Abstract
Dynamic contrast-enhanced magnetic resonance imaging (DCE MRI) of the breast is a routinely used imaging method which is highly sensitive for detecting breast malignancy. Specificity, though, remains suboptimal. Dynamic susceptibility contrast magnetic resonance imaging (DSC MRI), an alternative dynamic contrast imaging technique, evaluates perfusion-related parameters unique from DCE MRI. Previous work has shown that the combination of DSC MRI with DCE MRI can improve diagnostic specificity, though an additional administration of intravenous contrast is required. Dual-echo MRI can measure both T1W DCE MRI and T2*W DSC MRI parameters with a single contrast bolus, but has not been previously implemented in breast imaging. We have developed a dual-echo gradient-echo sequence to perform such simultaneous measurements in the breast, and use it to calculate the semi-quantitative T1W and T2*W related parameters such as peak enhancement ratio, time of maximal enhancement, regional blood flow, and regional blood volume in 20 malignant lesions and 10 benign fibroadenomas in 38 patients. Imaging parameters were compared to surgical or biopsy obtained tissue samples. Receiver operating characteristic (ROC) curves and area under the ROC curves were calculated for each parameter and combination of parameters. The time of maximal enhancement derived from DCE MRI had a 90% sensitivity and 69% specificity for predicting malignancy. When combined with DSC MRI derived regional blood flow and volume parameters, sensitivity remained unchanged at 90% but specificity increased to 80%. In conclusion, we show that dual-echo MRI with a single administration of contrast agent can simultaneously measure both T1W and T2*W related perfusion and kinetic parameters in the breast and the combination of DCE MRI and DSC MRI parameters improves the diagnostic performance of breast MRI to differentiate breast cancer from benign fibroadenomas.
Introduction
Magnetic resonance imaging (MRI) has become an important technique for breast cancer detection, diagnosis, and staging [1]. When lesion morphology is combined with the dynamic analysis of contrast kinetics within breast lesions, the overall sensitivity of MRI is nearly 90%, and specificity varies between 67% and 72% [2], [3]. Compared to all other imaging techniques (including ultrasonography and mammography), the negative predictive value of MRI remains the highest of all modalities [4], [5].
Conventional dynamic contrast enhanced MRI (DCE MRI) is the most widely used and clinically validated technique for breast cancer MRI. It not only provides morphological information, but also typically uses high spatial resolution to estimate T1W-related contrast uptake parameters. Despite high sensitivity, diagnostic specificity remains unsatisfactory [6], [7]. An alternative dynamic contrast technique, known as dynamic susceptibility-contrast MRI (DSC MRI) uses high temporal resolution to obtain perfusion-related parameters based on T2*measurements, such as relative regional blood volume (rBV)and relative regional blood flow (rBF) [8]–[10]. Perfusion-related parameters can differentiate malignant from benign lesions [8]–[10]. The combination of conventional DCE MRI and DSC MRI with two administrations of contrast agent (CA) has demonstrated the capability to substantially improve the diagnostic specificity of breast MRI [7], [11].
Using dual-echo MRI, and with a single administration of contrast agent, T1W and T2*W related measurements can be simultaneously acquired, with the first echo acquiring T1W (DCE MRI) data and the second echo (6.3 ms later) acquiring T2*W data (DSC MRI)[12]–[14]. To date, dual-echo MRI has not been performed in the breast. We have developed a dual gradient echo (GRE) sequence and have used it to evaluate both T1W and T2*W related parameters in differentiating breast cancer from the most common benign breast mass, fibroadenoma.
Methods
Ethics Statement
The study was approved by the ethics committee of the First Affiliated Hospital of Zhejiang Chinese Medical University, Hangzhou, China, and performed in accordance with the ethical guidelines of the Declaration of Helsinki. After a throughout explanation of the study to the patient, written informed consent was obtained from 37 patients and 1 guardian on the behalf of the minor.
Patients
Between May 2011 to March 2012, 46 patients with suspected breast cancer based on mammography and the BIRADS category being 4 or 5 underwent dynamic contrast enhanced MRI and dual-echo dynamic contrast enhanced MRI examination. Of the 46 patients, 38 patients had subsequent surgery or biopsy with pathologic correlation (mean age: 45 years, range: 15–65 years).There were 45 lesions evaluated in 38 patients, with 5 patients having two lesions and one patient having three lesions. In patients with multiple lesions, only the largest lesion was analyzed in this study. All patients had normal renal function (eGFR>60 ml/min/1.73 m2).
