Abstract
Poria cocos is a medicinal mushroom that is widely used in traditional Asian medicine. Here, we show that a characterized mixture of triterpenes extracted from P. cocos (PTE) and three purified triterpenes: pachymic acid (PA), dehydropachymic acid (DPA) and polyporenic acid C (PPAC) suppress the proliferation of the human pancreatic cancer cell lines Panc-1, MiaPaca-2, AsPc-1 and BxPc-3. Moreover, the most effective compound, PA, only slightly affects the proliferation of HPDE-6 normal pancreatic duct epithelial cells. The anti-proliferative effects of PTE on BxPc-3 cells are mediated by the cell cycle arrest at G0/G1 phase. DNA microarray analysis demonstrated that PTE significantly downregulates the expression of KRAS and matrix metalloproteinase-7 (MMP-7) in BxPc-3 cells. In addition, PTE and PA suppress the invasive behavior of BxPc-3 cells. The inhibition of invasiveness by PTE and PA was associated with the reduction of MMP-7 at the protein level and the role of MMP-7 further confirmed by the gene silencing of MMP-7 which also suppressed the invasiveness of BxPc-3 cells. In conclusion, triterpenes from P. cocos demonstrate anticancer and anti-invasive effects on human pancreatic cancer cells and can be considered as new therapeutic agents in the treatment of pancreatic cancer.
Keywords: triterpenes, Poria cocos, pancreatic cancer, pachymic acid, MMP-7
Introduction
Pancreatic cancer is the fourth leading cause of cancer-related deaths in the United States. As one of the most aggressive human cancers, the 5-year survival rate of pancreatic ductal adenocarcinoma (PDAC) is <5% (1). Recent studies suggested that bioactive compounds from mushrooms could protect against multiple myeloma, breast and skin cancer (2–5). Poria cocos (also known as Wolfiporia extensa) is a medicinal mushroom in the Polyporaceae family that grows in pine trees and its sclerotium is widely used in traditional Asian medicine for its sedative, diuretic, digestive and tonic effects (6–8). Although the anticancer activity of polysaccharides extracted from P. cocos is associated with the stimulation of immune response and these polysaccharides significantly enhance immunopotentiation (9), triterpenes isolated from P. cocos have a direct inhibitory effect on cancer cells through a variety of mechanisms including inhibition of cell proliferation, induction of apoptosis and suppression of invasive behavior (10–16). However the effect of triterpenes from P. cocos against pancreatic cancer remains to be evaluated and the mechanism determined.
In the present study, we evaluated the effects of a triterpene mixture extracted from P. cocos (PTE) and three purified triterpenes: pachymic acid (PA), dehydropachymic acid (DPA) and polyporenic acid C (PPAC), on growth and invasive behavior of human pancreatic cancer cell lines Panc-1, MiaPaca-2, BxPc-3 and AsPc-1 and normal pancreatic duct epithelial cell line HPDE-6. PTE as well as PA, DPA and PPAC inhibit growth of pancreatic cancer cells and PTE and PA significantly suppress invasive behavior of BxPc-3 cells by inhibiting expression of MMP-7. Taken together, our results indicate that triterpenes from P. cocos may be potentially exploited for the use in pancreatic cancer intervention.
Materials and methods
Reagents
Dried sclerotium of P. cocos from Fujian, P.R. China was provided by Professor Zhonglin Yang (China Pharmaceutical University, Nanjing, P.R. China). It was authenticated by School of Traditional Chinese Medicine at China Pharmaceutical University. Voucher specimens were deposited at State Key Laboratory of Natural Medicines, China Pharmaceutical University. DMSO was purchased from Sigma (St. Louis, MO). All other chemicals and reagents were of analytical grade. Anti-MMP-7 and anti-β-actin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA).
