Abstract
One third of the human population is currently infected by one or more species of parasitic helminths. Certain helminths establish long-term chronic infections resulting in a modulation of the host’s immune system with attenuated responsiveness to “bystander” antigens such as allergens or vaccines. In this study we investigated whether parasite-derived products suppress the development of allergic inflammation in a mouse model. We show that extract derived from adult male Oesophagostomum dentatum (eMOD) induced Th2 and regulatory responses in BALB/c mice. Stimulation of bone marrow-derived dendritic cells induced production of regulatory cytokines IL-10 and TGF-beta. In a mouse model of birch pollen allergy, co-administration of eMOD with sensitizing allergen Bet v 1 markedly reduced the production of allergen-specific antibodies in serum as well as IgE-dependent basophil degranulation. Furthermore, eMOD prevented the development of airway inflammation, as demonstrated by attenuation of bronchoalveolar lavages eosinophil influx, peribronchial inflammatory infiltrate, and mucus secretion in lungs and IL-4 and IL-5 levels in lung cell cultures. Reduced secretion of Th2-related cytokines by birch pollen-re-stimulated splenocytes and mesenteric lymph node cells was observed in eMOD-treated/sensitized and challenged mice in comparison to sensitized and challenged controls. The suppressive effects of eMOD were heat-stable. Immunization with model antigens in the presence of eMOD reduced production of antibodies to thymus-dependent but not to thymus-independent antigen, suggesting that suppression of the immune responses by eMOD was mediated by interference with antigen presenting cell or T helper cell function but did not directly suppress B cell function. In conclusion, we have shown that eMOD possesses immunomodulatory properties and that heat-stable factors in eMOD are responsible for the dramatic suppression of allergic responses in a mouse model of type I allergy. The identification and characterization of parasite-derived immune-modulating molecules might have potential for designing novel prophylactic/therapeutic strategies for immune-mediated diseases.
Introduction
Infections with helminth parasites represent a global health problem with more than one billion people infected worldwide. Exposure to helminth parasites has a major impact on the development and reactivity of the host’s immune system. In order to prevent their expulsion or reduce severe pathology, helminth parasites engage complex mechanisms to “act the innocent” in order to avoid attention and/or to actively manipulate the effector mechanism of the host immune system [1].
Even though they have common immunological features (elevated levels of IgE, eosinophilia, and production of Th2 cytokines such as IL-4 and IL-5), epidemiological studies revealed an inverse relationship between helminth infections and allergic diseases. For example, infections with Schistosoma haematobium or hookworms were associated with protection from atopic reactivity [2], [3]. Similarly, infection with A. lumbricoides or Trichuris trichiura was associated with lower prevalence of skin prick test reactivity [4], [5]. Studies reporting a significant increase in allergen skin sensitization following anthelmintic treatment provide additional evidence that helminth infection and allergic sensitization are likely to be interrelated. [6], [7], [8], [9]. Such observations recently received considerable interest, leading to intervention studies using worms as a therapy of immunological disorders. Due to the fact that T. suis, the pig whipworm, is likely to be non-pathogenic in human subjects, the majority of reported clinical trials have been performed with this parasite.
Notably, earlier clinical studies provided encouraging results on the beneficial effect of application of T. suis eggs to patients with multiple sclerosis [10] as well as to patients with Th1-mediated Crohn’s disease or Th2-mediated ulcerative colitis [11], [12], [13]. However, no beneficial effect of experimental T. suis infection was found in two clinical trials in allergic rhinitis [14], [15]. Similarly, experimental infection with the hookworm Necator americanus did not result in clinically significant improvement of airway responsiveness [16], [17] but induced regulatory responses in celiac individuals [18]. While clinical studies performed thus far have demonstrated the safety and provided evidence that controlled infections with helminth parasites are well tolerated, greater consistency and wider application may be achieved by identifying active parasite-derived substances which do not require live infections of patients with helminth parasites.
Several parasite-derived products have revealed their potential to inhibit immunopathology in various animal models. For example, soluble products from T. suis, Trichinella spiralis [19], or S. japonicum [20] suppressed clinical signs in murine experimental autoimmune encephalomyelitis. Moreover, mice treated with extracts of the tapeworm Hymenolepis diminuta were protected against colitis induced by DNBS [21]. Finally, excretory/secretory products derived from Nippostrongylus brasiliensis [22], Heligmosomoides polygyrus [23], Toxascaris leonina [24], or S. mansoni [25] suppressed allergic airway inflammation in mice.
Oesophagostomum dentatum, the nodule worm, is a gastrointestinal nematode parasite of pigs worldwide, which follows a direct transmission cycle. Ingested larvae invade the mucosa of cecum and colon, moult to L4 larvae and return to the intestinal lumen, where they mature. Adult male and female worms cause chronic patent infections without overt clinical signs [26].
In this study we investigated whether products derived from adult male O. dentatum (eMOD) modulate responses to sensitizing allergen in a mouse model of type I allergy. We found that co-administration of eMOD with the major birch pollen allergen Bet v 1 led to significant suppression of both humoral and cellular allergic responses, as well as airway eosinophilia. The allergy-protective effect of eMOD is mediated by heat-stable component, interfering possibly with antigen presenting cell or T cell function.
Materials and Methods
Animals
6–8 week-old female BALB/c mice were obtained from Charles River (Sulzfeld, Germany) and maintained under conventional housing conditions. Pigs were kept at the animal facilities of the Institute of Parasitology, University of Veterinary Medicine, Vienna. All experimental protocols were reviewed and approved by the Austrian Federal Ministry of Science and Research.
Preparation of Parasite Extract
Oesophagostomum dentatum, OD-Hann (Joachim et al. 1997) is routinely maintained by infection of parasite-free pigs. Adult male worms were isolated from the intestines of pigs during the patent stage of infection according to Slotved et al. [27]. Whole body extract from male worms (eMOD) was prepared by homogenization of worms isolated from the intestine of pigs during the patent stage of infection. Worms were extensively washed in PBS, snap-frozen in liquid nitrogen and homogenized mechanically in PBS, followed by centrifugation at 10,000×g for 15 min at 4°C. The supernatant was passed through a 0.22 µm filter and the total protein concentration was quantified by the bicinchoninic acid assay (BCA Protein Assay; Thermo Scientific) according to the manufacturer’s protocol. Extracts were tested in the Limulus Amoebocyte Lysate (LAL) assay (Endpoint Chromogenic LAL Assays; Lonza). The levels of endotoxin were below 0.1 EU in 1 µg of extract. Samples were stored at −80°C until required. Heat treatment was performed by incubation at 96°C for 15 min.
Generation and Stimulation of Bone Marrow Derived Dendritic Cells
Bone marrow derived dendritic cells (BM-DC) were generated as previously described [28]. Briefly, the bone marrow precursors were isolated from femurs and tibias of BALB/c mice. Cells were cultured at 2×105/ml in bacteriological Petri dishes in 10 ml culture medium with GM-CSF (20 ng/ml; Sigma-Aldrich). Fresh medium was added at days 3 and 6 and BM-DC were used on day 8 of culture. BM-DC (106 cells/ml) were stimulated with eMOD at a final concentration of 100 µg/ml or left untreated for 24 h, after which supernatants were tested by ELISA.