Dual-Echo based DCE MRI and DSC MRI
A dual-echo based gradient echo sequence has been implemented to acquire both DCE MR and DSC MR images simultaneously with the 1st echo as T1W and the 2nd echo as T2*W. The signal intensity of a GRE can be given by
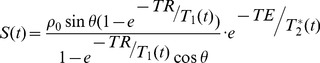 |
(1) |
Where, ρ0 denotes the spin density, θ denotes the flip angle, TR denotes the repetition time, TE denotes echo time, T1(t) denotes the longitude relaxation time at time t. The equation clearly shows that the signal of DCE MR and DSC MR images are determined by not only T1 component, but also the T2* effect. That is to say, there are errors associated with the commonly used CK model for perfusion which assumes a constant T2*, and the commonly used CK model for permeability which assume a constant T1. Simultaneously acquired T1W and T2W* data make it possible to correct the errors.
To take into account the T2* effect in permeability analysis, we first calculate T2* map by taking the advantage of the dual echo pulse sequence using Equation (2),
| (2) |
Where Secho1(t) and Secho2(t) are signal intensities from the 1st echo (TE1) and 2nd echo (TE2), respectively. To exclude T2* dependency in each pixel, the signal intensity S(t) of Equation (1) is divided by the corresponding T2* exponential component thereby obtaining only T1-dependent signal intensity ST1(t) for T1W DCE MR imaging,
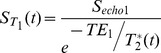 |
(3) |
To take into account the T1 effect on perfusion, we take the advantage of the dual-echo sequence to calculate only T2*-dependent signal intensity ST2*(t) using Equation (4),
| (4) |
MR Imaging Technique
All imaging was performed on a 3T whole-body MRI scanner (Verio, Siemens, Germany) with a sixteen-channel phased-array breast coil. Data were obtained for routine clinical diagnosis, which included routine clinical semi-quantitative DCE MRI in addition to the experimental dual-echo DCE/DSC MRI sequence.
Before injection, the following sequences were acquired: transverse Turbo Inversion Recovery Magnitude (TIRM) (TR = 4000 ms, TE = 70 ms, slice thickness = 4 mm, FOV = 340 mm×340 mm, average = 2, number of slices = 34, acquisition time = 168 s) and transverse diffusion weighted imaging (TR = 7000 ms, TE = 85 ms, slice thickness = 4 mm, FOV = 340 mm×340 mm, average = 3, number of slices = 24, acquisition time = 210 s).Then, a transverse dynamic contrast-enhanced (DCE) T1-weighted gradient-echo sequence(3D FLASH)(TR = 4.51 ms, TE = 1.61 ms, flip angle = 10°slice thickness = 1 mm, FOV = 340 mm×340 mm, average = 1,slab = 1, number of time points (measurements) = 7, acquisition time = 429 s) was acquired after administrating first gadolinium injection. Finally, using the slice where the lesion was the largest, dual-echo DCE/DSC gradient-echo was performed (TR = 25 ms, TE1 = 1.70 ms, TE2 = 8 ms, flip angle = 35°, slice thickness = 3.0 mm, matrix size = 256×216,FOV = 380 mm×380 mm, averages = 1,slices = 1, measurements = 220, acquisition time = 359 s) after second gadolinium injection. 15 minutes elapsed between the first gadolinium injection and second gadolinium injection. For both injections, gadolinium was given through a catheter placed within the antecubital vein at a dose of 0.1 mmol/kg via a power injector at a rate of 3 ml/s, followed by a 10 ml normal saline flush. The total administered dose of gadolinium contrast was 0.2 mmol/kg.
MR Image Analysis
Image data was saved and transferred to an offline workstation. Custom software written in house with Matlab 7.6 (MathWorks, Natick, Mass, USA) was used for subsequent analysis.
