Abstract
We combined in silico, in vivo, and in vitro studies to gain insights into age-dependent changes in acute inflammation in response to bacterial endotoxin (LPS). Time-course cytokine, chemokine, and NO2 −/NO3 − data from “middle-aged” (6–8 months old) C57BL/6 mice were used to re-parameterize a mechanistic mathematical model of acute inflammation originally calibrated for “young” (2–3 months old) mice. These studies suggested that macrophages from middle-aged mice are more susceptible to cell death, as well as producing higher levels of pro-inflammatory cytokines, vs. macrophages from young mice. In support of the in silico-derived hypotheses, resident peritoneal cells from endotoxemic middle-aged mice exhibited reduced viability and produced elevated levels of TNF-α, IL-6, IL-10, and KC/CXCL1 as compared to cells from young mice. Our studies demonstrate the utility of a combined in silico, in vivo, and in vitro approach to the study of acute inflammation in shock states, and suggest hypotheses with regard to the changes in the cytokine milieu that accompany aging.
Introduction
Inflammation ensues upon acute biological stress such as bacterial infection or tissue trauma [1]. In elderly individuals, the inflammatory response becomes radically altered, and is often accompanied by low grade, chronic, inflammation even in the absence of external stimulus [2]–[4]. Indeed, the linkage between inflammation and aging is close, prompting some authors to coin the term “inflamm-aging” for this complex process [4]. “Inflamm-aging” is associated with many functional alterations in both innate and adaptive immunity [5], [6]. Among the cellular components of the innate immune response, macrophages secrete a wide range of cytokines, chemokines and growth factors in response to pathogens, bacterial toxins, and host-derived damage-associated molecular pattern (DAMP) molecules [7], [8]. The effect of age on the production of these inflammatory mediators following macrophage activation by stimuli such as Gram-negative bacterial lipopolysaccharide (LPS) is not well defined [9]–[11].
To address the complexity of the acute inflammatory response in vivo, we have constructed mechanistic mathematical models of increasing complexity, using ordinary differential equations (ODE), which encompass the dynamics of relevant cells and cytokines as well as aspects of physiology and global tissue damage/dysfunction [12]–[15]. These computational models are capable of quantitative predictions, since they are calibrated to circulating levels of inflammatory mediators in mice [12], [16], rats [17], and swine [18]. These models’ ability to predict mortality is based on the incorporation of a global tissue damage/dysfunction variable, which is both a marker of the adverse effects of inflammation as well as a driver of further inflammation.
To address the effect of a chronic inflammatory perturbation on inflammation in vivo, we have developed a methodology that utilizes a combination of the mathematical model, automated data-fitting algorithms, and both in vivo and in vitro data. We previously utilized this approach to examine the controversial role of LPS or bacterial translocation in trauma/hemorrhage-induced inflammation [16]. Herein, we sought to use a similar approach to gain insight into the alterations characteristic of inflammation in aged mice. We hypothesized that we could obtain insights into cellular-level changes characteristic of the aged inflammatory response by examining the systemic responses of “middle-aged” mice, re-parameterizing our mathematical model for the levels of circulating inflammatory analytes in these mice, and deriving insights into the cellular and intracellular changes that could lead to these systemic alterations.
Materials and Methods
Ethics Statement
This study was performed in strict accordance with animal use protocols following approval of the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC, protocol number 13021433). These studies involved experimentally-induced acute inflammation (endotoxemia; see below). Clinical signs associated with this experimental paradigm include decreased food/water intake, lethargy, anorexia, piloerection, and weight loss. These pathophysiological changes mandated the classification of this study as Category E of Pain/Distress. Euthanasia via approved methods at a maximum of 90 min, or immediately upon observation of the above clinical signs, was the primary means of mitigating this degree of pain and distress.
Analgesics (opioids, steroids and non-steroidal anti-inflammatory drugs) have profound effect on the cardiorespiratory system and on the inflammatory response [19]. These effects include blockade of cytokine response and acceleration of the progression to shock [20]. In rodent models of sepsis, trauma-hemorrhagic shock and hepatic ischemia/reperfusion, these drugs are also known to significantly alter the inflammatory response and the degree of organ damage [21]. Because the study proposed in this protocol aim to unravel the mechanisms of inflammation-induced organ injury through the generation of mathematical models, administration of these drugs was contra-indicated, for they would alter the course of inflammation and injury.
Experimental Endotoxemia and Culture of Resident Peritoneal Cells
Male C57BL/6 mice were purchased from Charles Rivers (Wilmington, MA) and were acclimatized in specific pathogen-free conditions with 12-h light/dark facilities and received food and water ad libitum. Plasma levels of inflammatory mediators from young mice (n = 4, 8–12 weeks old, weighing 20–25 g) were used for the initial calibration of the mathematical model, as described elsewhere [12]. Plasma levels of inflammatory mediators from “Middle-aged” mice (n = 4, 6–8 months old, weighing 25–35 g) were used to re-parameterize the mechanistic mathematical model of acute inflammation that was originally calibrated for young mice (see Mathematical model of inflammation). In these initial studies, a limited number of plasma analytes (TNF-α, IL-6, IL-10, and NO2 −/NO3 −; see Cytokine and NO2−/NO3 − analysis) were assessed. These initial re-calibration studies led specific predictions regarding biological properties of young vs. middle-aged macrophages (see Mathematical model of inflammation).
To validate these predictions in vitro, additional mice (n = 16 for either young or middle-aged) were treated with sterile saline or 3 mg/kg LPS (Escherichia coli serotype O111:B4, catalog # L2630; Sigma-Aldrich, St. Louis, MO) intraperitoneally for 90 min. The animals were then euthanized by CO2 asphyxiation followed by cervical dislocation. Blood samples were collected via cardiac puncture and stored at −80°C until analysis for cytokine and NO2 −/NO3 −.
To obtain resident peritoneal cells, the peritoneal cavity was then flushed with cold saline (10 ml) via a large bore needle (18-gauge), avoiding traumatic perforation of the bowel. The abdomen was gently manipulated and the peritoneal fluid was aspirated back using a 26-gauge needle. Peritoneal cells were collected, washed and suspended in previously prepared DMEM (Dulbecco’s Modified Eagle’s Medium, Lonza, Walkersville, MD) cell culture media containing 10% heat-inactivated FCS, 1 mM glutamine, 50 U/ml penicillin and 50 µg/ml streptomycin.
Peritoneal cells (at a density of 2×105 cells per well) from both saline and LPS treated mice were plated on eight-well Lab Tek™ Chamber Slide™ tissue culture plates (Nalge Nunc International, Rochester, NY) and allowed to attach in a humidified atmosphere (5% CO2) for 2 h [22]. After adherence, the culture media was replaced to remove the non-adherent cells and cell monolayers (predominantly to be macrophages) were further incubated for 90 min. At the end of the experiment, the supernatants were collected from each well and stored at −80°C until analysis. To determine peritoneal cell count and viability, 10 µL of peritoneal fluid were mixed with an equivalent volume of 0.1% trypan blue pipetted into Countess® chamber slide, and analyzed using a Countess® Automated Cell Counter (Life Technologies, Grand Island, NY).