Extraction and purification
Pulverized sclerotium of P. cocos (2.0 kg) was extracted three times with 95% ethanol (10 l) under reflux for 3 h at room temperature. The ethanol solution was combined and evaporated in vacuum to give a crude extract (38 g). The crude extract was mixed with silica gel G (size: 200–300 mesh) and fractionated on silica column chromatography by gradient elution using petroleum ether and ethyl acetate (100:0→75:15→1:1). Fractions were collected, combined and subjected to further chromatography on a silica gel→H (size: 60–120 mesh) column by step gradients of cyclohexane-ethyl acetate (100:0→75:15). The collected fractions were combined on the basis of their thin-layer chromatography (TLC) characteristics to give three pooled fractions: pooled extracts A, B and C (PEA, PEB and PEC), listed in increasing order of polarity. Part of the PEB (PTE) was subjected to high-performance preparative liquid chromatography (Daojing, Japan, model: SPD-20A), from which three pure compounds, PPAC, DPA and PA (Fig. 1) were isolated with isocratic elution of CH3OH-H2O (85:15), with trifluoroacetic acid added at 0.05%. Quantification of HPLC analysis demonstrated that PTE contains 55.7% PA, 31.7% DPA and 4.1% PPAC. Identification of PA, DPA and PPAC was conducted by comparison of their physical and spectroscopic data (1H-, 13C-NMR and MS) with the corresponding compounds reported in the literatures. PTE, PA, DPA and PPAC were dissolved in DMSO at a concentration of 50 mg/ml and 50 mM, respectively then stored at −20°C.
Figure 1.
Structure of lanostane-type triterpenoids isolated from P. cocos.
Cell culture
The human pancreatic cancer cell lines Panc-1, MiaPaca-2, BxPc-3 and AsPc-1 were obtained from ATCC (Manassas, VA). Panc-1 cells were maintained in Dulbecco’s modified Eagle’s medium containing penicillin (50 U/ml), streptomycin (50 U/ml) and 10% fetal bovine serum (FBS). MiaPaca-2 cells were maintained in Dulbecco’s modified Eagle’s medium containing penicillin (50 U/ml), streptomycin (50 U/ml), 10% FBS and 2.5% horse serum (HS). BxPC-3 and AsPC-1 cells were maintained in RPMI-1640 medium containing penicillin (50 U/ml), streptomycin (50 U/ml) and 10% FBS. Media came from ATCC. Supplements, FBS and HS were obtained from Gibco BRL (Grand Island, NY). The normal human pancreatic duct epithelial cell line HPDE-6 was a generous gift from Dr Ming-Sound Tsao (University of Toronto, Toronto, Ontario, Canada). HPDE-6 cells were routinely cultured in keratinocyte serum-free (KSF) medium supplemented by epidermal growth factor and bovine pituitary extract. Medium and supplements came from Gibco BRL.
Cell proliferation and cell viability
Human pancreatic cancer cells and normal human pancreatic duct epithelial cell line were treated with indicated concentrations of PTE, PA, DPA or PPAC for 24–72 h and cell proliferation determined as described (17). Cell viability was determined after incubation with PTE, PA, DPA or PPAC for 24 h by staining with trypan blue as described (18). Data are the mean ± SD from three independent experiments.
Cell cycle analysis and invasive behavior assays
Cell cycle analysis of BxPc-3 cells incubated in the presence of PTE (0–5.0 μg/ml) for 24 h was evaluated as described (19). Cell invasion of BxPc-3 cells treated with PTE (0–5.0 μg/ml), PA (0–5.0 μM) or transfected with MMP-7 or control siRNA were performed as described (20). Data points represent the mean ± SD of three individual filters within one representative experiment repeated at least twice.
DNA microarrays
BxPc-3 cells were treated with PTE (0, 5.0 μg/ml) for 24 h and RNA isolated with RNeasy® Mini Kit (Qiagen, Valencia, CA). RNA quality was monitored and quantified using the Qubit® 2.0 Fluorometer (Invitrogen, Carlsbad, CA). Reverse transcription was performed with High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) using 2.0 μg total RNA. PCR analysis was performed on TaqMan® Array Human Pancreatic Adenocarcinoma and 7900HT Fast Real-Time PCR System according to the manufacturer’s protocol (Applied Biosystems). Analysis of the relative quantity gene expression (RQ) data was normalized by HPRT1 expression and was performed using the 2-ΔΔCt method (21).
