Abstract
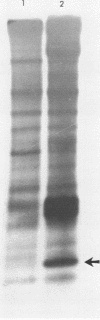
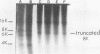
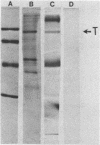
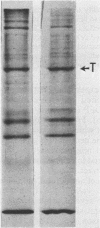
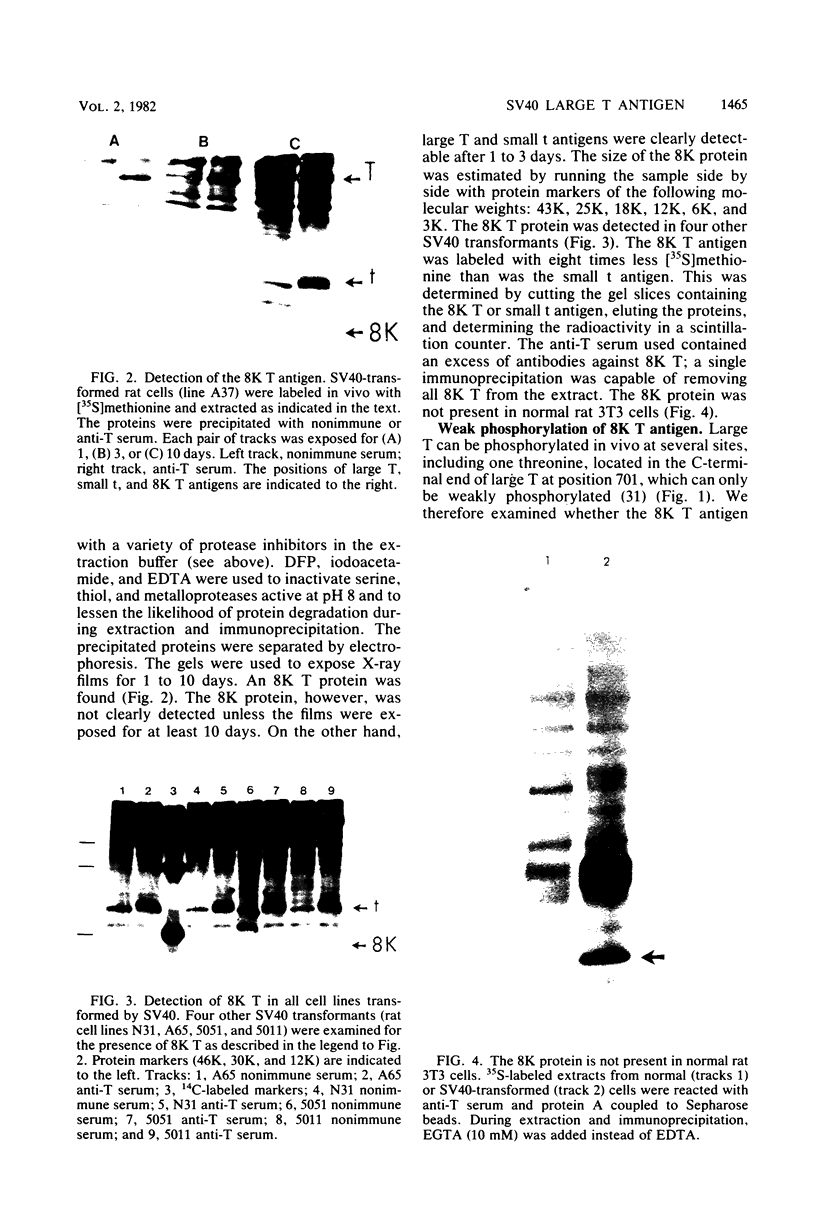
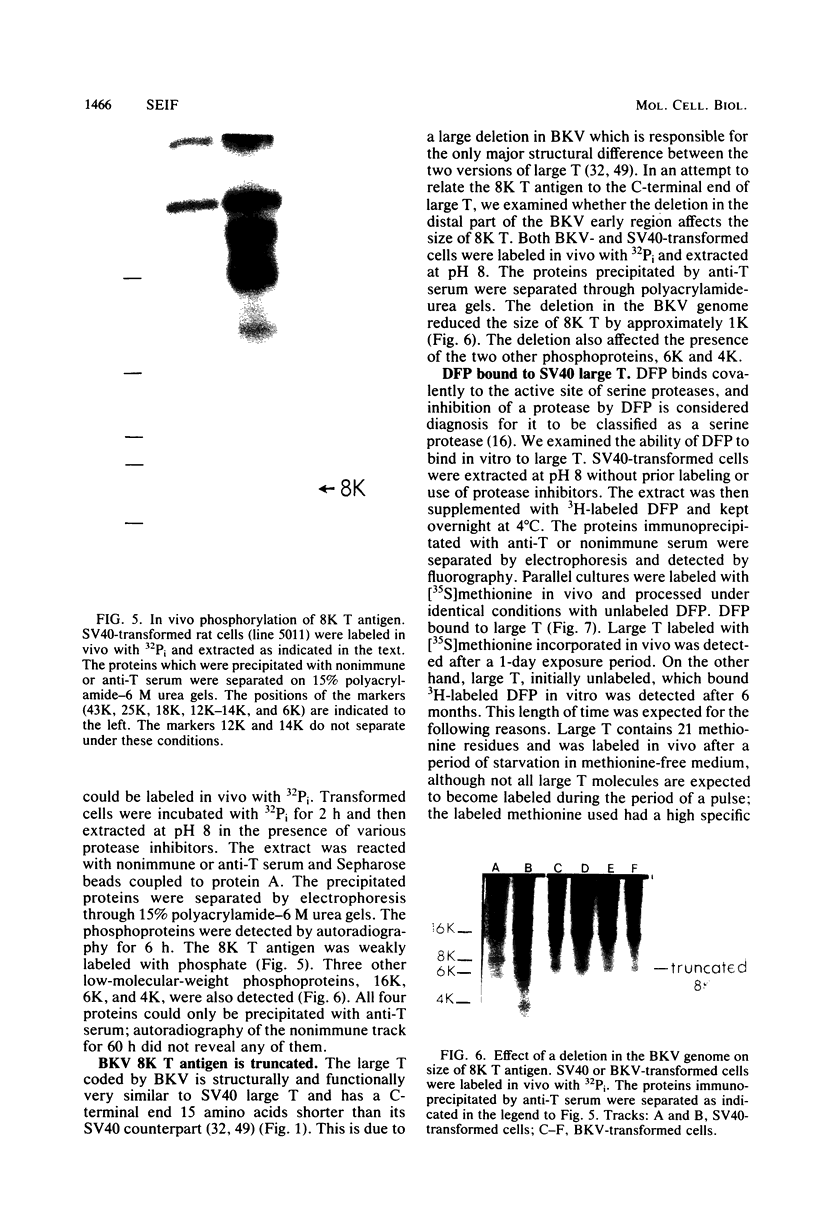
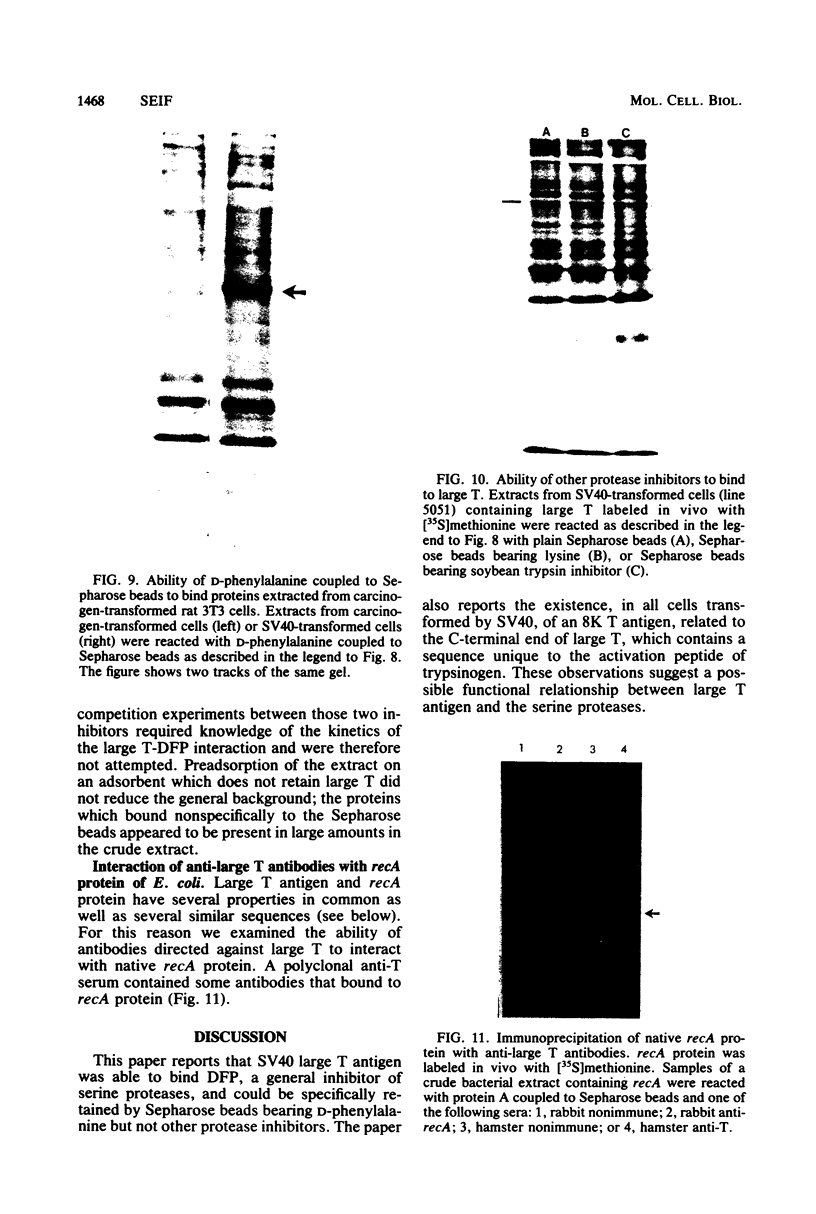
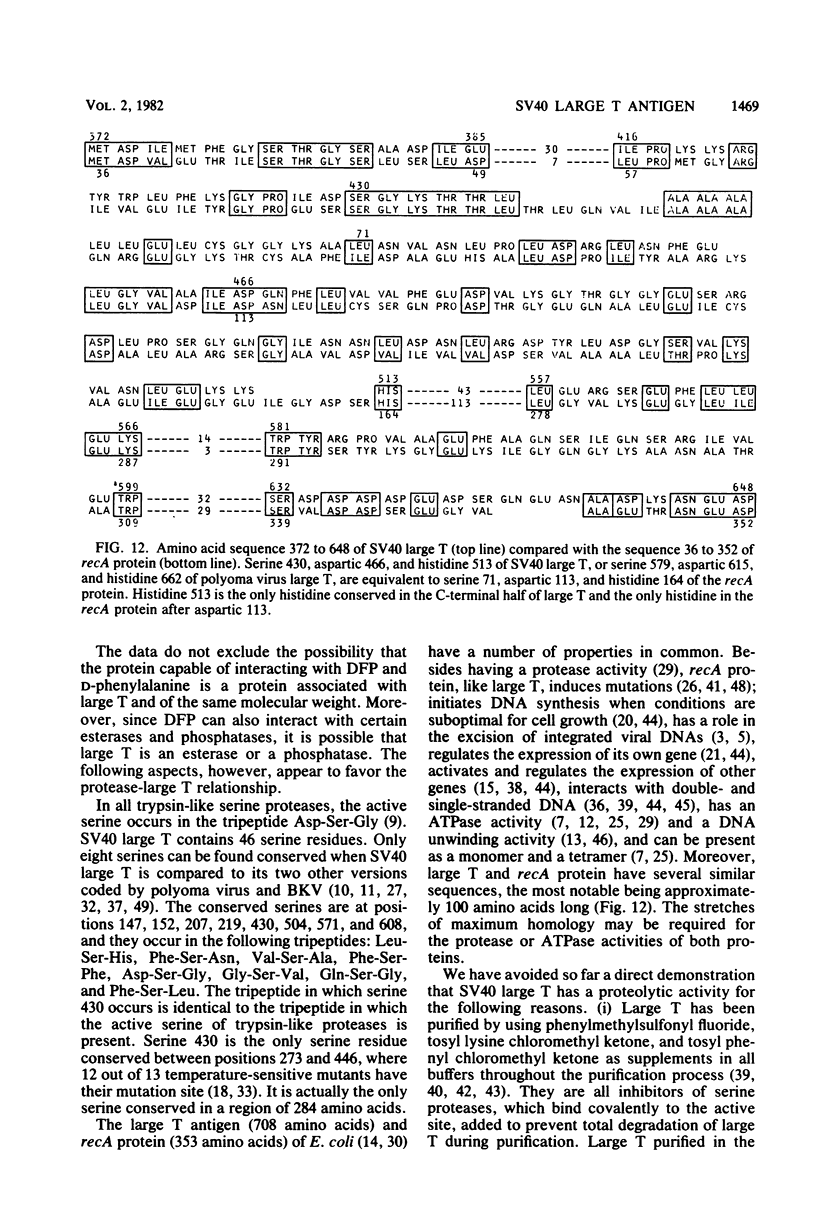
An 8,000-molecular-weight (8K) T antigen was found in all cells transformed by simian virus 40. The 8K T antigen was weakly labeled in vivo with [35S]methionine or 32Pi. A deletion in the human papovavirus BK genome, in the region coding for the carboxy-terminal end of the large T antigen, reduced the size of the 8K T antigen. The last 80 amino acids of the large T antigen include the sequence Asp-Asp-Asp-Asp unique to the activation peptide of trypsinogen. Large T antigen bound diisopropyl fluorophosphate and was retained by d-phenylalanine coupled to Sepharose beads, an affinity adsorbent that can retain chymotrypsin. The large T antigen and the recA protein of Escherichia coli, a known protease, have several properties in common as well as several similar sequences. Antibodies against large T antigen interacted with native recA protein.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abita J. P., Delaage M., Lazdunski M. The mechanism of activation of trypsinogen. The role of the four N-terminal aspartyl residues. Eur J Biochem. 1969 Apr;8(3):314–324. doi: 10.1111/j.1432-1033.1969.tb00530.x. [DOI] [PubMed] [Google Scholar]
- Alwine J. C., Khoury G. Simian virus 40-associated small RNA: mapping on the simian virus 40 genome and characterization of its synthesis. J Virol. 1980 Dec;36(3):701–708. doi: 10.1128/jvi.36.3.701-708.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. Studies on simian virus 40 excision from cellular chromosomes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):709–719. doi: 10.1101/sqb.1979.043.01.079. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Muello K., Melero J. A. Coordinate expression of the 48K host nuclear phosphoprotein and SV40 T ag upon primary infection of mouse cells. Virology. 1980 Apr 30;102(2):447–452. doi: 10.1016/0042-6822(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Clark A. J. Recombination deficient mutants of E. coli and other bacteria. Annu Rev Genet. 1973;7:67–86. doi: 10.1146/annurev.ge.07.120173.000435. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Crawford L. V., Berg P. Simian virus 40 mutants with deletions at the 3' end of the early region are defective in adenovirus helper function. J Virol. 1979 Jun;30(3):683–691. doi: 10.1128/jvi.30.3.683-691.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIE E. W., NEURATH H. Identification of a peptide released during autocatalytic activation of trypsinogen. J Biol Chem. 1955 Feb;212(2):515–529. [PubMed] [Google Scholar]
- Fiers W., Contreras R., Haegemann G., Rogiers R., Van de Voorde A., Van Heuverswyn H., Van Herreweghe J., Volckaert G., Ysebaert M. Complete nucleotide sequence of SV40 DNA. Nature. 1978 May 11;273(5658):113–120. doi: 10.1038/273113a0. [DOI] [PubMed] [Google Scholar]
- Friedmann T., Esty A., LaPorte P., Deininger P. The nucleotide sequence and genome organization of the polyoma early region: extensive nucleotide and amino acid homology with SV40. Cell. 1979 Jul;17(3):715–724. doi: 10.1016/0092-8674(79)90278-2. [DOI] [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A poly(dT)-stimulated ATPase activity associated with simian virus 40 large T antigen. J Biol Chem. 1979 Sep 10;254(17):8113–8116. [PubMed] [Google Scholar]
- Giacherio D., Hager L. P. A specific DNA unwinding activity associated with SV40 large T antigen. J Biol Chem. 1980 Oct 10;255(19):8963–8966. [PubMed] [Google Scholar]