Immunization
Mice were immunized by i.p. injections of 50 µg aluminium-precipitated eMOD (eMOD group) or sham treated with PBS-alum (sham group) on days 0 and 10. Blood, spleen and mesenteric lymph nodes (MLN) were collected on sacrifice (day 21). In order to investigate the impact of eMOD on third-party antigens, mice were immunized i.p. with 50 µg of eMOD (eMOD group); 10 µg of diphtheria toxoid-alum (DT group); 50 µg of eMOD plus 10 µg of diphtheria toxoid (eMOD/DT group); 200 µg of NIP-Ficoll (NIP group) or 50 µg of eMOD plus 200 µg of NIP-Ficoll (eMOD/NIP group) on days 0 and 7. Blood was collected on day 21. Blood samples were allowed to coagulate at room temperature for at least 4 h and centrifuged for 10 min at 1,500×g. Serum was collected and stored at −20°C for further analysis. Single-cell suspensions were prepared from spleens, lungs and MLN. Cells (2.5×107/ml) were stimulated with eMOD (100 µg/ml) or media alone in 96 well-plates at 37°C for 72 h in culture medium (RPMI 1640 supplemented with 10% heat-treated FCS, 2 mM L-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin). Cell-free supernatants were stored at −20°C for further analysis.
Mouse Model of Birch Pollen Induced Allergic Airway Inflammation
Induction of allergic airway inflammation was performed as previously described [29]. Briefly, mice were sensitized i.p. with three injections of 1 µg of recombinant Bet v 1 (Biomay, Vienna, Austria) precipitated with 100 µl of aluminium hydroxide (alum, Serva, Heidelberg, Germany) on days 0, 14, and 28; and then challenged intranasally with birch pollen extract (BP; Allergon, Välinge, Sweden; 100 µg/mouse in final volume of 30 µl) on three consecutive days (days 35, 36 and 37). Mice were terminally anesthetized 72 h after the final airway challenge (day 40).
Bronchoalvelar Lavage and Differential Cell Counts
Bronchoalveolar lavage fluid (BALF) was collected by cannulating the trachea and injecting/recovering 2×1 ml PBS containing a protease inhibitor cocktail (Complete® Protease Inhibitor cocktail Tablets; Roche, Mannheim, Germany). BALF was centrifuged at 300×g at 4°C for 5 min and cell-free supernatants were stored at −20°C for further analysis. The cell pellet was resuspended in 200 µl of PBS; total leukocytes were counted and cytospins (4×104 cells; Shandon Cytospin®, Shandon Southern Instruments, USA) were stained with haematoxylin and eosin (H&E Hemacolor®, Merck, Darmstadt, Germany) for differential cell counts (200 cells were counted per cytospin).
Lung Histology
Following bronchoalveolar lavage, the lungs were fixed with 10% formaldehyde-PBS and paraffin-embedded. Tissue sections were stained with H&E. Airway mucus occlusion was analyzed on periodic acid-Schiff-stained (PAS, Sigma-Aldrich) sections.
Immunoglobulin Levels in Blood Serum
Blood samples were taken by tail bleeding on the day of sacrifice and sera were stored at −20°C. Levels of antigen-specific serum antibodies were measured by ELISA. Microtitre plates (Nunc, Wiesbaden, Germany) were coated with eMOD (2 µg/ml), Bet v 1 (2 µg/ml), DT (10 µg/ml) or NIP-BSA (2 µg/ml) in coating buffer (0.1 M carbonate-bicarbonate buffer). Serum samples were diluted 1/100 for IgM and IgG, 1/1000 for IgG1, 1/500 for IgG2a, and 1/10 for IgE and IgA. Rat anti-mouse IgM, IgG, IgG1, IgG2a, IgE and IgA antibodies (1/500; Pharmingen, San Diego, CA) were applied and peroxidase-conjugated mouse anti-rat IgG antibodies (1/1000; Jackson, Immuno Labs., West Grove) were used for the detection. In order to measure specific IgE levels in sera, a rat basophil leukemia (RBL) cell mediator release assay was performed as previously described [30]. Briefly, RBL-2H3 cells were incubated with sera at a dilution of 1/100. Degranulation of RBL cells was induced by adding 0.3 µg of Bet v 1 diluted in 100 µl of Tyrode’s buffer. Data represent mean values ±SEM of percentages of total β-hexosaminidase activity after addition of 1% Triton X-100 and are shown after subtractions of baseline release levels obtained with pre-immune sera.
Cytokine Measurements
Levels of IL-4, IL-5, IL-10, and TGF-β in supernatants were measured by ELISA (Endogen, Cambridge, MA) according to the manufacturer’s instructions.
Statistical Analysis
All data are shown as mean ±SEM. Significance was analyzed using the non-parametric Mann-Whitney U-test (Graph Prism; Graph Pad Software, Inc, San Diego, CA). Differences were considered significant at p<0.05.
Results
Humoral and Cellular Responses induced by Immunization with Crude Extract of Male Oesophagostomum dentatum
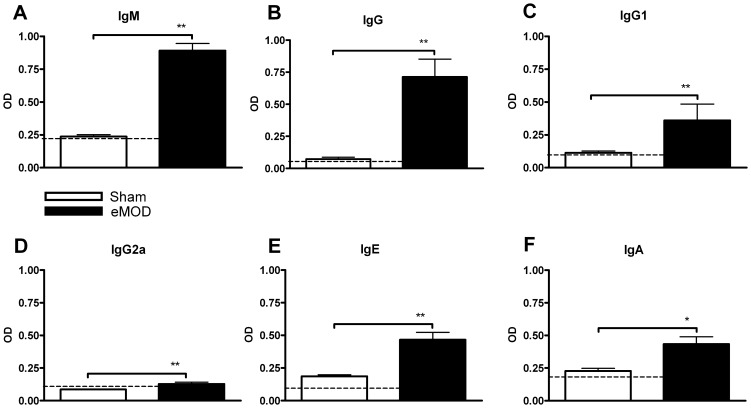
To analyze whether BALB/c mice can be primed in vivo to mount specific humoral and cellular immune responses to O. dentatum, mice were immunized twice, 10 days apart, with the crude extract of male O. dentatum (eMOD) in alum or with PBS in alum. At 11 days following the second immunization, O. dentatum-specific antibody production in serum and O. dentatum-specific cytokine production in eMOD re-stimulated spleen and MLN cell cultures were assessed. As shown in Fig. 1, by day 21 a substantial production of O. dentatum-specific antibodies was detected. Immunization with O. dentatum extract induced high levels of total IgG antibodies (Fig. 1 B) as well as specific IgG1 (Fig. 1 C). The production of anti-eMOD-IgG2a (Th1-associated isotype; Fig. 1 D) was significantly higher in O. dentatum-immunized mice compared to sham treated controls, even though the titres reached relatively low levels. Furthermore, immunization with O. dentatum induced high levels of specific IgM (Fig. 1 A), IgE (Fig. 1 E), and IgA (Fig. 1 F) antibodies in serum.
Figure 1. Antibody production in mice immunized with eMOD.
Mice were immunized intraperitoneally with 50 µg of eMOD-alum (dark bars) or sham treated with PBS-alum (white bars) on days 0 and 10. Serum was collected on day 21. The levels of eMOD-specific IgM (A), IgG (B), IgG1 (C), IgG2a (D), IgE (E), and IgA (F) were detected by ELISA. The dashed line indicates the background level of the assay. Results are representative of three repeat experiments each with four to six mice per group. Data are expressed as mean ±SEM. *p<0.05; **p<0.01.