Qualitative Assessment of Cultured Peritoneal Macrophages
At the end of the cell culture experiments the supernatants were collected and the purity and overall viability of the resident peritoneal macrophages were assessed. To do so, the wells were washed with PBS and fixed with 70% ethanol. Eight random wells from two chamber slides were selected from each group (young and middle-aged mice) and macrophages were identified in each well by characteristic morphology under light microscopy as [23].
Cytokine and NO2 −/NO3 − Analysis
For the initial calibration of the mathematical model, plasma samples from young mice (n = 4) treated with LPS in vivo were collected at 0, 60 min, 90 min, 4 h, 12 h and 24 h and assayed for IL-6, TNF-α, IL-10, and NO2 −/NO3 −. For the validation cohort of mice, plasma samples and cell culture supernatants (n = 16 per group for young and middle-aged mice, respectively) were assayed for 20 cytokines using a multiplex bead immunoassay system (Luminex™, Millipore, Billerica, MA). The cytokine assays included granulocyte-macrophage colony stimulating factor (GM-CSF), keratinocyte-derived chemokine (KC; CINC-1; CXCL1), interferon-γ (IFN-γ), IFN-γ-inducible protein-10 (IP-10; CXCL10), interleukin- (IL)-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12 p40, IL-12p70, IL-13, IL-17, monocyte chemotactic protein-1 (MCP-1; CCL2), monokine induced by gamma interferon (MIG; CXCL9), macrophage inflammatory protein-1α (MIP-1α), vascular endothelial growth factor (VEGF) and tumor necrosis factor-α (TNF-α). Plasma and supernatant NO2 −/NO3 − were measured by the nitrate reductase method using a commercially available kit (Cayman Chemical, Ann Arbor, MI).
Statistical Analysis
All data are expressed as mean ± SEM. Statistical analysis was performed by Student’s t-test or Two-Way ANOVA followed by Tukey post hoc or Holm-Sidak test as indicated using SigmaPlot (Systat Software, San Jose, CA), with P<0.05 considered to be significant.
Mathematical Model of Inflammation
The equations and specifications of the “young mouse-specific” mathematical model of inflammation have been described elsewhere [12]. Briefly, we constructed a mathematical model of acute inflammation that incorporates key cellular and molecular components of the acute inflammatory response. The mathematical model consists of a system of 17 nonlinear ordinary differential equations that describe the time course of these components. Included in the model equations are two systemic variables that represent mean arterial blood pressure and global tissue dysfunction and damage. “Global tissue damage/dysfunction” describes the overall health of the organism, since the hallmark of all physiological derangements accompanying sepsis and hemorrhagic shock is the eventual, sequential failure of multiple organs. Given the complexity of simulating individual organs, we approximated this process by treating it as a gradual, ongoing process occurring in the whole body and driven by inflammation. Thus, elevated and unrecoverable tissue damage/dysfunction served as a surrogate for death, while damage/dysfunction that tended to return to baseline over time was a proxy for survival.
Each equation was constructed from known interactions among model components as documented in the existing scientific literature. The model and parameters were specified in three stages. In the preliminary stage, the model was constructed so it could reproduce qualitatively several different scenarios reported in the literature. In this stage, experimentally determined values of parameters such as cytokine half-lives were used when available. In the second stage, the model was matched to our experimental data by adjusting some of the parameters using our qualitative understanding of the biological mechanisms together with the dynamics of the model, to attain desired time course shapes. In the third stage, the parameters were estimated by fitting the model to the experimental data and using a stochastic gradient descent algorithm that was developed by Immunetrics, Inc. (Pittsburgh, PA) [12].
Adaptation of “Young-specific” Mathematical Model to Account for Inflammation in Aging Mice
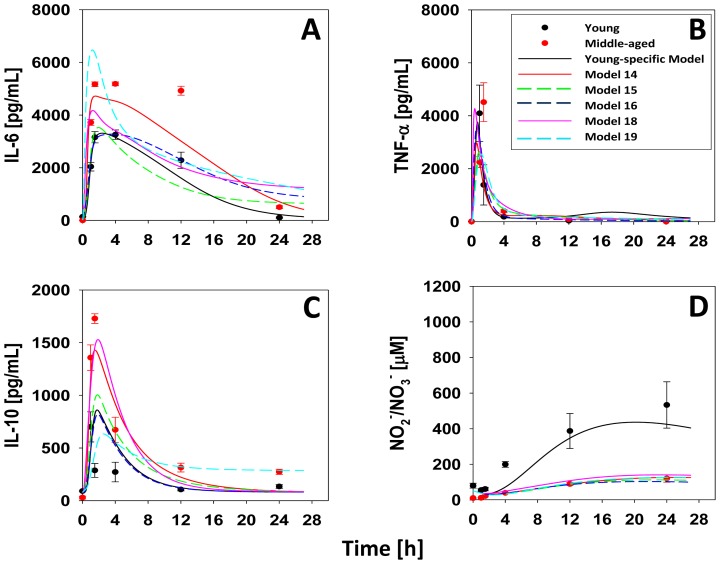
To recalibrate our mathematical model for data obtained in “middle-aged” mice we sought to find a minimal number of parameter changes that would allow our original model to reproduce accurately empirically observed time courses in aging-mice. To prevent the optimizing algorithm from exploiting the indirect action of unmeasured analytes, we attempted to confine our search to those parameters that appear directly in the equations of our measured analytes (IL-6, TNF-α, IL-10, and NO2 −/NO3 −). Our procedure was to impose a limit of N changes, run an optimizer bounded by this change limit, and evaluate the fit. We evaluated these fits with respect to quantitative error totals as well as general qualitative behavior. After evaluation, we increased the number of parameters to be optimized and repeated the process iteratively until sufficient fit quality was achieved. Each optimization pass was allowed to choose randomly which N of the candidate parameters to change. We performed numerous fits for each N to account for the stochastic nature of our gradient descent search. In order to obtain good fits with fewer than 10 changes, we found it necessary to add the parameters of the (unmeasured) activated macrophages (MA), activated neutrophils (NA), and iNOS equations to our initial set of parameters to be fit [12]. When these parameters were included in the search, we found that excellent fits could be obtained with fewer than eight parameter changes. We also observed that eliminating any one of these changes seems to result in a worse fit by both quantitative and qualitative measures. This procedure yielded five parameter sets with good fits to the data in middle-aged mice, with essentially equal measures of error with respect to experimental data (data not shown). The models exhibited similar behavior with regard to model output and predictions presented herein, with various “tradeoffs” relative to a hypothetical perfect fit. Each parameter set was based on similar, but not identical, changes to various constants (Table 1). We show these models and indicate the one judged as the best overall fit to the data by investigator consensus (necessary given the overall similarity of the quantitative measures used to distinguish among the models). Nonetheless, the model output and predictions of all models are shown (Fig. 1, lines).