Western blot analysis
BxPc-3 cells were treated with PTE (0–10 μg/ml), PA (0–10 μM), DPA (0–10 μM) or PPAC (0–10 μM) for 24 h. Whole cell extracts isolated from cells were prepared and western blot analysis with MMP-7 antibody was performed as previously described (17). Western blots were quantified with HP-Scanjet 550c and analyzed by UN-SCAN-IT software (Silk Scientific, Orem, UT).
siRNA transfection
BxPc-3 cells were transfected with human MMP-7 siRNA or control siRNA-A using siRNA Reagent System according to the manufacturer’s instructions at a final concentration of 60 nM (Santa Cruz, CA). After 48 h of transfection, the cells were harvested and MMP-7 knockdown was verified by western blot analysis.
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical comparison between groups of data was carried out using ANOVA. P<0.05 was considered to be significant.
Results
Triterpenes from P. cocos suppress proliferation of human pancreatic cancer cell lines
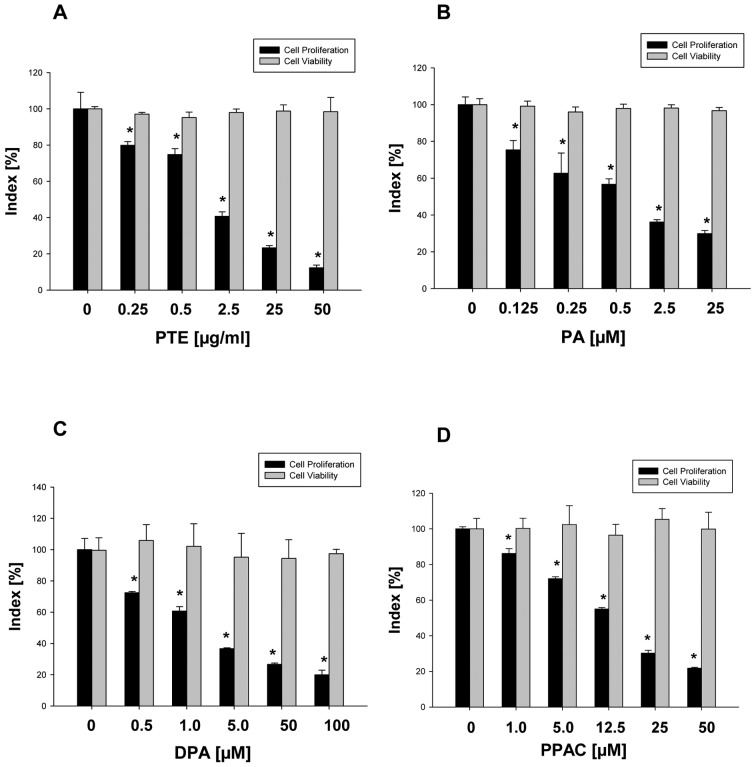
As previously demonstrated P. cocos triterpenes: PA, DPA and PPAC inhibited growth of human breast cancer, lung cancer and prostate cancer cells (11–13,22). To evaluate whether these triterpenes also affect growth of different human pancreatic cancer cell lines, Panc-1, MiaPaca-2, AsPc-1 and BxPc-3 cells were treated with PTE (0–80 μg/ml) for 24 and 48 h and the proliferation determined as described in Materials and methods. PTE suppresses proliferation of Panc-1 (IC50-24 h = 28.3 μg/ml, IC50-48 h = 24.5 μg/ml), MiaPaca-2 (IC50-24 h = 29.4 μg/ml, IC50-48 h = 23.0 μg/ml), AsPc-1 (IC50-24 h = 13.7 μg/ml, IC50-48 h = 11.3 μg/ml) and BxPc-3 cells (IC50-24 h = 1.2 μg/ml, IC50-48 h = 1.0 μg/ml). Therefore, BxPc-3 cells are most sensitive to the PTE as well as PA, DPA and PPAC treatment (Fig. 2). PA demonstrates the strongest activity against BxPc-3 cells with IC50 0.26 μM (24 h), IC50 0.29 μM (48 h), IC50 0.42 μM (72 h) but only partially affects proliferation of normal human pancreatic duct epithelial cell line HPDE-6 with IC50 41.6 μM (24 h), IC50 34.9 μM (48 h) and IC50 76.0 μM (72 h). Moreover, PTE, PA, DPA and PPAC treatment do not affect viability of BxPc-3 cells (Fig. 2), suggesting cytostatic effect of these P. cocos triterpenes on BxPc-3 cells.