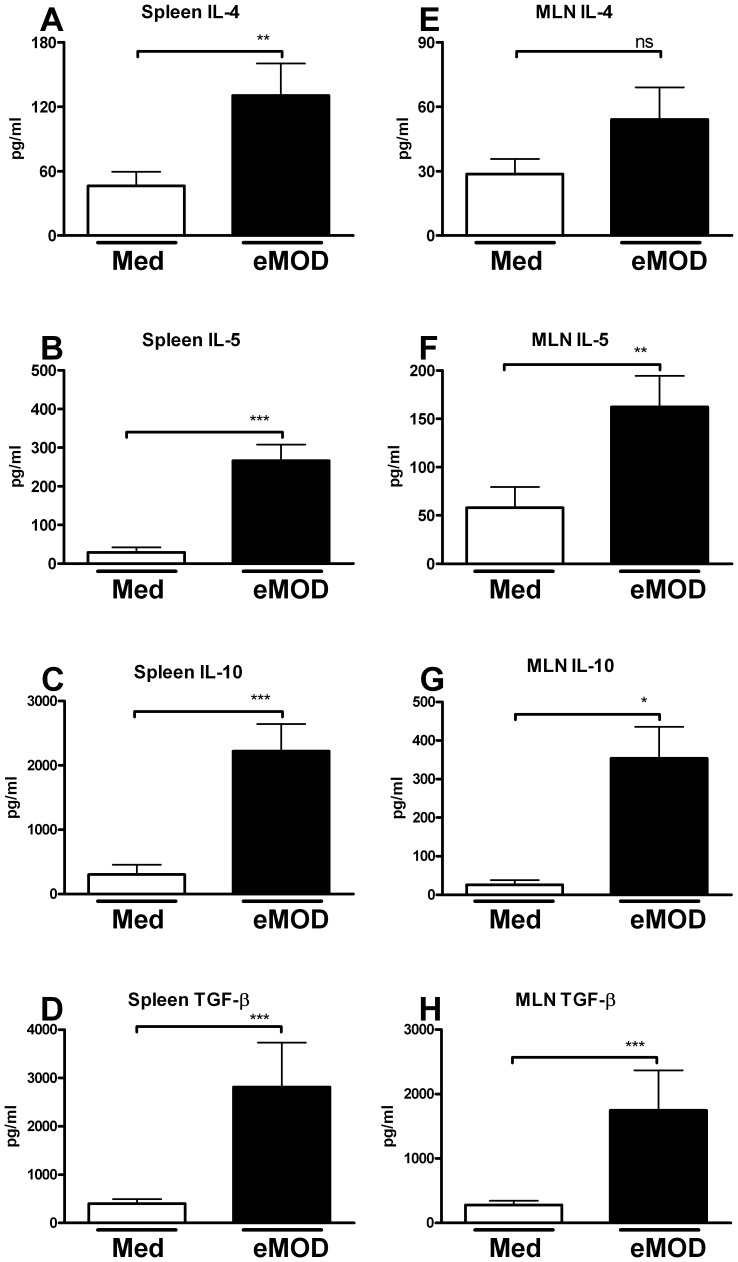
The production of the Th2-associated cytokines IL-4 and IL-5 as well as of regulatory cytokines IL-10 and TGF-β were significantly increased in spleen cell cultures derived from eMOD-immunized mice stimulated with eMOD, in comparison to cells stimulated with medium alone (Fig. 2 A–D). Similar results were obtained for MLN cell cultures (Fig. 2 E–H). Furthermore, immunization with eMOD in the absence of alum triggered the production of specific IgG in serum and re-stimulation of spleen cell cultures with eMOD in these mice led to significant induction of IL-4, IL-5, IL-10 and TGF-β (data not shown). Levels of IFN-γ did not differ between eMOD-stimulated and untreated cultures (data not shown).
Figure 2. Immunization with eMOD induces Th2 and regulatory responses.
Mice were immunized intraperitoneally with 50 µg of eMOD-alum on days 0 and 10. Spleen and MLN for cell preparation were collected on day 21. Cell cultures were stimulated with 100 µg of eMOD (dark bars; eMOD) or left untreated (white bars; Med) for 72 hours. Concentrations of IL-4 (A, E), IL-5 (B, F), IL-10 (C, G) and TGF-β (D, H) in the supernatants were measured by ELISA. Results represent three combined experiments each with four to six mice per group and are expressed as mean ±SEM. *p<0.05; **p<0.01; ***p<0.001.
Dendritic Cells Stimulated with eMOD Produce Regulatory Cytokines
Dendritic cells (DC) are professional antigen-presenting cells which are critical for initiating but also for regulating immune responses. In order to investigate whether DC are important players in induction of regulatory cytokines by eMOD, bone marrow-derived DC (BM-DC) from naïve BALB/c mice were stimulated with eMOD for 24 h. Cells stimulated with eMOD induced increased levels of IL-10 (up to 76-fold) and TGF-β (up to 6-fold) in comparison to cells stimulated with medium alone (Fig. 3). Levels of IL-12p40, IL-12p70 and TNF-α were not elevated in BM-DC cultures after stimulation with eMOD when compared to controls (data not shown).
Figure 3. Stimulation of BM-DC with eMOD results in the production of regulatory cytokines.
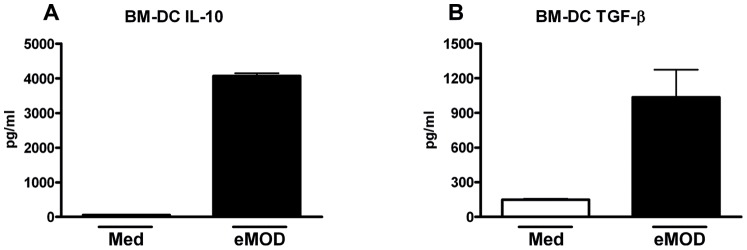
Bone marrow-derived dendritic cells (BM-DC) derived from naïve BALB/c mice were stimulated with 100 µg/ml of eMOD (dark bars; eMOD) or left untreated (white bars; Med) for 24 hours. Levels of IL-10 (A) and TGF-β (B) in the supernatants were measured by ELISA. Results are representative of three repeat experiments. Data are expressed as mean ±SEM.