Table 1. Characteristics of middle-aged specific mathematical models of inflammation.
| Model | Param. | h10cp | hn10 | kmd | kmpe | kn | knpe | xcp6 | |||||||
| Best | Param. Def. | Influence of TNF onIL-10 (Hill coefficient) | Influence of IL-10 on leukocyte activation (Hill coefficient) | Rate of activation of macrophages due to damage | Activation of macrophages by LPS | Death rate of neutrophils | Activation of neutrophils by LPS | Saturation constant for IL-6′s influence on TNF | |||||||
| Change in Middle- aged vs. Young | 8-fold lower | 5-fold lower | 3-fold lower | 9-fold higher | 16-fold higher | 5-fold higher | 11-fold higher | ||||||||
| Model 15 | Param. | h6cp | k10 | k10m0 | kn | s6 | xcp6 | ||||||||
| Param. Def. | Influence of TNF onIL-10 (Hill coefficient) | Regulates levels of IL-10 by modulating production/decay rates | Rate of IL-10 production in response to other cytokines | Death rate of neutrophils | Propensity to produce and secrete IL-6 at rest | Saturation constant for IL-6′s influence on TNF | |||||||||
| Change in Middle- aged vs. Young | 3-fold lower | 7-fold higher | 84-fold higher | 4-fold higher | 36-fold higher | 66-fold higher | |||||||||
| Model 16 | Param. | k6no | Kn | knpe | s6 | xcp6 | xm6 | ||||||||
| Param. Def. | Influence of NO2 −/NO3 − on IL-6 | Death rate of neutrophils | Activation ofneutrophils by LPS | Propensity to produce and secrete IL-6 at rest | Saturation constant for IL-6′s influence on TNF | Saturation constant for IL-6′s influence on macrophage activation | |||||||||
| Change in Middle- aged vs. Young | 26-fold higher | 19-fold higher | 6-fold higher | 46-fold higher | 50-fold higher | 3-fold higher | |||||||||
| Model 18 | Param. | hcp6 | hcp10 | kinosn | knpe | s6 | x6cp | xmpe | |||||||
| Param. Def. | Influence of IL-6 onTNF (Hill coefficient) | Influence of IL-10 onTNF (Hill coefficient) | Influence ofneutrophils on iNOS | Activation ofneutrophils by LPS | Propensity to produce and secrete IL-6 at rest | Saturation constant for TNF’s influence on IL-6 | Saturation constant for influence of LPS on macrophage activation | ||||||||
| Change in Middle- aged/vs. Young | 1.4-fold lower | 41-fold lower | 6-fold lower | 28-fold higher | 62-fold higher | 73-fold higher | 7-fold lower | ||||||||
| Model 19 | Param. | k6n | kn | s10 | xcp6 | xmd | |||||||||
| Param. Def. | Influence ofneutrophils on IL-6 | Death rate of neutrophils | Propensity to produce and secrete IL-10at rest | Saturation constant for IL-6′s influence on TNF | Saturation constant for influence of damage on macrophage activation | ||||||||||
| Change in Middle- aged vs. Young | 52-fold higher | 8-fold higher | 4-fold higher | 30-fold higher | 7-fold higher | ||||||||||
Model parameters (constants) changed in the middle-aged specific models are indicated, along with their biological significance. Param.: parameter. Def.: definition.
Figure 1. Data and model output for plasma cytokines and NO2 −/NO3 − in young and middle-aged mice subjected to LPS.
Young (C57BL/6; n = 4 animals per time point) and middle-aged mice (n = 4 per time point) were injected with saline or 3 mg/kg LPS as described in Materials and Methods. Plasma concentration as a function of time and age: (A) IL-6, (B) TNF-α, (C) IL-10 and (D) NO2 −/NO3 −. Symbols represent the mean ± SEM (P<0.05, analyzed by Two-way ANOVA followed by Holm-Sidak test for plasma levels of IL-6 and IL-10). For young mice, the line indicates the output of the baseline model of acute inflammation. For middle-aged mice, model re-calibration was carried out as described in the Materials and Methods, yielding five models (colored lines). “Best Model” indicates the model giving the best overall fit to the data.
Results
Age-associated Changes in Inflammatory Mediators of Endotoxemic Mice
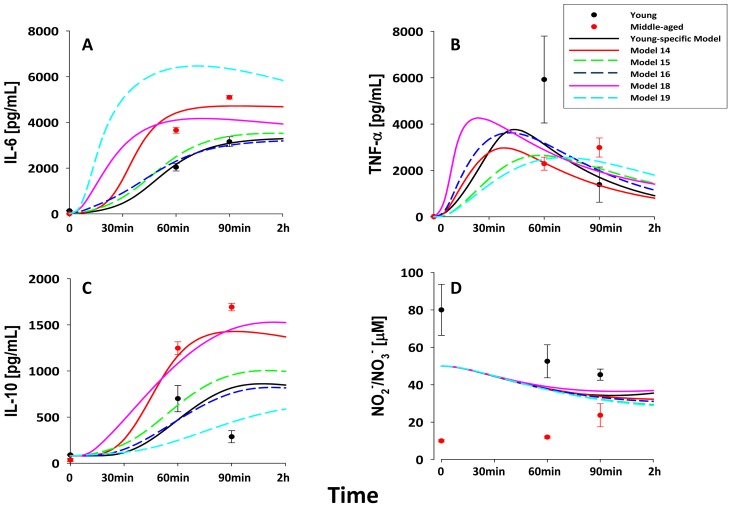
Prior studies had suggested that older mice exhibit dysregulated acute inflammation [24], [25]. We confirmed this finding initially by measuring plasma levels of IL-6 (Fig. 1A), TNF-α (Fig. 1B), IL-10 (Fig. 1C) and NO2 −/NO3 − (Fig. 1D) in “young” (2–3 month old) and “middle-aged” (6–8 month old) C57BL/6 mice subjected to bolus intraperitoneal injection of 3 mg/kg LPS. Based on Two-Way ANOVA, plasma levels of IL-6 and IL-10 were statistically significantly higher (P<0.05) in middle-aged mice when compared to similarly treated young mice, whereas NO2 −/NO3 − was statistically significantly higher (P<0.05) in young mice. In addition, we discerned significant (P<0.05) age-time interactions in the dynamics of IL-6 (at 60 min, 90 min, 4 h and 12 h), TNF-α (at 60 and 90 min), IL-10 (at 60 min, 90 min, 4 h and 12 h), and NO2 −/NO3 − (at 4 h, 12 h and 24 h) (Fig. 2, symbols), when comparing young vs. middle-aged mice post-LPS treatment.
