Abstract
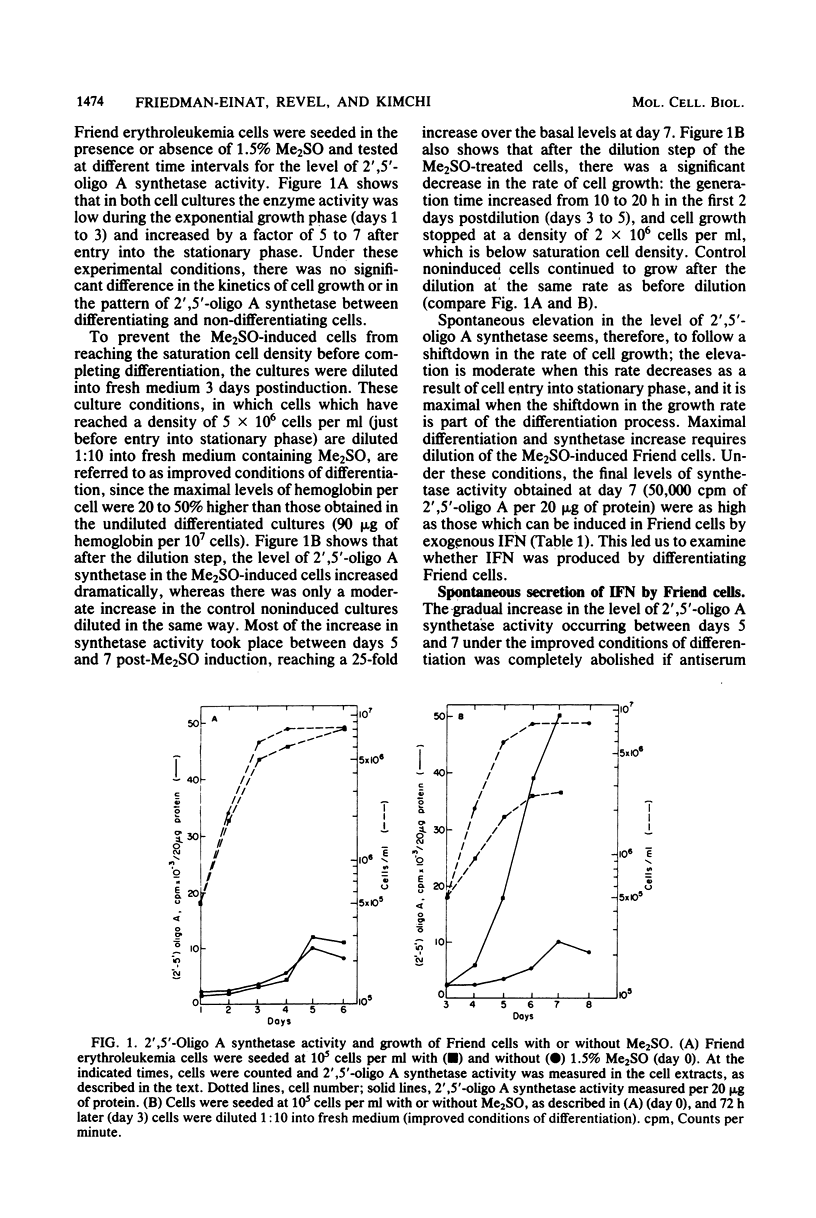
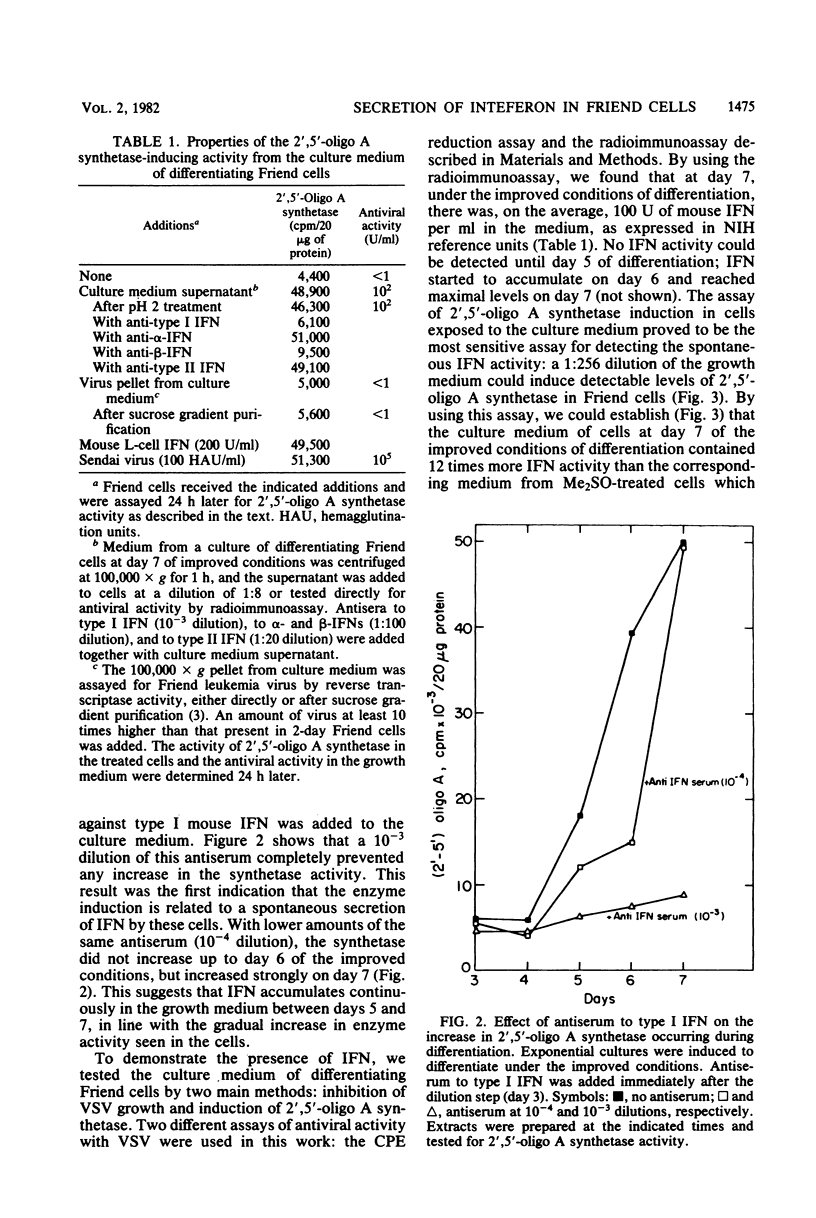
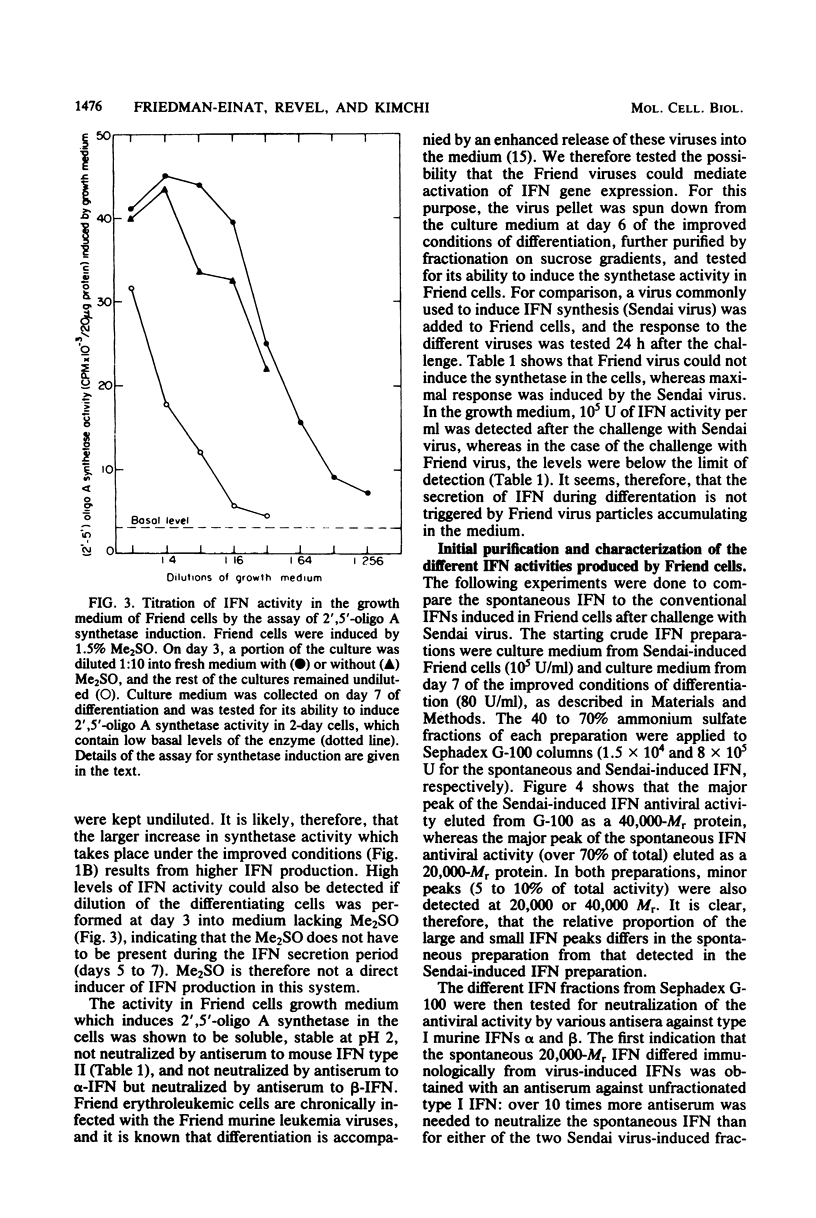
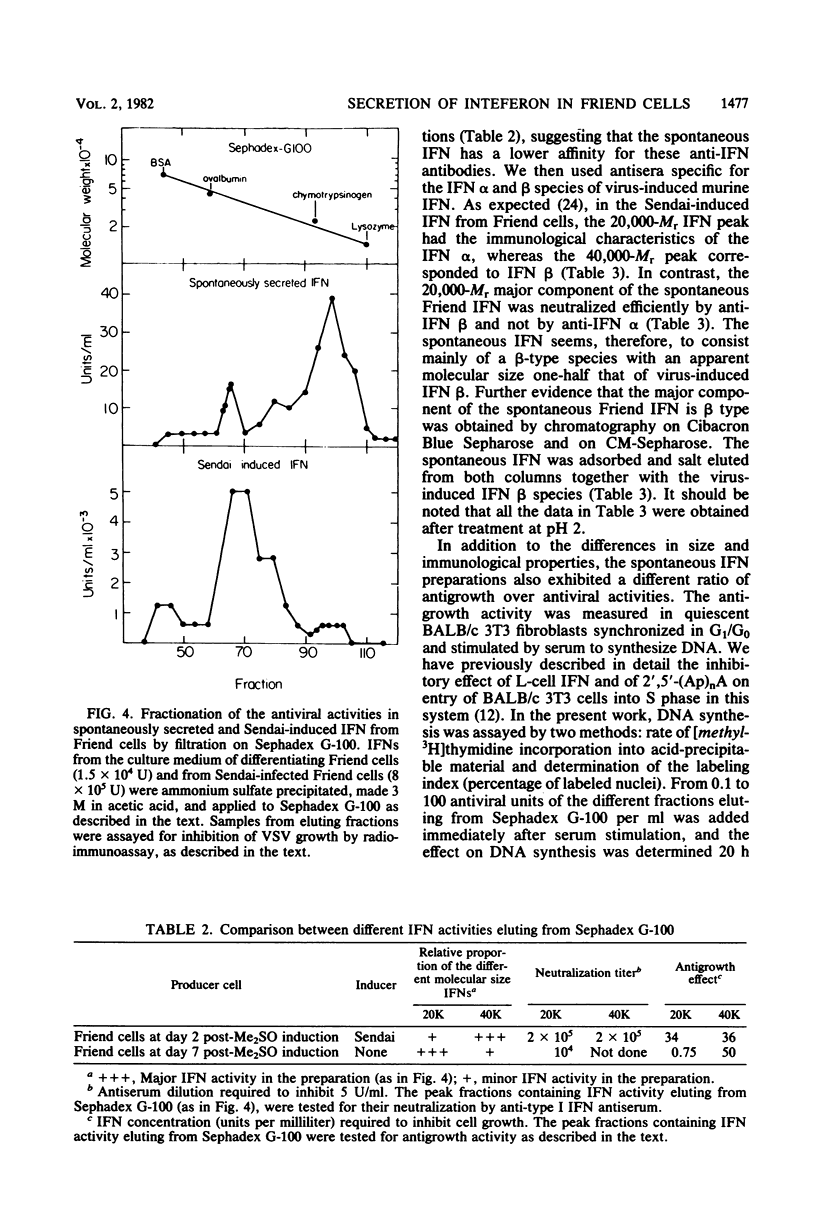
A gradual increase in the level of 2′,5′-oligoadenylate synthetase takes place in Friend erythroleukemia cells after a shiftdown in the rate of cell growth. The increase is about 5-fold after entry of cells into the stationary phase of growth, but much higher (25-fold) when reduction in growth accompanies cell differentiation. In the latter case, the enzyme increase is similar to that which can be induced in these cells by exogenous interferon (IFN). The increase in 2′,5′-oligoadenylate synthetase was shown to be due to a spontaneous secretion of IFN by the cells themselves: it is completely abolished if antiserum to murine type I IFN is added to the culture medium. In attempts to isolate some of this spontaneously secreted IFN, we show that it is stable at pH 2, not neutralized by antiserum to type II IFN, and that it also differs from the known IFN species induced by Sendai virus in Friend cells. The major component of this spontaneously secreted IFN is 20,000 Mr and differs from the corresponding virus-induced 20,000-Mr IFN by its lower affinity for antiserum to type I IFN and its antigenic characterization as β-murine IFN. The major component of the spontaneous IFN also exhibits a higher ratio of antigrowth to antiviral activity than the Sendai-induced IFNs. We suggest that Friend cells