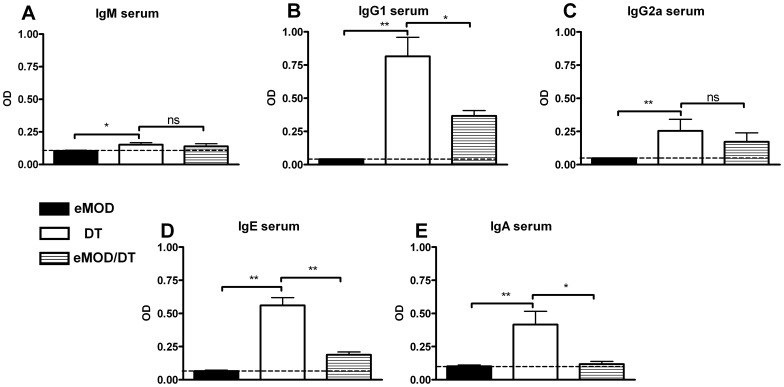
Application of eMOD during Sensitization Down-regulates Allergen-specific Antibody Production in a Mouse Model of Type I Allergy
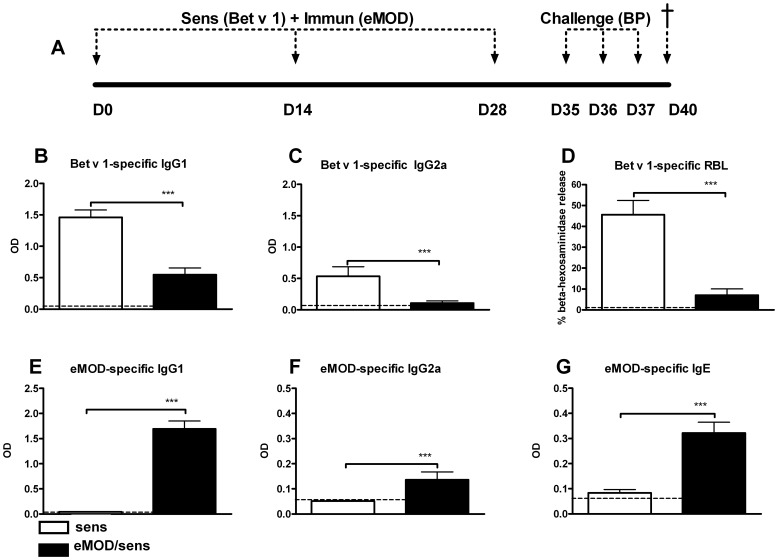
To study the effect of eMOD on allergen-mediated sensitization and intranasal challenge we used a mouse model of birch pollen allergy (Fig. 4 A). The application of eMOD together with Bet v 1 significantly reduced the development of Bet v 1-specific IgG1 as well as IgG2a isotypes in serum (Fig. 4 B, C). These data suggest that eMOD has a general effect on allergen-specific antibody production rather than an effect on the Th1/Th2 balance. Similarly, the application of eMOD during sensitization reduced the IgE-dependent basophil degranulation to Bet v1 compared with sensitized and challenged controls (Fig. 4 D). On the other hand, eMOD-specific antibody production in eMOD-treated, sensitized and challenged mice was significantly increased in comparison to sham-treated sensitized and challenged controls (Fig. 4 E–G).
Figure 4. Co-application of eMOD at sensitization suppresses Bet v 1-specific but not eMOD-specific humoral responses.
(A) A diagram of experimental setup. Mice were immunized intraperitoneally with 50 µg of eMOD admixed to 1 µg of Bet v 1-alum (black bars; eMOD/sens) on days 0, 14 and 28, then intranasally challenged with 100 µg of birch pollen extract (BP) on three consecutive days one week after the last sensitization (days 35–37) and sacrificed on day 40. Control mice (white bars; sens) were immunized with Bet v 1-alum admixed to PBS. Serum was collected at day 40. Levels of Bet v 1-specific and eMOD-specific IgG1 (B; E); IgG2a (C; F), and eMOD-specific IgE (G) were measured by ELISA. Functional IgE was measured by Bet v 1-mediated β-hexosaminidase release from rat basophil leukemia cells (D). The dashed line indicates the background level of the assay. Results represent three combined experiments each with five mice per group. Data are expressed as mean ±SEM. ***p<0.001.
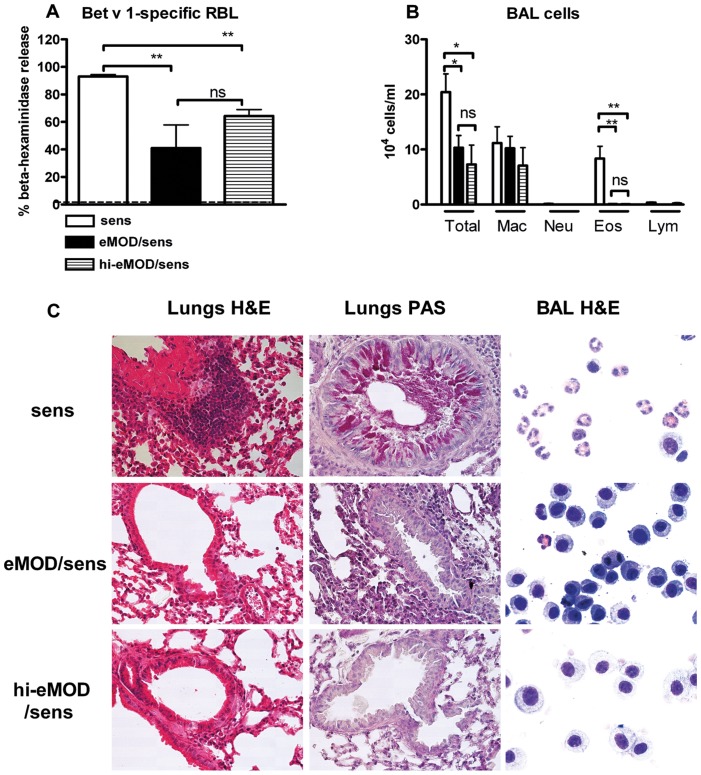
Application of eMOD during Sensitization with Bet v 1 Inhibits the Development of Allergic Airway Inflammation
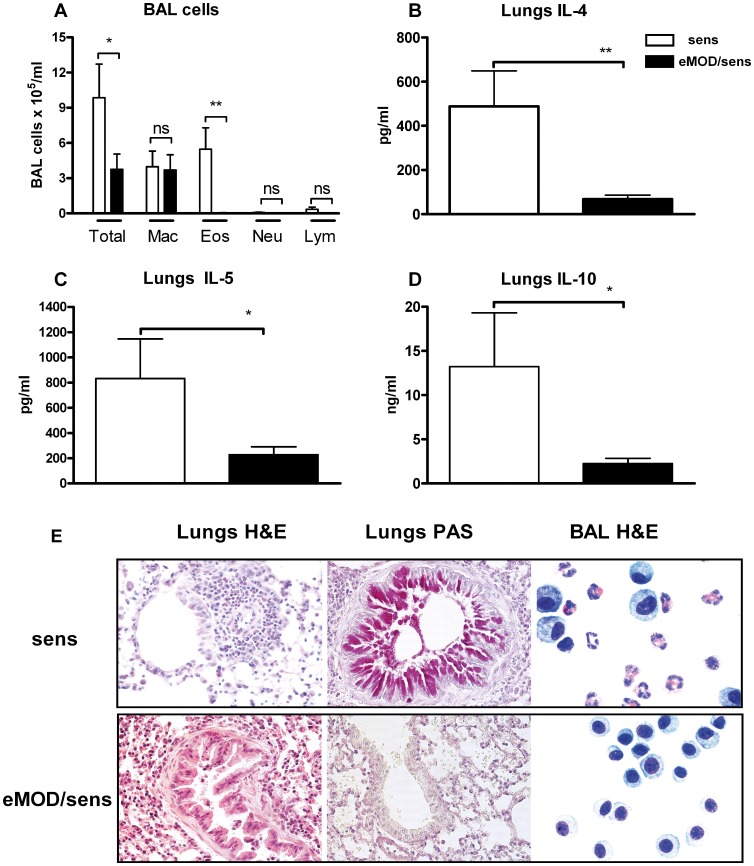
Three doses of eMOD during sensitization with Bet v 1 significantly suppressed the airway allergic inflammation measured by total cell numbers in the BALF after intranasal challenge with birch pollen extract (Fig. 5 A). The differential cell counting of cytospins revealed that the cellular infiltrates were reduced with eMOD administration, which was due primarily to the reduction of eosinophils (Fig. 5 A, E). H&E and PAS staining of formalin-fixed lung sections also showed a strong reduction of peribronchial and perivascular cellular infiltrations as well as reduced goblet cell hyperplasia and mucus production in mice treated with eMOD (Fig. 5 E). Administration of eMOD also suppressed production of IL-4, IL-5 as well as regulatory cytokine IL-10 in lung cells stimulated with birch pollen extract (Fig. 5 B–D).