Figure 2. Difference in plasma cytokines and NO2 −/NO3 − in young and middle-aged mice subjected to LPS.
Young (C57BL/6; n = 3–8 animals per time point) and middle-aged mice (n = 4 per time point) were injected with saline or 3 mg/kg LPS as described in Materials and Methods. Middle-aged mice had significantly higher levels of IL-6 (A), TNF-α (B), and IL-10(C) at 60 and 90 min when compared to young mice post-LPS treatment (P<0.05, analyzed by Two-way ANOVA).
Mathematical Modeling of Age-dependent Changes in Circulating Inflammatory Mediators
We next hypothesized that mathematical modeling could help us gain insights into the some of these complex changes in circulating inflammatory mediators. We have previously developed a mechanistic mathematical model in which dynamics of leukocytes and their products affect the whole-organism inflammatory response assessed as circulating levels of mediators, that we calibrated using data from young C57BL/6 mice [12], [16], [26], [27] (see Materials and Methods). Using this base mathematical model, we have previously developed a methodology by which to gain insight into the complex changes in the acute inflammatory response of gene-deficient animals (see Materials and Methods), which we utilized to study the complex alterations in the acute inflammatory response of CD14-deficient mice [16]. This methodology involves measuring the levels of key circulating inflammatory mediators (IL-6, TNF-α, IL-10, and NO2 −/NO3 −); re-parameterizing the base mathematical model for these new data by generating an ensemble of models with roughly equivalent fit to the data, as assessed by an error function; and finally, determining the consensus set of parameters that were altered in the automated fitting process in order to fit these new data. Since these parameters can be mapped to cellular- and molecular-level biological processes (e.g. the degree to which LPS will induce macrophages to produce TNF-α), this re-parameterization process allows us to generate hypotheses regarding the underlying differences in the inflammatory responses of the test animals (in the current study, middle-aged C57BL/6 mice) vs. the control animals (young C57BL/6 mice) based solely on systemic inflammatory mediator data.
Accordingly, we carried out the process described above (see Materials and Methods). The resulting ensemble of five mathematical models that fit the systemic IL-6, TNF-α, IL-10, and NO2 −/NO3 − data in middle-aged mice suggested that, at the cellular level, aged leukocytes produce higher levels of inflammatory cytokines, particularly IL-6, IL-10 and to a lesser degree TNF-α at baseline and in response to LPS. These models also predicted that leukocytes from middle-aged mice undergo increased death as compared to leukocytes from young mice (Table 1 and Fig. 1, lines).
In vitro Validation of in silico Predictions
We next sought to validate these mathematical model predictions by in vitro studies using resident macrophages harvested from peritoneal cavities of young and middle aged mice that received either intra-peritoneal saline or LPS. We first confirmed that treatment with LPS for 90 min would result in a broad-based induction of the inflammatory response in vivo. Accordingly, we expanded our analysis to additional animals and additional circulating inflammatory mediators (20 cytokines/chemokines as well as NO2 −/NO3 −) (Table 2). In support of our initial findings, middle-aged mice that were treated with LPS in vivo had statistically significantly higher plasma concentrations of IL-6, TNF-α, IL-10, MIG/CXCL9, MCP-1/CCL2, IL-1α, IL-17, and IL-12p40 when compared to LPS treated young mice. However, saline-treated (baseline) middle-aged mice had higher circulating levels of GM-CSF, IL-1β, IL-2, IL-4, IL-12p40, and VEGF when compared to saline-treated young mice. Moreover, saline-treated young mice exhibited statistically significantly higher circulating levels of NO2 −/NO3 − when compared to saline treated middle-aged mice (Table 2). Circulating plasma levels of IFN-γ, IL-12p70, IL-13, IL-17 and MIG were below the detection limit in both young and middle-aged mice after either saline or LPS treatment.
Table 2. Statistically significant plasma inflammatory mediators in both young and middle-aged mice after I.P saline or LPS treatment.
| Treatment | Inflammatory Mediators | Young (mean ± SEM) | Middle-aged (mean ± SEM) | P value (Student’s t-test) |
| Saline | GM-CSF | 0 | 20.2±3.5 | P<0.001 |
| IL-1β | 0 | 21.2±2.9 | P<0.001 | |
| IL-2 | 2.7±0.2 | 4.8±0.2 | P<0.001 | |
| IL-4 | 2.8±0.2 | 5.5±0.4 | P<0.001 | |
| IL-12p40 | 0 | 28.9±2.8 | P<0.001 | |
| VEGF | 1.2±0.2 | 2.8±0.3 | P<0.001 | |
| NO2 −/NO3 − | 36.9±1.9 | 22.1±1.1 | P<0.001 | |
| LPS | IL-6 | 14589.7±1583.9 | 33445.08±7281.9 | P = 0.004 |
| TNF-α | 93.6±18.1 | 332.6±63.7 | P<0.001 | |
| IL-10 | 251.9±40.3 | 458.9±67.2 | P = 0.016 | |
| MIG/CXCL9 | 100.9±17.5 | 4099.4±690.6 | P<0.001 | |
| MCP-1 | 18869.1±3153.4 | 34142.9±4515.6 | P = 0.012 | |
| IL-1α | 48.1±6 | 124.5±0.4 | P = 0.002 | |
| IL-17 | 0 | 5.5±0.9 | P<0.001 | |
| IL-12p40 | 104.1±14.3 | 183.2±21.3 | P = 0.006 | |
| NO2 −/NO3 − | 54.7±4.3 | 34.6±2.3 | P<0.001 | |
| GM-CSF | 59.4±5.9 | 35.4±4.8 | P = 0.005 |
Young and middle-aged mice (n = 16 each) were treated with either saline or LPS in vivo for 90 min., and circulating inflammatory mediators were assessed as described in Materials and Methods.