produce this specific type of IFN for the regulation of their growth and differentiation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C. Interferon-induced enzymatic activities and their role in the antriviral state. Cell. 1979 Jun;17(2):255–264. doi: 10.1016/0092-8674(79)90151-x. [DOI] [PubMed] [Google Scholar]
- Creasey A. A., Bartholomew J. C., Merigan T. C. Role of G0-G1 arrest in the inhibition of tumor cell growth by interferon. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1471–1475. doi: 10.1073/pnas.77.3.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarniecki C. W., Sreevalsan T., Friedman R. M., Panet A. Dissociation of interferon effects on murine leukemia virus and encephalomyocarditis virus replication in mouse cells. J Virol. 1981 Feb;37(2):827–831. doi: 10.1128/jvi.37.2.827-831.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube S. K., Pragnell I. B., Kluge N., Gaedicke G., Steinheider G., Ostertag W. Induction of endogenous and of spleen focus-forming viruses during dimethylsulfoxide-induced differentiation of mouse erythroleukemia cells transformed by spleen focus-forming virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1863–1867. doi: 10.1073/pnas.72.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G. Antitumor effects of interferon. Biochim Biophys Acta. 1978 Oct 27;516(2):231–247. doi: 10.1016/0304-419x(78)90009-4. [DOI] [PubMed] [Google Scholar]