Figure 5. Co-application of eMOD at sensitization reduces the development of airway inflammation.
Mice were immunized intraperitoneally with 50 µg of eMOD admixed to1 µg of Bet v 1-alum (black bars; eMOD/sens) on days 0, 14 and 28, then intranasally challenged with 100 µg of birch pollen extract (BP) on three consecutive days one week after the last sensitization (days 35–37) and sacrificed on day 40. Control mice (white bars; sens) were immunized with Bet v 1-alum admixed to PBS. Bronchoalveolar lavage fluid (BALF) and lung tissue for cell preparation and histology were collected at day 40. (A) The numbers of total and differential cells in BALF. Production of IL-4 (B), IL-5 (C) and IL-10 (D) in BP-re-stimulated lung cell cultures was assessed by ELISA. Results represent three combined experiments each with five mice per group. Data is expressed as mean ±SEM. *p<0.05; **p<0.01; Mac = macrophages; Eos = eosinophils, Neu = neutrophils; Lym = lymphocytes. (E) Representative haematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining of paraformaldehyde-fixed lung tissue sections at 40×magnification. Representative BALF cytospins were stained with H&E and are shown at 100×magnification.
Systemic Allergen-specific Recall Responses are Reduced by eMOD Treatment
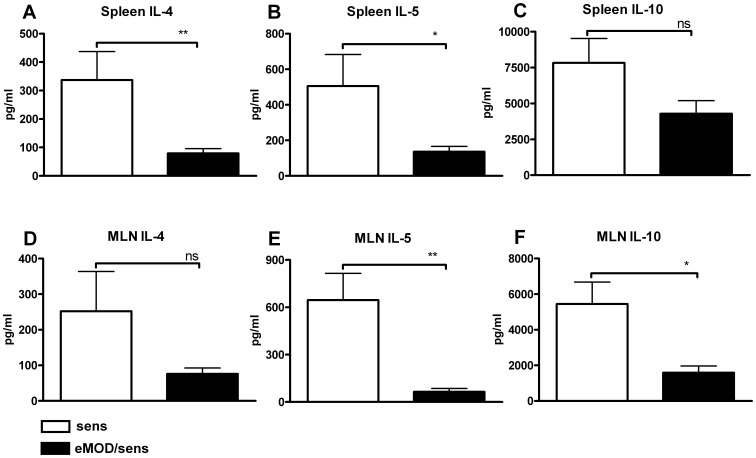
To determine whether the suppressive effects of eMOD on airway inflammation were associated with changes in systemic cellular responses, we examined allergen-specific recall responses in splenocytes and MLN cells. We found that IL-4, IL-5 and IL-10 were all suppressed in both birch pollen extract stimulated spleen (Fig. 6 A–C) and MLN (Fig. 6 D–F) cultures derived from eMOD-treated mice in comparison to controls. Levels of IFN-γ were found not to differ between mice that had received eMOD and control mice (data not shown). These results provide additional evidence that the shift to Th1 responsiveness does not account for the down-regulatory effects of eMOD on Bet v 1-specific Th2 responses.
Figure 6. Co-application of eMOD at sensitization reduces allergen-specific recall responses.
Mice were immunized intraperitoneally with 50 µg of eMOD admixed to 1 µg of Bet v 1 (black bars; eMOD/sens) on days 0, 14 and 28, then intranasally challenged with 100 µg of birch pollen extract (BP) on three consecutive days one week after the last sensitization (days 35–37). Control mice (white bars; sens) were immunized with Bet v 1 admixed to PBS only. On day 40, splenocytes and mesenteric lymph node (MLN) cells were stimulated with 50 µg/ml of BP extract for 72 hours. Levels of IL-4 (A, D), IL-5 (B, E) and IL-10 (C, F) were assessed by ELISA. Results are representative of at least two independent experiments each with five mice per group. Data is expressed as mean ±SEM. *p<0.05; **p<0.01.
The Potential of eMOD to Prevent the Development of Allergy is Mediated by Heat-stable Components
To elucidate whether heat-stable components of eMOD are responsible for the suppression of Bet v 1-specific responses, eMOD was heated at 96°C for 15 min to denaturate the protein components. As shown in Fig. 7, heat-treated eMOD could still reduce IgE-dependent basophil degranulation in response to Bet v1 (Fig. 7 A) and retained the ability to suppress allergen-induced airway eosinophilia (Fig. 7 B, C) and peribronchial and perivascular cellular infiltration and mucus production in lungs (Fig. 7 C).
Figure 7. The suppressive effects of eMOD are heat-stable.
Mice were immunized intraperitoneally with 1 µg of Bet v 1 admixed to 50 µg of eMOD (black bars; eMOD/sens) or heat-treated eMOD (striped bars; hi-eMOD/sens) on days 0, 14 and 28, then intranasally challenged with 100 µg of birch pollen extract (BP) on three consecutive days one week after the last sensitization (days 35–37). Control mice (white bars; sens) were immunized with Bet v 1 admixed to PBS. Serum, bronchoalveolar lavage fluid (BALF) and lung tissue for histology were collected at day 40. Functional IgE was measured by Bet v 1-mediated β-hexosaminidase release from rat basophil leukemia cells (A). The dashed line indicates the background level of the assay. (B) The numbers of total and differential cells in BALF. Results are representative of two experiments each with five mice per group. Data is expressed as mean ±SEM. **p<0.01; ns = not significant; Mac = macrophages; Eos = eosinophils, Neu = neutrophils; Lym = lymphocytes. (C) Representative haematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) staining of paraformaldehyde-fixed lung tissue sections at 40×magnification. Representative BALF cytospins were stained with H&E and are shown at 100×magnification.