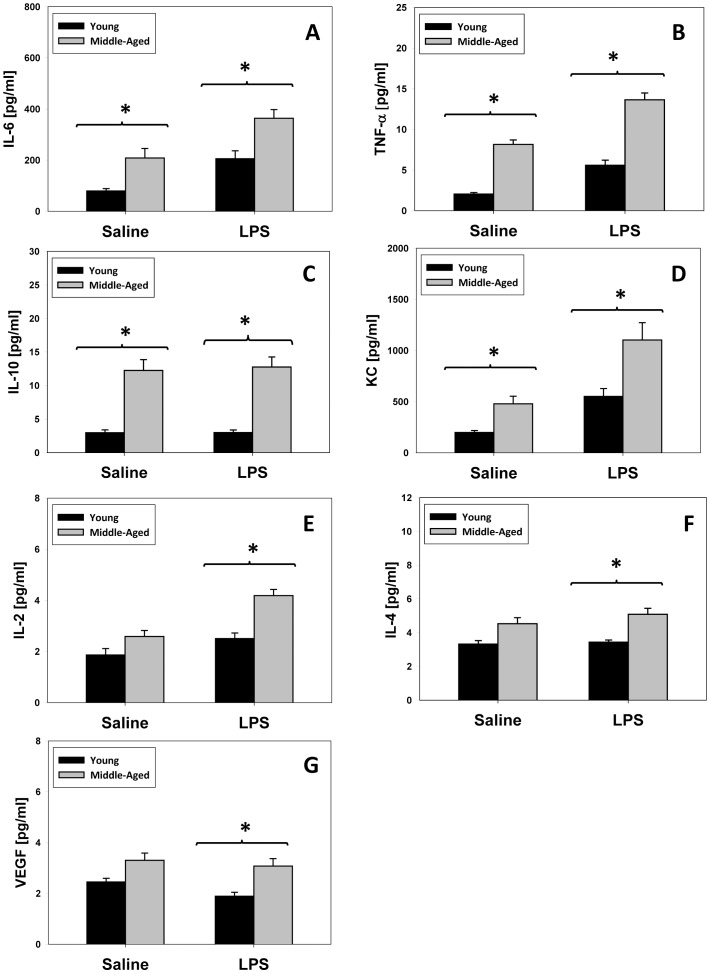
One key prediction of our re-parameterized mathematical model of inflammation was that leukocytes from middle-aged mice would exhibit a higher production of inflammatory cytokines. Resident macrophages from young and middle-aged mice were obtained by peritoneal lavage and the supernatants of adherent cells were assayed for the same 21 inflammatory mediators described in Table 2. In support of the in silico predictions, macrophages from saline-treated middle-aged mice produced elevated levels of IL-6 (Fig. 3A), TNF-α (Fig. 3B), IL-10 (Fig. 3C) and KC (Fig. 3D) when compared with similarly treated young mice. However, macrophages from middle-aged mice subjected to 3 mg/kg LPS in vivo secreted statistically significantly elevated levels of IL-6 (Fig. 3A), TNF-α (Fig. 3B), IL-10 (Fig. 3C), KC (Fig. 3D), IL-2 (Fig. 3E), IL-4 (Fig. 3F) and VEGF (Fig. 3G) as compared to similarly-treated macrophages from young mice. We note that IL-2, IL-4, and VEGF are not included in the mathematical model of inflammation [12].
Figure 3. In vitro cytokine concentrations after in vivo treatment with either saline or LPS in young and middle aged mice.
Young (n = 16) and middle-aged mice (n = 16) were injected with saline or 3 m/kg LPS and peritoneal macrophages were harvested as described in Materials and Methods. Middle-aged mice had significantly higher levels (*P<0.001 vs. young mice) of IL-6 (A), TNF-α (B), IL-10 (C) and KC (D) after in vivo treatment with saline followed by in vitro treatment with culture media. IL-6 (A), TNF-α (B), IL-10 (C), KC (D), IL-2 (E), IL-4 (F) and VEGF (G) concentrations were significantly higher (*P<0.001 vs. young mice) after in vivo LPS treatment followed by in vitro treatment with culture media.
Given the mathematically-predicted, age-dependent difference in baseline and in vivo LPS-induced production of inflammatory mediators, we next sought to determine if the actual degree of LPS-induced stimulation of these mediators differed between young and middle-aged mice. Accordingly, we calculated the fold change difference among those mediators determined to be statistically significantly different between young and middle-aged mice. This fold change difference was calculated as follows:
Figure S1 shows fold change difference between young and middle-aged mice. This analysis showed a statistically significant (P = 0.004) higher fold change difference for TNF-α in young mice when compared to middle-aged mice, whereas there was no statistical differences in the other inflammatory mediators.
Only one of the five middle aged-specific mathematical models invoked a relatively minor effect on kinosn, a parameter governing the influence of neutrophils on iNOS. Largely in agreement with the predictions of the mathematical model, there were no statistically significant differences in NO2 −/NO3 − levels in macrophages isolated from young or middle-aged mice that received either saline or LPS in vivo.
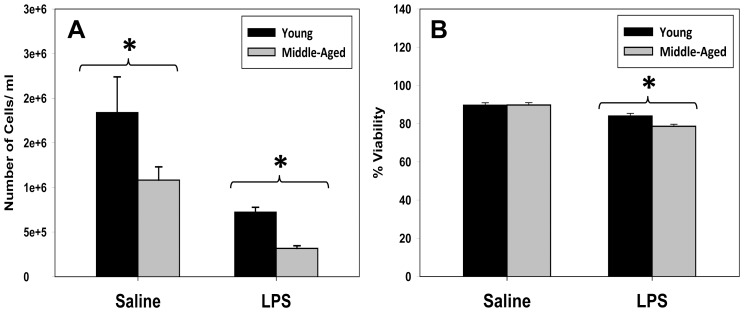
Another key prediction derived from the re-parameterization of our mathematical model of inflammation was that leukocytes from middle-aged mice would exhibit a shorter lifespan as compared to leukocytes from young mice. Fig. 4 shows the total peritoneal cell count (Fig. 4A) and cell viability (Fig. 4B) in young and middle-aged mice after treatment with either saline or 3 mg/kg LPS in vivo. In support of the in silico prediction, peritoneal cells from young mice that were treated with saline or LPS in vivo had statistically significantly higher cell counts when compared to saline treated middle-aged mice (Fig. 4A). Peritoneal cells from LPS-treated, but not saline-treated middle-aged mice exhibited significantly lower cell viability when compared to cells from LPS-treated young mice (Fig. 4B).
Figure 4. Total peritoneal cell count and cell viability.
(A): Middle-aged mice that received in vivo LPS had significantly lower (*P<0.001) cell count when compared to similarly treated young mice. (B): LPS-treated middle-aged mice had significantly lower (*P = 0.008) cell viability when compared to LPS treated young mice.
Discussion
Aging and the diseases that stem from it are consuming an ever-larger portion of healthcare expenditures [28]. Recent genome-wide association studies suggest that inflammation and cellular senescence are common mechanisms in aging-associated diseases [29]. Such studies raise the hypothesis that dysregulated inflammation is at least part of the cause of aging-related diseases [2], [3], [30], [31], which has been supported by studies demonstrating inflammation-counterbalancing mechanisms in centenarians [32]. It has also been hypothesized that an age-dependent increase in pro-inflammatory tendency was favored in an environment in which infection was prevalent, but that elevated inflammation is predominantly detrimental in a modern world in which infection is much less prevalent [33]. Importantly, these changes in inflammation can be observed by middle age in both humans and experimental animals, and are not merely a hallmark of the very aged [34]–[36].