Peak enhancement ratio (PER) and time from contrast agent arrival to peak enhancement (Tmax) are semi-quantitative T1W related parameters and they are often used in DCE MRI. Relative regional blood flow and regional flood volume are T2*W related parameters (perfusion parameters) and the most relevant parameters obtained in dynamic susceptibility-contrast MRI (DSC MRI). The semi-quantitative T1W related parameters, including PER and Tmax of conventional DCE MRI (c-PER, c-Tmax) and dual-echo MRI (d-PER, d-Tmax), and the T2*W related parameters such as rBV and rBFof dual-echo MRI (d-rBV, d-rBF) were calculated[15]–[19]. In the calculating of dual-echo MRI parameters, T2* effects were removed in T1W related parameters analysis and T1 effect would be taken into account in perfusion analysis.
Statistical Analysis
The parametric variables were compared using one-way ANOVA. Pathology results from tissue sampling were considered the gold standard. The mean and variance were used in this setting. Receiver operating curves (ROC) and the area under the ROC curve (AUROC) were calculated as a descriptive tool to assess the overall discrimination of individual parameters and combined parameters. Sensitivity, specificity and Kappa statistic were used with respect to the diagnostic performance. Analysis was performed with SPSS 19 software (SPSS Inc., Chicago, IL). A P value of less than 0.05 was considered to indicate a statistically significant difference.
Results
Pathology Results
Of the 38 lesions sampled and analyzed, 20/38 (52.6%) were malignant and 18/38 (47.4%) were benign. Of the 20 malignancies, 14 patients had invasive ductal cancer (moderately or poorly differentiated), 3 patients had invasive lobular carcinoma, 2 patients had ductal carcinoma in situ (DCIS) and one patient had micro papillary carcinoma. Of the 18 benign lesions included 10 fibroadenomas, 4 cyclomastopathy, 3 plasma cell mastitis, and 1 galactoma.
Comparison of the Semi-quantitative Conventional DCE MRI and Dual-echo MRI Parameters between Breast Cancer and Fibroadenoma
Breast cancers displayed a lower value of Tmax with conventional DCE MRI (c-Tmax) than fibroadenomas (P = 0.014). Breast cancers also had lower values of Tmax, rBF and rBV with dual-echo MRI (d-Tmax, d-rBF and d-rBV) than fibroadenomas (P = 0.017, P = 0.029, P = 0.046, respectively). No significant difference regarding peak enhancement ratio (PER) of conventional DCE MRI and dual-echo MRI (P = 0.423, 0.252, respectively) was found between breast cancers and fibroadenoma (Table 1).
Table 1. Semi-quantitative conventional DCE MRI and Dual-echo MRI parameters of breast lesions.
| Parameter | Fibroadenoma (n = 10) | Malignancy (n = 20) | t | p |
| c-PER | 1.6(1.12–2.09) | 1.44(1.23–1.65) | 0.663 | 0.423 |
| c-Tmax | 272(234–311) s | 219(194–244) s | 6.99 | 0.014* |
| d-PER | 0.456(0.303–0.608) | 0.612(0.428–0.796) | 1.367 | 0.252 |
| d-Tmax | 183(173–193) s | 168(155–175) s | 6.420 | 0.017* |
| d-rBF | 0.015(0.009–0.022) | 0.044(0.027–0.062) | 5.319 | 0.029* |
| d-rBV | 0.005(0.001–0.008) | 0.012(0.007–0.017) | 4.306 | 0.046* |
Mean value and 95% confidence interval are given for each parameter.