Figure 2.
Effect of PTE, PA, DPA and PPAC on growth and viability of BxPc-3 cells. BxPc-3 cells were treated with (A) PTE (0–50 μg/ml), (B) PA (0–25 μM), (C) DPA (0–100 μM) or (D) PPAC (0–50 μM) for 24 h. Cell proliferation and viability were determined as described in Materials and methods. Each bar represents the mean ± SD of three experiments. Similar results were obtained in three independent experiments. Statistical analysis by ANOVA. *P<0.05.
Effect of PTE and PA on the cell cycle and invasive behavior of BxPc-3 cells
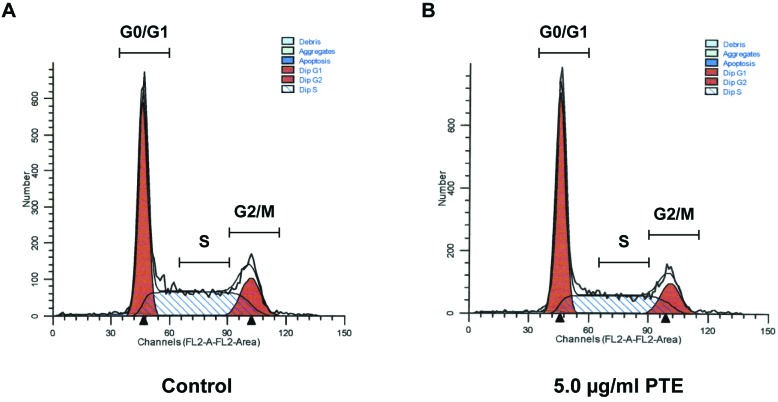
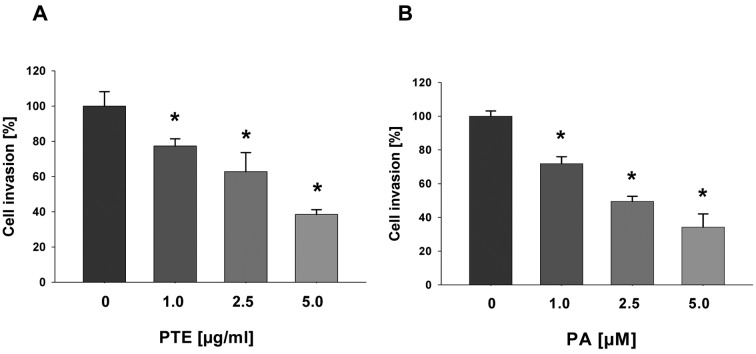
In order to evaluate whether the cytostatic effect of PTE on pancreatic cancer cells is associated with the cell cycle arrest, BxPc-3 cells were treated with PTE as described in Materials and methods. Cell cycle analysis revealed that PTE induces significant cell cycle arrest at G0/G1 phase from 44.15% (control) to 47.66% (5.0 μg/ml) (Fig. 3 and Table I). To evaluate whether PTE and PA suppress invasive behavior of pancreatic cancer cells, BxPc-3 cells were treated with PTE (0–5.0 μg/ml) and PA (0–5.0 μM) for 24 h and cell invasion was determined as described Materials and methods. As seen in Fig. 4, both PTE and PA markedly inhibit cell invasion through Matrigel. Together, our data indicate that triterpenes from P. cocos not only inhibit cell proliferation through cell cycle arrest at G0/G1 phase but also inhibit invasive behavior of BxPc-3 cells.
Figure 3.
Effect of PTE on cell cycle distribution. BxPc-3 cells were treated with (A) vehicle or (B) PTE (5.0 μg/ml) for 24 h. Cell cycle analysis was performed as described in Materials and methods. Representative image is shown.
Table I.
Effect of PTE on cell cycle distribution.
| PTE (μg/ml) | G0/G1 | S | G2/M |
|---|---|---|---|
| 0 | 44.15±1.06 | 40.98±1.01 | 14.87±0.10 |
| 2.5 | 44.79±0.20 | 40.54±1.18 | 14.67±0.98 |
| 5.0 | 47.66±1.57a | 38.63±1.46 | 13.70±1.04 |
Cell cycle analysis was performed as described in Materials and methods. Cell cycle distribution G0/G1, S and G2/M phase in %. The data are mean ± SD from three experiments. Statistical significance;
P<0.05.