Monocytes and macrophages play central roles in the initiation and resolution of inflammation in response to various insults [37], including LPS-induced endotoxic shock [8]. Monocytes and macrophages originate from a common myeloid progenitor cell in the bone marrow and, under normal circumstances; monocytes circulate in the bloodstream for a very short time before undergoing spontaneous apoptosis [37], [38]. In response to differentiation factors, monocytes escape their apoptotic fate by differentiating into tissue-resident macrophages [39]. Studies of age-related differences in macrophage production of pro-inflammatory and anti-inflammatory cytokines in response to acute stimulation in vitro have yielded conflicting results, highlighting the complexity of inflammation in the aging process. Indeed, some groups have reported a decrease in levels of IL-1β [40], IL-6 [41], and TNF-α [42], while increases in levels of IL-1β, IL-6, and TNF-α [43] have been reported by others.
Remarkable advances have been made in revealing molecular mechanism underlying the acute inflammatory response, and yet the complexity of this process – with its redundancy, positive and negative feedback loops, and emergent system behavior that cannot be readily discerned from a reductionist analysis of individual pathways – makes inflammation the prototypical case study for the application of systems and computational biology [44], [45]. These approaches, which include various “omics” methods as well as computational modeling, are being utilized to address the complexity of inflammation in the aging process [46].
Accordingly, we hypothesized that we could gain cellular-level insights into the altered inflammatory response that accompanies aging using a mechanistic computational model that simulates whole-animal inflammation as a consequence of inflammatory cell activation by LPS [12], [16], [26], [27]. We utilized mouse endotoxic shock as a simplified paradigm of acute inflammation driven in large part by monocyte/macrophages [8]. In essence, the variables and parameters in our mathematical model represent different aspects of cellular and sub-cellular biology of inflammation (e.g. growth and decay rates of cells and cytokines), and is predicated on the systemic spillover of inflammatory mediators into the circulation. This latter feature – the connection between local and systemic inflammation – has allowed us to calibrate this model using data on circulating inflammatory mediators. We have previously utilized this mathematical model to show commonality among the responses to LPS, surgical trauma, and trauma/hemorrhage in young C57BL/6 mice [12]. Using this mathematical model, we have also gained insights into the characteristics of systemic inflammation in young CD14-deficient mice vs. young C57BL/6 controls, including the lack of a role for endogenous LPS in trauma/hemorrhage-induced inflammation [16].
We reasoned that we could gain mechanistic insights into the role of LPS in the inflammatory response by obtaining data on circulating cytokines and NO2 −/NO3 − in middle-aged mice, re-calibrating our model, and determining which parameters were changed relative to young mice. In initial studies, middle-aged mice subjected to 3 mg/kg LPS exhibited higher circulating levels of IL-6 and IL-10, and blunted NO2 −/NO3 − when compared to young mice, and in subsequent studies we verified that a broader set of mediators was likewise altered in middle-aged mice. We then re-calibrated the baseline (young C57BL/6 mouse-specific) mathematical model of inflammation using automated algorithms to yield an ensemble of “aging-specific” models. These models were interrogated as to changes in various parameters (corresponding to biological mechanisms) compared to the “young-specific” model. The ensemble of five mathematical models that best fit these data suggested that aged leukocytes will produce elevated baseline levels of IL-6 (and to a lesser degree, TNF-α), elevated LPS-induced production of pro-inflammatory mediators and undergo increased cell death as compared to young leukocytes, in agreement with the literature [36], [47].
To validate key in silico predictions about cellular-level changes in acute inflammation that occur with aging, predictions which were based on in vivo data (levels of circulating inflammatory mediators), we carried out in vitro time-course studies on peritoneal macrophages from C57BL/6 young and middle-aged mice. To assess the universality of these predictions, and to gain further insights into changes in acute inflammation that are characteristic of aging, we surveyed a larger number of inflammatory mediators. These in vitro results largely validated the mathematical model prediction, as observed by elevated levels of IL-6, TNF-α, IL-10 and KC in resident peritoneal cells from middle-aged mice as compared to cells from similarly-treated young mice. Though aging can lead to reduced macrophage NO production [48], our algorithms suggested at best a minor role for reduced expression of iNOS by neutrophils, and this prediction was supported by the lack of statistically significant differences in the production of NO2 −/NO3 − between young and middle-aged peritoneal macrophages. In addition, the statistically significant fold change difference in TNF-α production suggests that macrophages in young mice have greater responsiveness to LPS in vivo, with regard to this key inflammatory cytokine, when compared to similarly treated macrophages in middle-aged mice. Importantly, other inflammatory mediators that differ significantly between young and middle-aged mice are induced by LPS to roughly the same extent. Another key in silico prediction was that the lifespans of leukocytes derived from older mice would be shorter, a prediction supported by in vitro cell qualitative assessment and cell viability as well.
Our experimental studies, like our mathematical model, were focused on cellular-level phenomena that drive systemic changes in inflammatory mediators. In the present study, we therefore sought to stimulate animals in vivo and then examine the responses in vitro with the least further perturbation, in order to verify predictions derived from the mathematical model. In separate studies we also treated cultured macrophages with LPS in vitro (data not shown). We observed a roughly similar increase in the levels of inflammatory mediators when we added LPS in vitro to macrophages derived from mice that were previously treated in vivo with saline (data not shown). Interestingly, in vitro LPS treatment of macrophages derived from either young or middle-aged mice previously treated in vivo with LPS resulted in decrease in levels of inflammatory mediators, suggesting that the phenomenon of LPS tolerance [49], [50] occurs to an approximately equal degree in young vs. middle-aged mice (data not shown).
We recognize that there are several limitations to the current study. We note that there is discrepancy in the literature regarding the definition of “middle-aged” mice. While some authors report “middle-age” as early as 8 month old [51], [52], others have considered 10–12 month as the appropriate range [53], [54]. Yet other investigators have gone beyond these age ranges and used 17–18 month mice to refer to “middle-age” [55], [56]. In our study, we used 6–8 month old mice to characterize the inflammatory response associated with the upper age range for the mature adult group, which is typically approximately 6 months [57] . We suggest that our reported changes in the acute inflammatory response might best be seen as characteristic of the continuum of aging. Another limitation of our study lies with the cell type being studied. Like all mathematical models, our model is, by necessity, an abstraction of the full scope of the biology of acute inflammation. Thus, variables such as “macrophages” may represent multiple inflammatory cell types (e.g. dendritic cells). Also important is the fact that our mathematical model is based on a single compartment which obeys the mean-field approximation, i.e. the whole organism is abstracted as a “well-mixed bag”; hence, peritoneal inflammatory cells, chief among the being peritoneal macrophages, are the best analog of the “macrophage” variable in our model. Another limitation of our study is that while the mathematical model predicted an increase in cell death of leukocytes in middle-aged mice, it did not infer mechanisms by which cell death ensue in aged leukocytes. In the current study, Trypan blue stain was used to give a rough estimate of the viability of cultured macrophages and to validate the mathematical model prediction. Future studies will be aimed at understanding the mechanism of reduced macrophage viability, in concert with mathematical models of increased detail (e.g. including mechanistic details based on our prior work on modeling apoptosis [58] and the role of NO in modulating this form of programmed cell death [59]. In addition, we also note that macrophage quantification by morphology alone would yield rough estimation of the total macrophage count and that techniques such as FACS and macrophage-specific markers would be more accurate in macrophage quantification.
