunity induced by “Leptomonas pessoai” against Trypanosoma cruzi in mice. The Journal of Protozoology 21: 579–584. [DOI] [PubMed] [Google Scholar]
- 44. Csete M, Lev BI, Pereira ME (1985) An influenza virus model for Trypanosoma cruzi infection: interactive roles for neuraminidase and lectin. Current Topics in Microbiology and Immunology 117: 153–165. [DOI] [PubMed] [Google Scholar]
- 45. de Souza Tde A, Graca-de Souza VK, Lancheros CA, Monteiro-Goes V, Krieger MA, et al. (2011) Identification, molecular and functional characterization of calmodulin gene of Phytomonas serpens 15 T that shares high similarity with its pathogenic counterparts Trypanosoma cruzi . The Protein Journal 30: 212–219. [DOI] [PubMed] [Google Scholar]
- 46. Briones MR, Egima CM, Eichinger D, Schenkan S (1995) Trans-sialidase genes expressed in mammalian forms of Trypanosoma cruzi evolved from ancestor genes expressed in insect forms of the parasite. Journal of Molecular Evolution 41: 120–131. [DOI] [PubMed] [Google Scholar]
- 47. Gazzinelli RT, Romanha AJ, Fontes G, Chiari E, Gazzinelli G, et al. (1991) Distribution of carbohydrates recognized by the lectins Euonymus europaeus and concanavalin A in monoxenic and heteroxenic trypanosomatids. The Journal of Protozoology 38: 320–325. [DOI] [PubMed] [Google Scholar]
- 48. Marques AF, Nohara L, Ganiko L, Silva LS, Travassos LR, et al. (2010) Alpha-Gal-Based Vaccine Provides Full Protection Against Trypanosoma cruzi Infection in Mice. Glycobiology 20: 1528–1528. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tomatoes ( Lycopersicum esculentum ) infected with Phytomonas serpens . A and B . (A) Tomatoes infected. (B) Living culture flagellates forms of P. serpens (original magnification 400 X). Arrows indicate local infection on the fruit.
(TIF)
Heat-inactivated TS does not modify the life span of platelets. C57BL/6 mice were inoculated i.p. with 50 µg of recombinant TS heat-inactivated. Platelets counts were determined 24 h later. Values represent the mean ± standard error and are representative of two independent experiments; using 4 mice per group Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. Asterisks indicate significant differences (p<0.05) when compared with control group (PBS).
(TIF)
Oral exposure to P. serpens does not induce alterations in megakariocyte counts. The mice received by gavage 2×108 living P. serpens parasites four times at weekly intervals and an i.p. 1 week later the mice were inoculated i.p. with 50 µg of recombinant TS. Megakariocyte counts were determined 24 h later. Values represent the mean ± standard error and are representative of two independent experiments, using 12 mice per group. Results were analyzed by analysis of variance (ANOVA) followed by Bonferroni multiple comparisons test. PBS (phosphate-buffered saline, pH 7.2) and PS (immunized with P. serpens).
(TIF)