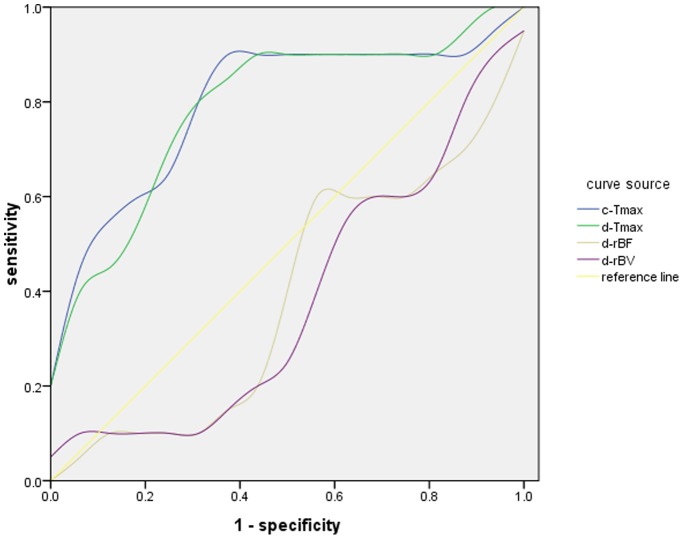
ROC analysis of both semi-quantitative conventional DCE MRI and dual-echo MRI parameters are given in Table 2, with ROC curves shown in Figure 1. The most relevant factors for discriminating breast cancer from fibroadenoma, based on the areas under the ROC curve (AUROC) were c-Tmax, d-Tmax, d-rBF and d-rBV, (0.800, 0.794, 0.613,0.619, respectively). Using ROC analysis, a c-Tmax<249 s had a sensitivity of 90% and a specificity of 68.8% for predicting malignancy, a d-Tmax<183(s) had a sensitivity of 80% and a specificity of 75% for predicting malignancy, a d-rBF<0.024 had a sensitivity of 50% and a specificity of 80% for predicting malignancy, a d-rBV<0.005 had a sensitivity of 60% and a specificity of 80% for predicting malignancy.
Table 2. ROC analysis of the semi-quantitative conventional DCE MRI and dual-echo MRI parameters.
| Parameters | AUROC | p | Cut-offValue | Sensitivity | Specificity |
| c-PER | 0.594 (0.363–0.825) | 0.429 | 1.454 | 70% | 56.3% |
| c-Tmax | 0.800 (0.606–0.994) | 0.011 | 249s | 90% | 68.8% |
| d-PER | 0.513 (0.274–0.751) | 0.916 | 0.473 | 60% | 62.5% |
| d-Tmax | 0.794 (0.605–0.983) | 0.013 | 183 s | 80% | 75% |
| d-rBF | 0.613 (0.383–0.842) | 0.029 | 0.024 | 50% | 80% |
| d-rBV | 0.619 (0.391–0.846) | 0.056 | 0.005 | 60% | 80% |
Figure 1. The ROC curves of c-Tmax,d-Tmax,d-rBF and d-rBV.
Combining the d-Tmax of dual-echo MRI with rBV, sensitivity is 90% and specificity becomes 70%. When combining the three factors of Tmax, rBF and rBV, sensitivity remains unchanged at 90% but specificity increases to 80% (Table3).
Table 3. Accuracy of combining of Tmax, rBF and rBV of Dual-echo DCE MRI to differentiate breast cancers from fibroadenomas.
| Joint Parameters | Sensitivity* | Specificity** | Kappa | p |
| d-Tmax, d-rBF | 90% (18/20) | 70% (7/10) | 0.615 | 0.001 |
| d-Tmax, d-rBV | 85% (17/20) | 80% (8/10) | 0.634 | 0.000 |
| d-Tmax, d-rBFand d-rBV | 90% (18/20) | 80% (8/10) | 0.615 | 0.001 |
Data in parenthesis were positive cases tested by imaging vs gold standard.
Data in parenthesis were negative cases tested by imaging vs gold standard.
Discussion
Conventional T1W DCE MRI typically uses high spatial resolution to estimate T1W related semi-quantitative parameters such as c-PER and c-Tmax. Despite high sensitivity, diagnostic specificity remains unsatisfactory [7], [11].The addition of perfusion-related parameters obtained from T2*W DSC MRI, such as rBF and rBV[8]–[10], has been shown to increase examination sensitivity and specificity. Improved specificity likely is due in part to an increased number of capillaries and a greater mean capillary diameter in malignant tissues compared to those in benign tissues [7].
It has been shown that simultaneous measurement of T1W and T2*W parameters can improve the accuracy of both perfusion parameters and permeability kinetics using dynamic contrast enhancement [14]. In particular, T2* effects of gadolinium contrast agents, which are more pronounced with increased concentrations, can be measured to reduce underestimation of peak enhancement and overestimation of permeability [14]. A dual-echo gradient echo sequence is a requirement to perform simultaneous T1W and T2*W measurements using a single contrast dose, and eliminates motion artifact between measurements, since echoes are spaced less than 7 ms apart. Despite the attractiveness of such a technique, dual-echo MRI has not been performed in the breast until now due to various technical challenges[20]–[22].Furthermore, the effects of T1-corrected perfusion and T2*-corrected T1W semi-quantitative parameters have never been evaluated within breast tissue. In fact, there are no comparison studies between normal, benign, or malignant breast with regard to T2* effects on routinely-acquired T1W dynamic perfusion parameters, and systemic errors can be considerable (particularly within tumors) when this effect is not considered. The dual-echo technique magnetic resonance sequence and analysis software we have implemented leverages multiple recent techniques developed by our group for imaging the breast[23]–[25] to explore the clinical potential of reducing the false positive rate in clinical breast MRI.