Figure 4.
Effect of PTE and PA on invasive behavior of BxPC-3 cells. Invasion of BxPc-3 cells through Matrigel was determined after 24 h of incubation in the presence of (A) PTE (0–5.0 μg/ml) or (B) PA (0–5.0 μM) as described in Materials and methods. Each bar represents the mean ± SD of three experiments. Statistical analysis by ANOVA. *P<0.05.
Triterpenes from P. cocos downregulate MMP-7 expression in BxPc-3 cell line
To identify possible molecular targets of triterpenes from P. cocos, we treated BxPc-3 cells with PTE and performed DNA-microarray analysis using Array Human Pancreatic Adenocarcinoma genes as described in Materials and methods. In addition to KRAS (0.74±0.02, P<0.05 vs. control), PTE also markedly suppresses expression of MMP-7 (0.67±0.14, P<0.05 vs control) (Table II). Since MMP-7 is significantly overexpressed in pancreatic cancer samples when compared to pseudotumoral chronic pancreatitis (23), we further determined if PTE, PA, DPA and PPAC inhibit MMP-7 at the translation level as well. Western blot analysis shows that in addition to PTE and PA, PPAC inhibits protein expression of MMP-7 in BxPc-3 cells, whereas DPA has no effect (Fig. 5).
Table II.
Effect of PTE on the expression of human pancreatic adenocarcinoma genes.
| Gene | Description | Fold change |
|---|---|---|
| BIRC5 | Baculoviral IAP repeat-containing 5 (survivin) | 0.85±0.12 |
| CCNB1 | Cyclin B1 | 0.87±0.03 |
| HSP90AA1 | Heat shock protein 90 kDa α (cytosolic), class A member 1 | 0.89±0.02 |
| IL-6 | Interleukin-6 | 0.86±0.04 |
| KRAS | V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | 0.86±0.04a |
| MMP-7 | Matrix metalloproteinase-7 | 0.67±0.14a |
| MMP-9 | Matrix metalloproteinase-9 | 0.79±0.08 |
| RELB | Transcription factor RelB | 0.79±0.10 |
| TGFB3 | Transforming growth factor β-3 | 0.82±0.02 |
DNA-microarray analysis was performed as described in Materials and methods. BxPc-3 cells were treated with PTE (0 and 5.0 μg/ml) for 24 h. Data are the means ± SD (n=3). Fold change PTE vs. control. Statistical significance.
P<0.05.
Figure 5.
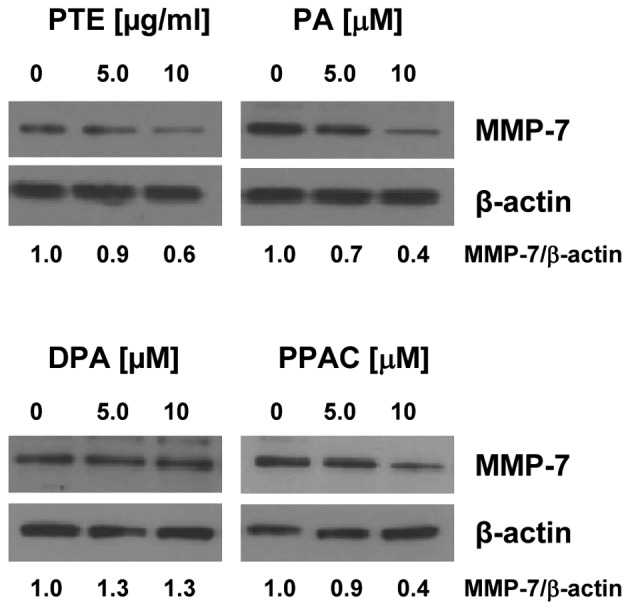
Effect of PTE, PA, DPA and PPAC on the expression of MMP-7 in BxPc-3 cells. BxPc-3 cells were treated with PTE (0–5.0 μg/ml), PA (0–5.0 μM), DPA (0–5.0 μM) or PPAC (0–5.0 μM) for 24 h and the expression of MMP-7 was evaluated by western blot analysis as described in Materials and methods. Similar results were obtained in at least two additional experiments.