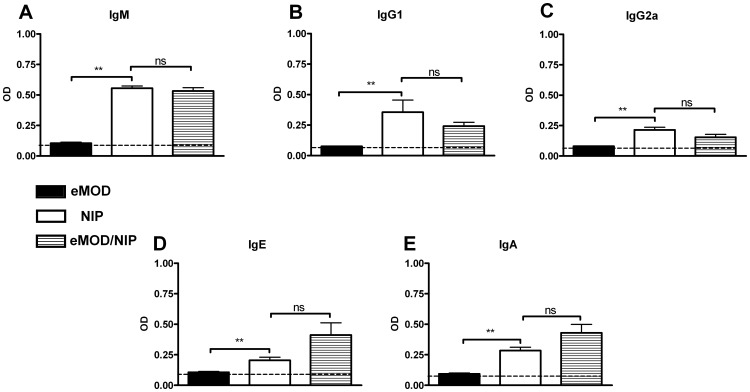
Co-administration of eMOD during Immunization Suppresses Humoral Responses to Thymus-Dependent but not to Thymus-independent Model Antigen
To distinguish if the suppression of allergy is due to modulation of T helper cell or B cell function, we measured the impact of eMOD on responses to a thymus-dependent and -independent model antigens DT and NIP-Ficoll, respectively. Immunization with DT led to the production of DT-specific IgM, IgG (predominantly IgG1 but also IgG2a), IgE and IgA in serum, as expected (Fig. 8 A–E). When DT was co-administered with eMOD, anti-DT IgG1 (Fig. 8 B), IgE (Fig. 8 D) and IgA (Fig. 8 E) levels were significantly reduced. The responses to DT-specific IgM (Fig. 8 A) and IgG2a (Fig. 8 C) were not significantly influenced by eMOD. Next we investigated the impact of eMOD on humoral responses to thymus-independent model antigen NIP-Ficoll. Immunization of mice with NIP-Ficoll led to the production of specific IgM (Fig. 9 A), IgG1 (Fig. 9 B), IgG2a (Fig. 9 C), IgE (Fig. 9 D), and IgA (Fig. 9 E) antibodies in sera. These antibody responses were comparable between mice immunized with NIP-Ficoll and mice immunized with NIP-Ficoll admixed to eMOD (Fig. 9 A–E). Thus, immune responses to thymus independent antigen NIP-Ficoll which activates B cells and antibody production in the absence of T cell support were similar between NIP-Ficoll-immunized mice irrespective of the simultaneous administration of eMOD.
Figure 8. Co-application of eMOD suppresses humoral responses to thymus-dependent diphtheria toxoid antigen.
Mice were immunized intraperitoneally with 50 µg of eMOD (dark bars; eMOD) or 10 µg of diphtheria toxoid-alum (white bars; DT) or 50 µg of eMOD admixed to 10 µg of diphtheria toxoid-alum (striped bars; eMOD/DT) on days 0 and 7. Sera were collected on day 21. Levels of DT-specific IgM (A), IgG1 (B), IgG2a (C), IgE (D), and IgA (E) were detected by ELISA. The dashed line indicates the background level of the assay. Results are representative of two experiments each with five mice per group and data are expressed as mean ±SEM. *p<0.05; **p<0.01; ns = not significant.
Figure 9. Co-application of eMOD does not interfere with humoral responses to thymus-independent NIP-Ficoll antigen.
Mice were immunized intraperitoneally with 50 µg of eMOD (dark bars; eMOD) or 200 µg of NIP-Ficoll (white bars; NIP) or 50 µg of eMOD admixed to 200 µg of NIP-Ficoll (striped bars; eMOD/NIP) on days 0 and 7. Sera were collected on day 21. Levels of NIP-specific IgM (A), IgG1 (B), IgG2a (C), IgE (D), and IgA (E) were detected by ELISA. The dashed line indicates the background level of the assay. Results are representative of two experiments each with five mice per group and data are expressed as mean ±SEM. **p<0.01; ns = not significant.
Discussion
Our study has investigated the immune response to eMOD, i.e. a extract from male O. dentatum, and its interactions with the immune system in three different experimental settings: i) sensitization and aerosol challenge with birch pollen allergen (type I allergy); ii) immunization with the thymus-dependent model antigen DT; and iii) immunization with the thymus-independent model antigen NIP-Ficoll.
Type 2 immune responses, characterized by the induction of cytokines IL-4, IL-5, the antibody isotypes IgG1 and IgE, as well as expanded populations of eosinophils, are induced by and confer protection against helminth infections in both humans and animals [31]. Here we have shown that live infection is not a prerequisite for induction of type 2 immune response. Immunization of BALB/c mice with eMOD led to induction of both humoral and cellular type 2 responses. In serum, eMOD-specific IgG responses consisted predominantly of IgG1 (Th2-associated isotype). Similarly, eMOD stimulation of splenocytes and MLN cells derived from eMOD-immunized mice led to the production of IL-4 and IL-5 (Th2-associated cytokines). Mechanisms leading to the adaptive Th2 immunity are mainly dependent on early IL-4 production, as well as the essential participation of dendritic cells [32]. The induction and maintenance of Th2 responses in this new eMOD model would be well worth exploring in future work.
The regulatory cytokines IL-10 and TGF-β were also induced in eMOD-re-stimulated spleen and MLN cell cultures. Chronic helminth infections generally induce multiple regulatory pathways, involving regulatory T cells, B cells, dendritic cells, and macrophages, with IL-10 and TGF-β playing an important role [33], [34], [35]. Furthermore, responsiveness to vaccine antigens such as BCG was attenuated in helminth infected subjects with concomitant increase in levels of TGF-β [36].
Despite the fact that both helminth infections and external allergens induce similar immunological responses, epidemiological data indicate that infections with certain parasites, such as hookworms or schistosomes, can protect from allergic disorders [37]. Furthermore, excretory-secretory products from H. polygyrus can also suppress allergen-specific Th2 responses and pathology in the OVA-induced mouse model of type I allergy [23]. In our current study we investigated whether eMOD can protect against allergic disease in a model of clinically relevant birch pollen allergy. Co-administration of eMOD with recombinant allergen Bet v 1 significantly suppressed allergen specific immune responses. The fact that only low levels of Bet v 1-specific antibodies were detected in serum of eMOD/Bet v 1-treated mice in comparison to sensitized controls suggest that the presence of eMOD during the allergen-sensitization phase might interfere with the development of Bet v 1-specific responses, resulting in significantly reduced eosinophilia and mucus production after the intranasal challenge with birch pollen extract.
Helminth parasites produce enzymes with protease activity that facilitate their entry into the host [38]. Thus, proteases in eMOD could be responsible for the modification/digestion of Bet v 1 protein in vivo, preventing antigen-presenting cells, such as DC, from stimulating T cells. However, the fact that heat-treatment preserves the immunomodulatory effects of eMOD suggests that enzymatic effects do not play a major role in our model and suppression is likely to be mediated by heat-stable non-protein compounds.
We found that eMOD reduced production of both Th2-associated IgG1 and IgE as well as Th1-associated IgG2a to Bet v 1 in serum, suggesting that allergy inhibition was not achieved by immune deviation toward Th1 as previously suggested in other settings [39], [40]. This is of particular relevance since recent animal studies have shown that allergen-specific Th1 cells are recruited to the lung of sensitized and challenged mice, contributing to the development of severe airway inflammation and causing acute lung pathology [41], [42]. Thus, suppression of allergen-specific responses, rather than immune deviation toward allergen-specific Th1 responses might be the strategy of choice for the prevention of allergy.
Interestingly, eMOD suppresses Bet v 1-specific antibody production in serum but simultaneously induces production of eMOD-specific antibodies. Similarly, excretory/secretory products derived from N. brasiliensis (NES) were shown to induce NES-specific Th2 responses and at the same time inhibit the development of OVA-specific responses [22]. Again, these data suggest that eMOD prevents the development of allergy without evoking a general suppression of the immune system, indicating that this extract could be used for allergen-specific prevention strategies.