In conclusion, this study supports the concept of acute inflammation in older mice as a complex, dysregulated state in which imbalances in the cytokine network may lead to inappropriate, inadequate, or harmful responses. Moreover, our results demonstrate the utility of a combined in silico, in vivo, and in vitro approach to the study of acute inflammation.
Supporting Information
Fold change difference in inflammatory mediators between young and middle-aged mice. Young mice had a statistically significant (P = 0.004) higher fold change in TNF-α when compared to middle-aged mice (panel B), whereas no statistical differences were observed in the other inflammatory mediators.
(TIF)
Funding Statement
This work was supported by National Institutes of Health grant P50-GM-53789. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Nathan C (2002) Points of control in inflammation. Nature 19–26 420: 846–852. [DOI] [PubMed] [Google Scholar]
- 2. Ferrucci L, Ble A, Bandinelli S, Lauretani F, Suthers K, et al. (2004) A flame burning within. Aging Clin Exp Res 16: 240–243. [DOI] [PubMed] [Google Scholar]
- 3. Plackett TP, Boehmer ED, Faunce DE, Kovacs EJ (2004) Aging and innate immune cells. J Leukoc Biol 76: 291–299. [DOI] [PubMed] [Google Scholar]
- 4. Franceschi C, Bonafe M, Valensin S, Olivieri F, De LM, et al. (2000) Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 908 244–54 244–254. [DOI] [PubMed] [Google Scholar]
- 5. Schumer W (1988) Metabolic and immunologic alterations of sepsis in the elderly. Prog Clin Biol Res 264 223–31 223–231. [PubMed] [Google Scholar]
- 6. Saito H, Sherwood ER, Varma TK, Evers BM (2003) Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev 124: 1047–1058. [DOI] [PubMed] [Google Scholar]
- 7. Adams DO, Hamilton TA (1984) The cell biology of macrophage activation. Annu Rev Immunol 2: 283–318. [DOI] [PubMed] [Google Scholar]
- 8. Morrison DC, Ryan JL (1987) Endotoxins and disease mechanisms. Annu Rev Med 38: 417–432. [DOI] [PubMed] [Google Scholar]
- 9. Hasegawa Y, Sawada M, Ozaki N, Inagaki T, Suzumura A (2000) Increased soluble tumor necrosis factor receptor levels in the serum of elderly people. Gerontology 46: 185–188. [DOI] [PubMed] [Google Scholar]
- 10. Beharka AA, Meydani M, Wu D, Leka LS, Meydani A, et al. (2001) Interleukin-6 production does not increase with age. J Gerontol A Biol Sci Med Sci 56: B81–B88. [DOI] [PubMed] [Google Scholar]
- 11. Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S (2004) Innate immunity in aging: impact on macrophage function. Aging Cell 3: 161–167. [DOI] [PubMed] [Google Scholar]
- 12. Chow CC, Clermont G, Kumar R, Lagoa C, Tawadrous Z, et al. (2005) The acute inflammatory response in diverse shock states. Shock 24: 74–84. [DOI] [PubMed] [Google Scholar]
- 13. Kumar R, Clermont G, Vodovotz Y, Chow CC (2004) The dynamics of acute inflammation. J Theor Biol 230: 145–155. [DOI] [PubMed] [Google Scholar]
- 14. Vodovotz Y, Clermont G, Chow C, An G (2004) Mathematical models of the acute inflammatory response. Curr Opin Crit Care 10: 383–390. [DOI] [PubMed] [Google Scholar]
- 15. Vodovotz Y, Clermont G, Hunt CA, Lefering R, Bartels J, et al. (2007) Evidence-based modeling of critical illness: an initial consensus from the Society for Complexity in Acute Illness. J Crit Care 22: 77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prince JM, Levy RM, Bartels J, Baratt A, Kane JM, et al. (2006) In silico and in vivo approach to elucidate the inflammatory complexity of CD14-deficient mice. Mol Med 12: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daun S, Rubin J, Vodovotz Y, Roy A, Parker R, et al. (2008) An ensemble of models of the acute inflammatory response to bacterial lipopolysaccharide in rats: results from parameter space reduction. J Theor Biol 253: 843–853. [DOI] [PubMed] [Google Scholar]
- 18. Nieman G, Brown D, Sarkar J, Kubiak B, Ziraldo C, et al. (2012) A two-compartment mathematical model of endotoxin-induced inflammatory and physiologic alterations in swine. Crit Care Med 40: 1052–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greeneltch KM, Haudenschild CC, Keegan AD, Shi Y (2004) The opioid antagonist naltrexone blocks acute endotoxic shock by inhibiting tumor necrosis factor-alpha production. Brain Behav Immun 18: 476–484. [DOI] [PubMed] [Google Scholar]
- 20. Ocasio FM, Jiang Y, House SD, Chang SL (2004) Chronic morphine accelerates the progression of lipopolysaccharide-induced sepsis to septic shock. J Neuroimmunol 149: 90–100. [DOI] [PubMed] [Google Scholar]
- 21. Ito Y, Katagiri H, Ishii K, Kakita A, Hayashi I, et al. (2003) Effects of selective cyclooxygenase inhibitors on ischemia/reperfusion-induced hepatic microcirculatory dysfunction in mice. Eur Surg Res 35: 408–416. [DOI] [PubMed] [Google Scholar]
- 22. Zykova SN, Jenssen TG, Berdal M, Olsen R, Myklebust R, et al. (2000) Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 49: 1451–1458. [DOI] [PubMed] [Google Scholar]
- 23. Ochi H, Ishijima SA, Abe S, Tameike A, Yamaguchi H, et al. (1998) Morphological effects of itraconazole on murine macrophage. FEMS Immunol Med Microbiol 20: 181–189. [DOI] [PubMed] [Google Scholar]
- 24. Tateda K, Matsumoto T, Miyazaki S, Yamaguchi K (1996) Lipopolysaccharide-induced lethality and cytokine production in aged mice. Infect Immun 64: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gomez CR, Nomellini V, Faunce DE, Kovacs EJ (2008) Innate immunity and aging. Exp Gerontol 43: 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lagoa CE, Bartels J, Baratt A, Tseng G, Clermont G, et al. (2006) The role of initial trauma in the host’s response to injury and hemorrhage: insights from a correlation of mathematical simulations and hepatic transcriptomic analysis. Shock 26: 592–600. [DOI] [PubMed] [Google Scholar]
- 27. Torres A, Bentley T, Bartels J, Sarkar J, Barclay D, et al. (2009) Mathematical modeling of posthemorrhage inflammation in mice: studies using a novel, computer-controlled, closed-loop hemorrhage apparatus. Shock 32: 172–178. [DOI] [PubMed] [Google Scholar]
- 28. Yang Z, Norton EC, Stearns SC (2003) Longevity and health care expenditures: the real reasons older people spend more. J Gerontol B Psychol Sci Soc Sci 58: S2–10. [DOI] [PubMed] [Google Scholar]