Gene silencing of MMP-7 inhibits invasive behavior of BxPc-3 cell line
To determine whether invasive behavior of BxPc-3 cells is associated with the expression of MMP-7, we silenced MMP-7 with siRNA as described in Materials and methods. As shown in Fig. 6, knockdown of MMP-7 inhibits cell invasion by >50% in comparison with negative control cells transfected with scrambled siRNA. These results further confirm that MMP-7 greatly contributes to the invasive behavior and its deletion limits the invasive capacity of BxPc-3 cells.
Figure 6.

MMP-7 gene silencing suppresses invasiveness of BxPc-3 cells. BxPc-3 cells were transfected with scrambled siRNA (siRNA-A) or MMP-7 siRNA as described in Materials and methods. (A) Western blot analysis of MMP-7 was evaluated as described in Fig. 5. (B) Invasion of BxPc-3 cells through Matrigel was determined as described in Fig. 4. Each bar represents the mean ± SD of three experiments. Statistical analysis by ANOVA. *P<0.05.
Discussion
The present study demonstrates that triterpenes extracted from P. cocos suppress growth and invasive behavior of human pancreatic cancer cells and only slightly affecting normal pancreatic cells. Interestingly, invasive BxPc-3 cells are the most sensitive to the treatment of a characterized triterpene mixture (PTE) as well as purified triterpenes PA, DPA and PPAC (Fig. 1). Here we show that PA is the most potent triterpene responsible for the anticancer activity of the PTE mixture (containing 55.7% PA, 31.7% DPA and 4.1% PPAC) because PA inhibits proliferation of BxPc-3 cells with the IC50 value (0.26 μM) when compared to DPA (1.02 μM) and PPAC (21.76 μM).
To elucidate molecular mechanisms related to the inhibition of invasiveness of pancreatic cancer cells we used BxPc-3 cells. This cell line is the only Smad4-deficient among the four pancreatic cancer cell lines we investigated (24,25). Moreover, the loss of Smad4 is associated with a higher likelihood of metastasis, poor outcome following surgical resection (26) and predict a worse prognosis in patients with pancreatic cancer (27). Here we demonstrate, for the first time, that both PTE and PA significantly suppress invasive behavior of BxPc-3 cells. Evidently, this suppression is correlated with downregulation of MMP-7 expression. MMP-7 is overexpressed in pancreatic cancer (28), correlates with decreased survival (29,30) and contributes to cancer progression by supporting tumor size and metastasis in vivo(31). Therefore, MMP-7 is a suitable target involved in disease progression and the downregulation of MMP-7 expression by PA may be a useful strategy for pancreatic cancer metastasis intervention. Interestingly, PA also suppressed invasiveness through the inhibition of expression another matrix metalloproteinase, MMP-9 in breast cancer cells (12).
As recently demonstrated, PA was detected in urine and plasma of rats feed P. cocos(32), indicating that PA can be easily absorbed into blood. Therefore, the bioavailability of PA further promotes employment of PA or other P. cocos triterpenes for the treatment of different cancers including pancreatic cancer.
In conclusion, our study provides new evidence that the mixture or purified triterpenes extracted from mushroom P. cocos inhibits growth and invasiveness of pancreatic cancer cells. Moreover, we identified MMP-7 as a target of P. cocos triterpenes in pancreatic cancer cells. Further studies are in progress to investigate the exact mechanism of the inhibition of MMP-7 expression and the evaluation of the anticancer and anti-metastatic activity of PA in vivo.