- Gresser I., Tovey M. G., Bandu M. E., Maury C., Brouty-Boyé D. Role of interferon in the pathogenesis of virus diseases in mice as demonstrated by the use of anti-interferon serum. I. Rapid evolution of encephalomyocarditis virus infection. J Exp Med. 1976 Nov 2;144(5):1305–1315. doi: 10.1084/jem.144.5.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Kimchi A. Increased levels of interferon-induced (2'--5') oligo-isoadenylate synthetase in mature T-lymphocytes and in differentiated Friend-erythroleukemic cells. J Interferon Res. 1981;1(4):559–569. doi: 10.1089/jir.1981.1.559. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shulman L., Schmidt A., Chernajovsky Y., Fradin A., Revel M. Kinetics of the induction of three translation-regulatory enzymes by interferon. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3208–3212. doi: 10.1073/pnas.76.7.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Lapidot Y., Rapoport S., Panet A., Revel M. Antimitogenic effects of interferon and (2'-5')-oligoadenylate in synchronized 3T3 fibroblasts. FEBS Lett. 1981 Nov 16;134(2):212–216. doi: 10.1016/0014-5793(81)80604-7. [DOI] [PubMed] [Google Scholar]
- Kimchi A., Shure H., Revel M. Regulation of lymphocyte mitogenesis by (2'--5') oligo-isoadenylate. Nature. 1979 Dec 20;282(5741):849–851. doi: 10.1038/282849a0. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Conscience J. F., Skoultchi A., McMillan P., Revel M., Ruddle F. H. Effect of interferon on dimethyl sulfoxide-stimulated Friend erythroleukemic cells: ultrastructural and biochemical study. J Virol. 