In view of the high amounts of IL-10 and TGF-β produced in eMOD-stimulated cell cultures, it can be expected that Treg (Foxp3+) cells will be increased by expansion or by de novo induction. There are numerous studies showing the importance of Treg cells in modulating host immune responses. In several animal infection models, helminth parasites such as H. polygyrus [43], Litomosomoides sigmodontis [44] or Schistosoma mansoni [45], induce regulatory responses via different regulatory cells, most prominently the CD4+CD25+Foxp3+ population [35]. Excretory/secretory products of H. polygyrus induced Foxp3 expression in T cells in vitro through the TGF-β pathway and are able to suppress airway allergy [46]. However, using Foxp3-eGFP mice we have shown recently that eMOD does not have a potential to expand and/or induce de novo T cells Foxp3 expression in vitro (data not shown). Similarly, Ilic et al. have shown that products derived from T. spiralis induce high levels of IL-10 but they do not impact on the existing Foxp3+ cell population or induce Foxp3+ cells de novo [47].
Regulatory cytokine IL-10 has been shown to play an important role in control of allergic airway disease by helminth infections or by helminth-derived products [48], [49]. Interestingly, levels of IL-10 were down-regulated similarly to IL-4 and IL-5 in birch pollen-stimulated splenocytes derived from eMOD-treated and sensitized mice. This observation is in agreement with our previous studies, in which prevention of allergy by application of probiotic bacteria was associated with reduced levels of IL-10 in allergen-re-stimulated splenocytes [50], [51].
Pre-existing helminth infections interfere not only with the development of allergy but also with the efficacy of different vaccines, including BCG [36], [52], [53], [54], [55], influenza [56], tetanus [57], [58] or malaria vaccine candidates [59], [60]. On the basis of these reports we investigated whether responses to both T cell-dependent (DT) and T cell-independent (NIP-Ficoll) model antigens can be modulated by eMOD. We show that immunization of mice with DT antigen admixed to eMOD resulted in significant reduction of DT-specific IgG1 and IgE. In contrast, eMOD has no impact on T cell-independent DT-specific IgM and NIP-Ficoll specific antibody production. NIP-Ficoll is a hapten coupled to polymeric molecule leading to B cell receptor cross-linking, partial B cell maturation, class switching and antibody production without the requirement of antigen presentation to T helper cells by antigen presenting cells (APC) [61], [62]. Thus, our data suggest that rather than impairing B cell responses, eMOD might interfere with T cell functions directly or through the effects on APCs, such as DC. Indeed, we have shown that stimulation of BM-DC leads to production of the regulatory milieu which might be responsible for suppression of T cell responses. Along these lines, Boitelle et al. report that helminth products interfered with the efficient expansion of OVA specific CD4 T cells in vivo [63] and infection with S. ratti directly suppresses antigen-specific proliferation of T helper cells [64]. Studies investigating the impact of eMOD on antigen uptake by dendritic cells and their antigen presentation to T cells are now under way.
Trujillo-Vargas et al. also showed that heat-treated and proteinase K-digested NES retained the ability to suppress OVA-induced airway eosinophilia, but did not retain the ability to suppress IgE responses, implicating both heat-stable and heat-labile suppressive compounds in NES [22]. In our study, heat-treatment of eMOD retained the protective effects against birch pollen allergy, as Bet v 1-specific RBL release, infiltration of eosinophils in BALF, and reduced peribronchial inflammatory infiltrate and mucus hypersecretion were markedly reduced in mice treated with both native and heat-treated eMOD in comparison to sensitized and challenged control animals. These data indicate that non-protein components, such as carbohydrates, may play an important role in allergy suppression. Previous data, based on lectin binding studies, indicate that O. dentatum displays stage-specific glycoconjugates [65], [66]. More recently, structural data on glycan decorations of O. dentatum structures were analyzed and galactosylated fucose epitopes have been identified [67]. We are currently carrying out experiments to identify the compounds in eMOD with immunomodulatory potential.
Taken together, we clearly established that eMOD inhibits the development of allergen-specific immune responses including antibody production, cellular recall responses, and airway eosinophilia in a mouse model of allergy. Furthermore, the allergy-protective effects of eMOD are mediated by heat-stable compounds which might interfere with antigen presenting cells or T cell function. Understanding of the molecular cross-talk between chronic helminth infections and the host immune system as well as identification of parasite-derived molecules involved in immunomodulation could now pave the way to novel prophylactic/therapeutic strategies for immune dysfunctions such as allergies and autoimmunity.
Acknowledgments
We thank Erika Garner-Spitzer, Joanna Jasinska and Jonas Hohlweg for invaluable assistance and helpful discussions. We also thank to Michael Duchêne for critical review of the manuscript.
Funding Statement
This work was financially supported by SFB grant “Towards prevention and therapy of allergy”, F46 from Austrian Science Fund. The Austrian Science Fund had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Maizels RM, Hewitson JP, Smith KA (2012) Susceptibility and immunity to helminth parasites. Curr Opin Immunol 24: 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hartgers FC, Obeng BB, Kruize YC, Duijvestein M, de Breij A, et al. (2008) Lower expression of TLR2 and SOCS-3 is associated with Schistosoma haematobium infection and with lower risk for allergic reactivity in children living in a rural area in Ghana. PLoS Negl Trop Dis 2: e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flohr C, Tuyen LN, Lewis S, Quinnell R, Minh TT, et al. (2006) Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: A cross-sectional study. J Allergy Clin Immunol 118: 1305–1311. [DOI] [PubMed] [Google Scholar]
- 4. Rodrigues LC, Newcombe PJ, Cunha SS, Alcantara-Neves NM, Genser B, et al. (2008) Early infection with Trichuris trichiura and allergen skin test reactivity in later childhood. Clin Exp Allergy 38: 1769–1777. [DOI] [PubMed] [Google Scholar]
- 5.Alcantara-Neves NM, Veiga RV, Dattoli VC, Fiaccone RL, Esquivel R, et al.. (2012) The effect of single and multiple infections on atopy and wheezing in children. J Allergy Clin Immunol 129: 359–367, 367 e351–353. [DOI] [PMC free article] [PubMed]
- 6. Lynch NR, Hagel I, Perez M, Di Prisco MC, Lopez R, et al. (1993) Effect of anthelmintic treatment on the allergic reactivity of children in a tropical slum. J Allergy Clin Immunol 92: 404–411. [DOI] [PubMed] [Google Scholar]
- 7. van den Biggelaar AH, Rodrigues LC, van Ree R, van der Zee JS, Hoeksma-Kruize YC, et al. (2004) Long-term treatment of intestinal helminths increases mite skin-test reactivity in Gabonese schoolchildren. J Infect Dis 189: 892–900. [DOI] [PubMed] [Google Scholar]
- 8. Flohr C, Tuyen LN, Quinnell RJ, Lewis S, Minh TT, et al. (2010) Reduced helminth burden increases allergen skin sensitization but not clinical allergy: a randomized, double-blind, placebo-controlled trial in Vietnam. Clin Exp Allergy 40: 131–142. [DOI] [PubMed] [Google Scholar]