Acknowledgments
We thank Dr Anita Thyagarajan-Sahu for her technical assistance with the cell cycle analysis, Dr Ming-Sound Tsao for kindly providing the normal human pancreatic duct epithelial cell line HPDE-6, Dr Yaqiong Wang, Professor Zhonglin Yang for kindly providing the dried sclerotium of P. cocos, Dr Yaqiong Wang and Professor Ping Li for their helpful suggestions on extraction, purification and identification of triterpenes from P. cocos. This study was supported by research grants from China Scholarship Council and EcoNugenics, Inc., Santa Rosa, CA, USA. One of the authors, I. Eliaz, acknowledges his interest as the formulator and owner of EcoNugenics, Inc.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Rhee YH, Jeong SJ, Lee HJ, et al. Inhibition of STAT3 signaling and induction of SHP1 mediate antiangiogenic and antitumor activities of ergosterol peroxide in U266 multiple myeloma cells. BMC Cancer. 2012;12:28. doi: 10.1186/1471-2407-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CC, Yang HL, Way TD, et al. Inhibition of cell growth and induction of apoptosis by Antrodia camphorata in HER-2/neuoverexpressing breast cancer cells through the induction of ROS, depletion of HER-2/neu and disruption of the PI3K/Akt signaling pathway. Evid Based Complement Alternat Med. 2012;2012:702857. doi: 10.1155/2012/702857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuo YC, Lai CS, Tsai CY, Nagabhushanam K, Ho CT, Pan MH. Inotilone suppresses phorbol ester-induced inflammation and tumor promotion in mouse skin. Mol Nutr Food Res. 2012;56:1324–1332. doi: 10.1002/mnfr.201200073. [DOI] [PubMed] [Google Scholar]
- 5.Torkelson CJ, Sweet E, Martzen MR, et al. Phase 1 clinical trial of Trametes versicolor in women with breast cancer. ISRN Oncol. 2012;2012:251632. doi: 10.5402/2012/251632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rios JL. Chemical constituents and pharmacological properties of Poria cocos. Planta Med. 2011;77:681–691. doi: 10.1055/s-0030-1270823. [DOI] [PubMed] [Google Scholar]
- 7.Lee SM, Lee YJ, Yoon JJ, Kang DG, Lee HS. Effect of Poria cocos on hypertonic stress-induced water channel expression and apoptosis in renal collecting duct cells. J Ethnopharmacol. 2012;141:368–376. doi: 10.1016/j.jep.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 8.Zhao YY, Feng YL, Du X, Xi ZH, Cheng XL, Wei F. Diuretic activity of the ethanol and aqueous extracts of the surface layer of Poria cocos in rat. J Ethnopharmacol. 2012;144:775–778. doi: 10.1016/j.jep.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Zhang L, Cheung PC. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated beta-(1→3)-d-glucan from Poria cocos. Int Immunopharmacol. 2010;10:398–405. doi: 10.1016/j.intimp.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 10.Kikuchi T, Uchiyama E, Ukiya M, et al. Cytotoxic and apoptosis-inducing activities of triterpene acids from Poria cocos. J Nat Prod. 2011;74:137–144. doi: 10.1021/np100402b. [DOI] [PubMed] [Google Scholar]
- 11.Ling H, Zhou L, Jia X, Gapter LA, Agarwal R, Ng KY. Polyporenic acid C induces caspase-8-mediated apoptosis in human lung cancer A549 cells. Mol Carcinog. 2009;48:498–507. doi: 10.1002/mc.20487. [DOI] [PubMed] [Google Scholar]
- 12.Ling H, Zhang Y, Ng KY, Chew EH. Pachymic acid impairs breast cancer cell invasion by suppressing nuclear factor-kappaB-dependent matrix metalloproteinase-9 expression. Breast Cancer Res Treat. 2011;126:609–620. doi: 10.1007/s10549-010-0929-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhou L, Zhang Y, Gapter LA, Ling H, Agarwal R, Ng KY. Cytotoxic and anti-oxidant activities of lanostane-type triterpenes isolated from Poria cocos. Chem Pharm Bull. 2008;56:1459–1462. doi: 10.1248/cpb.56.1459. [DOI] [PubMed] [Google Scholar]