The combination of conventional DCE MRI and DSC MRI with two administrations of contrast agent has demonstrated substantially improvement in the diagnostic specificity of breast MRI [7], [11], [12]. Our data show that dual-echo MRI can simultaneously measure the T1W and T2*W related parameters and improves the accuracy of differentiating breast cancer from fibroadenomas. Dual-echo MRI with a single administration of contrast agent has the advantage of eliminating the expense of a second contrast dose administration, the elimination of a second imaging sequence. Furthermore, the dual-contrast dose technique necessitates a delay between contrast dose administrations to allow washout of residual contrast from the first administration; the dual-echo MRI technique obviates this delay, which typically is 15 minutes. Finally, reduction of total contrast agent administered improves the safety profile of the examination.
This study has several limitations. Only a small number of patients were included, as the intent was an initial investigation into the feasibility and applicability of dual-echo MRI in the human breast. A larger number of patients would permit improved sensitivity, specificity, and accuracy estimations in various subtypes of malignant and benign lesions. Furthermore, our current implementation of dual-echo MRI only allows a single slice to be acquired through a lesion with sufficient temporal and spatial resolution. However, we anticipate that with further iterations in sequence design and improvements in MRI technology a substantially larger imaging volume is achievable.
Conclusions
In conclusion, we show that dual-echo MRI with a single administration of contrast agent can simultaneously measure both T1W and T2*W related kinetic and perfusion parameters in the breast. We further show that combining T1W DCE MRI measurement of contrast kinetics with T2*W DSC MRI perfusion measurements improves the diagnostic performance of breast MRI to differentiate breast cancer from benign fibroadenomas.
Funding Statement
This research was supported by the Zhejiang Provincial Bureau of Health, Hangzhou, China (2011KYA121). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kuhl CK (2007) Current status of breast MR imaging. Part 2. Clinical applications. Radiology 244: 672–691. [DOI] [PubMed] [Google Scholar]
- 2. Bluemke DA, Gatsonis CA, Chen MH, DeAngelis GA, DeBruhl N, et al. (2004) Magnetic resonance imaging of the breast prior to biopsy. JAMA 292: 2735–2742. [DOI] [PubMed] [Google Scholar]
- 3. Peters NH, Borel RI, Zuithoff NP, Mali WP, Moons KG, et al. (2008) Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology 246: 116–124. [DOI] [PubMed] [Google Scholar]
- 4. Moy L, Elias K, Patel V, Lee J, Babb JS, et al. (2009) Is breast MRI helpful in the evaluation of inconclusive mammographic findings? AJR Am J Roentgenol 193: 986–993. [DOI] [PubMed] [Google Scholar]
- 5. Vassiou K, Kanavou T, Vlychou M, Poultsidi A, Athanasiou E, et al. (2009) Characterization of breast lesions with CE-MR multimodal morphological and kinetic analysis: comparison with conventional mammography and high-resolution ultrasound. Eur J Radiol 70: 69–76. [DOI] [PubMed] [Google Scholar]
- 6. Gilles R, Meunier M, Lucidarme O, Zafrani B, Guinebretiere JM, et al. (1996) Clustered breast microcalcifications: evaluation by dynamic contrast-enhanced subtraction MRI. J Comput Assist Tomogr 20: 9–14. [DOI] [PubMed] [Google Scholar]
- 7. Kvistad KA, Rydland J, Vainio J, Smethurst HB, Lundgren S, et al. (2000) Breast lesions: evaluation with dynamic contrast-enhanced T1-weighted MR imaging and with T2*-weighted first-pass perfusion MR imaging. Radiology 216: 545–553. [DOI] [PubMed] [Google Scholar]
- 8. Benner T, Heiland S, Erb G, Forsting M, Sartor K (1997) Accuracy of gamma-variate fits to concentration-time curves from dynamic susceptibility-contrast enhanced MRI: influence of time resolution, maximal signal drop and signal-to-noise. Magn Reson Imaging 15: 307–317. [DOI] [PubMed] [Google Scholar]