- 29.Jeck WR, Siebold AP, Sharpless NE (2012) Review: A Meta-Analysis of GWAS Studies and Age-Associated Diseases. Aging Cell. 10.1111/j.1474–9726. [DOI] [PMC free article] [PubMed]
- 30. Franceschi C, Bonafe M, Valensin S (2000) Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine 18: 1717–1720. [DOI] [PubMed] [Google Scholar]
- 31. Schroder AK, Rink L (2003) Neutrophil immunity of the elderly. Mech Ageing Dev 124: 419–425. [DOI] [PubMed] [Google Scholar]
- 32. Ostan R, Bucci L, Capri M, Salvioli S, Scurti M, et al. (2008) Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation 15: 224–240. [DOI] [PubMed] [Google Scholar]
- 33. Gurven M, Kaplan H, Winking J, Finch C, Crimmins EM (2008) Aging and inflammation in two epidemiological worlds. J Gerontol A Biol Sci Med Sci 63: 196–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kohman RA, Crowell B, Kusnecov AW (2010) Differential sensitivity to endotoxin exposure in young and middle-age mice. Brain Behav Immun 24: 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alvarez-Rodriguez L, Lopez-Hoyos M, Munoz-Cacho P, Martinez-Taboada VM (2012) Aging is associated with circulating cytokine dysregulation. Cell Immunol 273: 124–132. [DOI] [PubMed] [Google Scholar]
- 36. Kang SC, Matsutani T, Choudhry MA, Schwacha MG, Rue LW, et al. (2004) Are the immune responses different in middle-aged and young mice following bone fracture, tissue trauma and hemorrhage? Cytokine 26: 223–230. [DOI] [PubMed] [Google Scholar]
- 37. Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5: 953–964. [DOI] [PubMed] [Google Scholar]
- 38. Fahy RJ, Doseff AI, Wewers MD (1999) Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol 163: 1755–1762. [PubMed] [Google Scholar]
- 39. Wiktor-Jedrzejczak W, Gordon S (1996) Cytokine regulation of the macrophage (M phi) system studied using the colony stimulating factor-1-deficient op/op mouse. Physiol Rev 76: 927–947. [DOI] [PubMed] [Google Scholar]
- 40. Inamizu T, Chang MP, Makinodan T (1985) Influence of age on the production and regulation of interleukin-1 in mice. Immunology 55: 447–455. [PMC free article] [PubMed] [Google Scholar]
- 41. Effros RB, Svoboda K, Walford RL (1991) Influence of age and caloric restriction on macrophage IL-6 and TNF production. Lymphokine Cytokine Res 10: 347–351. [PubMed] [Google Scholar]
- 42. Bradley SF, Vibhagool A, Kunkel SL, Kauffman CA (1989) Monokine secretion in aging and protein malnutrition. J Leukoc Biol 45: 510–514. [DOI] [PubMed] [Google Scholar]
- 43. Fagiolo U, Cossarizza A, Scala E, Fanales-Belasio E, Ortolani C, et al. (1993) Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol 23: 2375–2378. [DOI] [PubMed] [Google Scholar]
- 44. Zak DE, Aderem A (2009) Systems biology of innate immunity. Immunol Rev 227: 264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vodovotz Y, Csete M, Bartels J, Chang S, An G (2008) Translational systems biology of inflammation. PLoS Comput Biol 4: e1000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cevenini E, Bellavista E, Tieri P, Castellani G, Lescai F, et al. (2010) Systems biology and longevity: an emerging approach to identify innovative anti-aging targets and strategies. Curr Pharm Des 16: 802–813. [DOI] [PubMed] [Google Scholar]
- 47. Turnbull IR, Clark AT, Stromberg PE, Dixon DJ, Woolsey CA, et al. (2009) Effects of aging on the immunopathologic response to sepsis. Crit Care Med 37: 1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Alvarez E, Machado A, Sobrino F, Santa MC (1996) Nitric oxide and superoxide anion production decrease with age in resident and activated rat peritoneal macrophages. Cell Immunol 169: 152–155. [DOI] [PubMed] [Google Scholar]
- 49. West MA, Heagy W (2002) Endotoxin tolerance: A review. Crit Care Med 30: S64–S73. [PubMed] [Google Scholar]
- 50. Cavaillon JM, Adib-Conquy M (2006) Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care 10: 233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang H, Li C, Wang H, Mei F, Liu Z, et al. (2013) Cuprizone-induced demyelination in mice: age-related vulnerability and exploratory behavior deficit. Neurosci Bull 29: 251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. L’Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, et al. (2013) Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/beta-catenin dysregulation. J Neurosci 33: 1462–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang RL, Chopp M, Roberts C, Wei M, Wang X, et al. (2012) Sildenafil enhances neurogenesis and oligodendrogenesis in ischemic brain of middle-aged mouse. PLoS One 7: e48141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Aujard F, Herzog ED, Block GD (2001) Circadian rhythms in firing rate of individual suprachiasmatic nucleus neurons from adult and middle-aged mice. Neuroscience 106: 255–261. [DOI] [PubMed] [Google Scholar]
- 55.Sinha-Hikim I, Sinha-Hikim AP, Parveen M, Shen R, Goswami R, et al.. (2013) Long-Term Supplementation With a Cystine-Based Antioxidant Delays Loss of Muscle Mass in Aging. J Gerontol A Biol Sci Med Sci. [DOI] [PMC free article] [PubMed]
- 56. Yang MJ, Sim S, Jeon JH, Jeong E, Kim HC, et al. (2013) Mitral and tufted cells are potential cellular targets of nitration in the olfactory bulb of aged mice. PLoS One 8: e59673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Flurkey K, Currer JM, Harrison DE (2007) The Mouse in Aging Research. In: Fox JG, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, et al. The Mouse in Biomedical Research 2nd Edition. Burlington: American College Laboratory Animal Medicine (Elsevier). 637–672.
- 58. Bagci EZ, Vodovotz Y, Billiar TR, Ermentrout GB, Bahar I (2006) Bistability in apoptosis: roles of bax, bcl-2, and mitochondrial permeability transition pores. Biophys J 90: 1546–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bagci EZ, Vodovotz Y, Billiar TR, Ermentrout B, Bahar I (2008) Computational insights on the competing effects of nitric oxide in regulating apoptosis. PLoS One 3: e2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fold change difference in inflammatory mediators between young and middle-aged mice. Young mice had a statistically significant (P = 0.004) higher fold change in TNF-α when compared to middle-aged mice (panel B), whereas no statistical differences were observed in the other inflammatory mediators.
(TIF)