- Horii T., Ogawa T., Ogawa H. Organization of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):313–317. doi: 10.1073/pnas.77.1.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. A. recA-dependent DNA replication in the absence of protein synthesis: characteristics of a dominant lethal replication mutation, dnaT, and requirement for recA+ function. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):537–549. doi: 10.1101/sqb.1979.043.01.059. [DOI] [PubMed] [Google Scholar]
- Little J. W., Edmiston S. H., Pacelli L. Z., Mount D. W. Cleavage of the Escherichia coli lexA protein by the recA protease. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3225–3229. doi: 10.1073/pnas.77.6.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May E., Kress M., Daya-Grosjean L., Monier R., May P. Mapping of the viral mRNA encoding a super-T antigen of 115,000 daltons expressed in simian virus 40-transformed rat cell lines. J Virol. 1981 Jan;37(1):24–35. doi: 10.1128/jvi.37.1.24-35.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P. L., Fanica M., Devoret R. Cleavage of lambda repressor and induction of recA protein synthesis elicited by aflatoxin B1 metabolites in Escherichia coli. Carcinogenesis. 1980;1(10):837–848. doi: 10.1093/carcin/1.10.837. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A. Role of proteolytic enzymes in biological regulation (a review). Proc Natl Acad Sci U S A. 1976 Nov;73(11):3825–3832. doi: 10.1073/pnas.73.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Wabiko H., Tsurimoto T., Horii T., Masukata H., Ogawa H. Characteristics of purified recA protein and the regulation of its synthesis in vivo. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):909–915. doi: 10.1101/sqb.1979.043.01.099. [DOI] [PubMed] [Google Scholar]