1977 Sep;23(3):799–810. doi: 10.1128/jvi.23.3.799-810.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks P. A., Rifkind R. A. Erythroleukemic differentiation. Annu Rev Biochem. 1978;47:419–448. doi: 10.1146/annurev.bi.47.070178.002223. [DOI] [PubMed] [Google Scholar]
- Pickering L. A., Kronenberg L. H., Stewart W. E., 2nd Spontaneous production of human interferon. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5938–5942. doi: 10.1073/pnas.77.10.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben R. C., Rifkind R. A., Marks P. A. Chemically induced murine erythroleukemic differentiation. Biochim Biophys Acta. 1980 Sep 22;605(3):325–346. doi: 10.1016/0304-419x(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Sokawa Y., Watanabe Y., Watanabe Y., Kawade Y. Interferon suppresses the transition of quiescent 3T3 cells to a growing state. Nature. 1977 Jul 21;268(5617):236–238. doi: 10.1038/268236a0. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Chernajovsky Y., Zeevi M., Shulman L., Soreq H., Nir U., Wallach D., Perricaudet M., Tiollais P., Revel M. Two interferon mRNAs in human fibroblasts: in vitro translation and Escherichia coli cloning studies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y. Antigenicity of mouse interferons: two distinct molecular species common to interferons of various sources. Virology. 1981 Jun;111(2):312–319. doi: 10.1016/0042-6822(81)90335-4. [DOI] [PubMed] [Google Scholar]