- 9. Henderson E, Rutt BK, Lee TY (1998) Temporal sampling requirements for the tracer kinetics modeling of breast disease. Magn Reson Imaging 16: 1057–1073. [DOI] [PubMed] [Google Scholar]
- 10. Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R (1999) Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab 19: 701–735. [DOI] [PubMed] [Google Scholar]
- 11. Huang W, Fisher PR, Dulaimy K, Tudorica LA, O'Hea B, et al. (2004) Detection of breast malignancy: diagnostic MR protocol for improved specificity. Radiology 232: 585–591. [DOI] [PubMed] [Google Scholar]
- 12. de Bazelaire C, Rofsky NM, Duhamel G, Zhang J, Michaelson MD, et al. (2006) Combined T2* and T1 measurements for improved perfusion and permeability studies in high field using dynamic contrast enhancement. Eur Radiol 16: 2083–2091. [DOI] [PubMed] [Google Scholar]
- 13. Taillieu F, Salomon LJ, Siauve N, Clement O, Faye N, et al. (2006) Placental perfusion and permeability: simultaneous assessment with dual-echo contrast-enhanced MR imaging in mice. Radiology 241: 737–745. [DOI] [PubMed] [Google Scholar]
- 14. Ludemann L, Prochnow D, Rohlfing T, Franiel T, Warmuth C, et al. (2009) Simultaneous quantification of perfusion and permeability in the prostate using dynamic contrast-enhanced magnetic resonance imaging with an inversion-prepared dual-contrast sequence. Ann Biomed Eng 37: 749–762. [DOI] [PubMed] [Google Scholar]
- 15. Mussurakis S, Gibbs P, Horsman A (1998) Primary breast abnormalities: selective pixel sampling on dynamic gadolinium-enhanced MR images. Radiology 206: 465–473. [DOI] [PubMed] [Google Scholar]
- 16. Evelhoch JL (1999) Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging 10: 254–259. [DOI] [PubMed] [Google Scholar]
- 17. Liney GP, Gibbs P, Hayes C, Leach MO, Turnbull LW (1999) Dynamic contrast-enhanced MRI in the differentiation of breast tumors: user-defined versus semi-automated region-of-interest analysis. J Magn Reson Imaging 10: 945–949. [DOI] [PubMed] [Google Scholar]
- 18. Barbier EL, Lamalle L, Decorps M (2001) Methodology of brain perfusion imaging. J Magn Reson Imaging 13: 496–520. [DOI] [PubMed] [Google Scholar]
- 19. Yang X, Knopp MV (2011) Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol 2011: 732848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shames DM, Kuwatsuru R, Vexler V, Muhler A, Brasch RC (1993) Measurement of capillary permeability to macromolecules by dynamic magnetic resonance imaging: a quantitative noninvasive technique. Magn Reson Med 29: 616–622. [DOI] [PubMed] [Google Scholar]
- 21. Kim EJ, Kim DH, Lee SH, Huh YM, Song HT, et al. (2004) Simultaneous acquisition of perfusion and permeability from corrected relaxation rates with dynamic susceptibility contrast dual gradient echo. Magn Reson Imaging 22: 307–314. [DOI] [PubMed] [Google Scholar]
- 22. Makkat S, Luypaert R, Sourbron S, Stadnik T, De Mey J (2007) Quantification of perfusion and permeability in breast tumors with a deconvolution-based analysis of second-bolus T1-DCE data. J Magn Reson Imaging 25: 1159–1167. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Yu Y, Zhang Y, Bao S, Wu C, et al. (2009) A clinically feasible method to estimate pharmacokinetic parameters in breast cancer. Med Phys 36: 3786–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang H, Miao Y, Zhou K, Yu Y, Bao S, et al. (2010) Feasibility of high temporal resolution breast DCE-MRI using compressed sensing theory. Med Phys 37: 4971–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yu Y, Jiang Q, Miao Y, Li J, Bao S, et al. (2010) Quantitative analysis of clinical dynamic contrast-enhanced MR imaging for evaluating treatment response in human breast cancer. Radiology 257: 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]