- Reddy V. B., Ghosh P. K., Lebowitz P., Piatak M., Weissman S. M. Simian virus 40 early mRNA's. I. Genomic localization of 3' and 5' termini and two major splices in mRNA from transformed and lytically infected cells. J Virol. 1979 Apr;30(1):279–296. doi: 10.1128/jvi.30.1.279-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy V. B., Thimmappaya B., Dhar R., Subramanian K. N., Zain B. S., Pan J., Ghosh P. K., Celma M. L., Weissman S. M. The genome of simian virus 40. Science. 1978 May 5;200(4341):494–502. doi: 10.1126/science.205947. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L., Phizicky E. M. Activity of the Escherichia coli recA-gene product. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):917–920. doi: 10.1101/sqb.1979.043.01.100. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidtmann K. H., Kaiser A., Carbone A., Walter G. Phosphorylation of threonine in the proline-rich carboxy-terminal region of simian virus 40 large T antigen. J Virol. 1981 Apr;38(1):59–69. doi: 10.1128/jvi.38.1.59-69.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. A rapid enzymatic DNA sequencing technique: determination of sequence alterations in early simian virus 40 temperature sensitive and deletion mutants. Nucleic Acids Res. 1980 May 24;8(10):2225–2240. doi: 10.1093/nar/8.10.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Seif R., Martin R. G. Growth state of the cell early after infection with simian virus 40 determines whether the maintenance of transformation will be A-gene dependent or independent. J Virol. 1979 Aug;31(2):350–359. doi: 10.1128/jvi.31.2.350-359.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shibata T., DasGupta C., Cunningham R. P., Radding C. M. Purified Escherichia coli recA protein catalyzes homologous pairing of superhelical DNA and single-stranded fragments. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1638–1642. doi: 10.1073/pnas.76.4.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda E., Arrand J. R., Griffin B. E. Polyoma virus. The early region and its T-antigens. Nucleic Acids Res. 1979 Oct 25;7(4):839–857. doi: 10.1093/nar/7.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soprano K. J., Rossini M., Croce C., Baserga R. The role of large T antigen in simian virus 40-induced reactivation of silent rRNA genes in human-mouse hybrid cells. Virology. 1980 Apr 30;102(2):317–326. doi: 10.1016/0042-6822(80)90099-9. [DOI] [PubMed] [Google Scholar]
- Spillman T., Giacherio D., Hager L. P. Single strand DNA binding of simian virus 40 tumor antigen. J Biol Chem. 1979 Apr 25;254(8):3100–3104. [PubMed] [Google Scholar]
- Tenen D. G., Garewal H., Haines L. L., Hudson J., Woodard V., Light S., Livingston D. M. Purification of simian virus 40 tumor antigen from a line of simian virus 40-transformed human cells. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3745–3749. doi: 10.1073/pnas.74.9.3745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R., Fey G., Graessmann A. Biological activity of purified simian virus 40 T antigen proteins. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1279–1283. doi: 10.1073/pnas.75.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Weinstock G. M., McEntee K., Lehman I. R. ATP-dependent renaturation of DNA catalyzed by the recA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):126–130. doi: 10.1073/pnas.76.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West S. C., Cassuto E., Mursalim J., Howard-Flanders P. Recognition of duplex DNA containing single-stranded regions by recA protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2569–2573. doi: 10.1073/pnas.77.5.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]