- 14.Mizushina Y, Akihisa T, Ukiya M, et al. A novel DNA topoisomerase inhibitor: dehydroebriconic acid, one of the lanostane-type triterpene acids from Poria cocos. Cancer Sci. 2004;95:354–360. doi: 10.1111/j.1349-7006.2004.tb03215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong R, Shen MH, Xie XH, Ruan SM. Inhibition of breast cancer metastasis via PITPNM3 by pachymic acid. Asian Pac J Cancer Prev. 2012;13:1877–1880. doi: 10.7314/apjcp.2012.13.5.1877. [DOI] [PubMed] [Google Scholar]
- 16.Ling H, Jia X, Zhang Y, et al. Pachymic acid inhibits cell growth and modulates arachidonic acid metabolism in nonsmall cell lung cancer A549 cells. Mol Carcinog. 2010;49:271–282. doi: 10.1002/mc.20597. [DOI] [PubMed] [Google Scholar]
- 17.Jiang J, Slivova V, Harvey K, Valachovicova T, Sliva D. Ganoderma lucidum suppresses growth of breast cancer cells through the inhibition of Akt/NF-kappaB signaling. Nutr Cancer. 2004;49:209–216. doi: 10.1207/s15327914nc4902_13. [DOI] [PubMed] [Google Scholar]
- 18.Sliva D, Jedinak A, Kawasaki J, Harvey K, Slivova V. Phellinus linteus suppresses growth, angiogenesis and invasive behaviour of breast cancer cells through the inhibition of AKT signalling. Br J Cancer. 2008;98:1348–1356. doi: 10.1038/sj.bjc.6604319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sliva D, Harvey K, Mason R, Lloyd F, Jr, English D. Effect of phosphatidic acid on human breast cancer cells exposed to doxorubicin. Cancer Invest. 2001;19:783–790. doi: 10.1081/cnv-100107739. [DOI] [PubMed] [Google Scholar]
- 20.Lloyd FP, Jr, Slivova V, Valachovicova T, Sliva D. Aspirin inhibits highly invasive prostate cancer cells. Int J Oncol. 2003;23:1277–1283. [PubMed] [Google Scholar]
- 21.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 22.Gapter L, Wang Z, Glinski J, Ng KY. Induction of apoptosis in prostate cancer cells by pachymic acid from Poria cocos. Biochem Biophys Res Commun. 2005;332:1153–1161. doi: 10.1016/j.bbrc.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 23.Bournet B, Pointreau A, Souque A, et al. Gene expression signature of advanced pancreatic ductal adenocarcinoma using low density array on endoscopic ultrasound-guided fine needle aspiration samples. Pancreatology. 2012;12:27–34. doi: 10.1016/j.pan.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res. 2011;17:483–493. doi: 10.1158/1078-0432.CCR-10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hlavaty J, Petznek H, Holzmuller H, et al. Evaluation of a gene-directed enzyme-product therapy (GDEPT) in human pancreatic tumor cells and their use as in vivo models for pancreatic cancer. PLoS One. 2012;7:e40611. doi: 10.1371/journal.pone.0040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tascilar M, Skinner HG, Rosty C, et al. The SMAD4 protein and prognosis of pancreatic ductal adenocarcinoma. Clin Cancer Res. 2001;7:4115–4121. [PubMed] [Google Scholar]
- 27.Singh P, Srinivasan R, Wig JD. SMAD4 genetic alterations predict a worse prognosis in patients with pancreatic ductal adenocarcinoma. Pancreas. 2012;41:541–546. doi: 10.1097/MPA.0b013e318247d6af. [DOI] [PubMed] [Google Scholar]
- 28.Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437–1444. doi: 10.1172/JCI15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fukushima H, Yamamoto H, Itoh F, et al. Association of matrilysin mRNA expression with K-ras mutations and progression in pancreatic ductal adenocarcinomas. Carcinogenesis. 2001;22:1049–1052. doi: 10.1093/carcin/22.7.1049. [DOI] [PubMed] [Google Scholar]
- 30.Jones LE. Comprehensive analysis of matrix metalloproteinase and tissue inhibitor expression in pancreatic cancer: increased expression of matrix metalloproteinase-7 predicts poor survival. Clin Cancer Res. 2004;10:2832–2845. doi: 10.1158/1078-0432.ccr-1157-03. [DOI] [PubMed] [Google Scholar]
- 31.Fukuda A, Wang SC, Morris JP, IV, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19:441–455. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ling Y, Chen M, Wang K, et al. Systematic screening and characterization of the major bioactive components of Poria cocos and their metabolites in rats by LC-ESI-MS(n) Biomed Chromatogr. 2012;26:1109–1117. doi: 10.1002/bmc.1756. [DOI] [PubMed] [Google Scholar]