- 9. Endara P, Vaca M, Chico ME, Erazo S, Oviedo G, et al. (2010) Long-term periodic anthelmintic treatments are associated with increased allergen skin reactivity. Clin Exp Allergy 40: 1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleming JO, Isaak A, Lee JE, Luzzio CC, Carrithers MD, et al. (2011) Probiotic helminth administration in relapsing-remitting multiple sclerosis: a phase 1 study. Mult Scler 17: 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Summers RW, Elliott DE, Qadir K, Urban JF Jr, Thompson R, et al. (2003) Trichuris suis seems to be safe and possibly effective in the treatment of inflammatory bowel disease. Am J Gastroenterol 98: 2034–2041. [DOI] [PubMed] [Google Scholar]
- 12. Summers RW, Elliott DE, Urban JF Jr, Thompson R, Weinstock JV (2005) Trichuris suis therapy in Crohn's disease. Gut 54: 87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Summers RW, Elliott DE, Urban JF Jr, Thompson RA, Weinstock JV (2005) Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology 128: 825–832. [DOI] [PubMed] [Google Scholar]
- 14.Bager P, Arnved J, Ronborg S, Wohlfahrt J, Poulsen LK, et al. (2012) Trichuris suis ova therapy for allergic rhinitis: a randomized, double-blind, placebo-controlled clinical trial. J Allergy Clin Immunol 125: 123–130 e121–123. [DOI] [PubMed]
- 15. Bourke CD, Mutapi F, Nausch N, Photiou DM, Poulsen LK, et al. (2012) Trichuris suis ova therapy for allergic rhinitis does not affect allergen-specific cytokine responses despite a parasite-specific cytokine response. Clin Exp Allergy 42: 1582–1595. [DOI] [PubMed] [Google Scholar]
- 16. Feary J, Venn A, Brown A, Hooi D, Falcone FH, et al. (2009) Safety of hookworm infection in individuals with measurable airway responsiveness: a randomized placebo-controlled feasibility study. Clin Exp Allergy 39: 1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Feary JR, Venn AJ, Mortimer K, Brown AP, Hooi D, et al. (2010) Experimental hookworm infection: a randomized placebo-controlled trial in asthma. Clin Exp Allergy 40: 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McSorley HJ, Gaze S, Daveson J, Jones D, Anderson RP, et al. (2011) Suppression of inflammatory immune responses in celiac disease by experimental hookworm infection. PLoS One 6: e24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kuijk LM, Klaver EJ, Kooij G, van der Pol SM, Heijnen P, et al. (2012) Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol 51: 210–218. [DOI] [PubMed] [Google Scholar]
- 20. Zheng X, Hu X, Zhou G, Lu Z, Qiu W, et al. (2008) Soluble egg antigen from Schistosoma japonicum modulates the progression of chronic progressive experimental autoimmune encephalomyelitis via Th2-shift response. J Neuroimmunol 194: 107–114. [DOI] [PubMed] [Google Scholar]
- 21. Johnston MJ, Wang A, Catarino ME, Ball L, Phan VC, et al. (2010) Extracts of the rat tapeworm, Hymenolepis diminuta, suppress macrophage activation in vitro and alleviate chemically induced colitis in mice. Infect Immun 78: 1364–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trujillo-Vargas CM, Werner-Klein M, Wohlleben G, Polte T, Hansen G, et al. (2007) Helminth-derived products inhibit the development of allergic responses in mice. Am J Respir Crit Care Med 175: 336–344. [DOI] [PubMed] [Google Scholar]
- 23.McSorley HJ, O'Gorman MT, Blair N, Sutherland TE, Filbey KJ, et al.. (2012) Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol. [DOI] [PMC free article] [PubMed]
- 24. Lee KH, Park HK, Jeong HJ, Park SK, Lee SJ, et al. (2008) Immunization of proteins from Toxascaris leonina adult worm inhibits allergic specific Th2 response. Vet Parasitol 156: 216–225. [DOI] [PubMed] [Google Scholar]
- 25. Cardoso LS, Oliveira SC, Goes AM, Oliveira RR, Pacifico LG, et al. (2010) Schistosoma mansoni antigens modulate the allergic response in a murine model of ovalbumin-induced airway inflammation. Clin Exp Immunol 160: 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gasser RB, Cottee P, Nisbet AJ, Ruttkowski B, Ranganathan S, et al. (2007) Oesophagostomum dentatum: potential as a model for genomic studies of strongylid nematodes, with biotechnological prospects. Biotechnol Adv 25: 281–293. [DOI] [PubMed] [Google Scholar]
- 27. Slotved HC, Barnes EH, Bjorn H, Christensen CM, Eriksen L, et al. (1996) Recovery of Oesophagostomum dentatum from pigs by isolation of parasites migrating from large intestinal contents embedded in agar-gel. Vet Parasitol 63: 237–245. [DOI] [PubMed] [Google Scholar]
- 28. Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, et al. (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223: 77–92. [DOI] [PubMed] [Google Scholar]
- 29. Schabussova I, Hufnagl K, Tang ML, Hoflehner E, Wagner A, et al. (2012) Perinatal maternal administration of Lactobacillus paracasei NCC 2461 prevents allergic inflammation in a mouse model of birch pollen allergy. PLoS One 7: e40271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hufnagl K, Wagner B, Winkler B, Baier K, Hochreiter R, et al. (2003) Induction of mucosal tolerance with recombinant Hev b 1 and recombinant Hev b 3 for prevention of latex allergy in BALB/c mice. Clin Exp Immunol 133: 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Allen JE, Maizels RM (2011) Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11: 375–388. [DOI] [PubMed] [Google Scholar]
- 32. Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, et al. (2010) CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med 207: 2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Doetze A, Satoguina J, Burchard G, Rau T, Loliger C, et al. (2000) Antigen-specific cellular hyporesponsiveness in a chronic human helminth infection is mediated by T(h)3/T(r)1-type cytokines IL-10 and transforming growth factor-beta but not by a T(h)1 to T(h)2 shift. Int Immunol 12: 623–630. [DOI] [PubMed] [Google Scholar]
- 34. Smits HH, Everts B, Hartgers FC, Yazdanbakhsh M (2010) Chronic helminth infections protect against allergic diseases by active regulatory processes. Curr Allergy Asthma Rep 10: 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor MD, van der Werf N, Maizels RM T cells in helminth infection: the regulators and the regulated. Trends Immunol 33: 181–189. [DOI] [PubMed] [Google Scholar]
- 36. Elias D, Britton S, Aseffa A, Engers H, Akuffo H (2008) Poor immunogenicity of BCG in helminth infected population is associated with increased in vitro TGF-beta production. Vaccine 26: 3897–3902. [DOI] [PubMed] [Google Scholar]
- 37. Flohr C, Quinnell RJ, Britton J (2009) Do helminth parasites protect against atopy and allergic disease? Clin Exp Allergy 39: 20–32. [DOI] [PubMed] [Google Scholar]
- 38. Donnelly S, Dalton JP, Loukas A (2006) Proteases in helminth- and allergen- induced inflammatory responses. Chem Immunol Allergy 90: 45–64. [DOI] [PubMed] [Google Scholar]
- 39. Lack G, Bradley KL, Hamelmann E, Renz H, Loader J, et al. (1996) Nebulized IFN-gamma inhibits the development of secondary allergic responses in mice. J Immunol 157: 1432–1439. [PubMed] [Google Scholar]
- 40. Gavett SH, O'Hearn DJ, Li X, Huang SK, Finkelman FD, et al. (1995) Interleukin 12 inhibits antigen-induced airway hyperresponsiveness, inflammation, and Th2 cytokine expression in mice. J Exp Med 182: 1527–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hansen G, Berry G, DeKruyff RH, Umetsu DT (1999) Allergen-specific Th1 cells fail to counterbalance Th2 cell-induced airway hyperreactivity but cause severe airway inflammation. J Clin Invest 103: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Randolph DA, Carruthers CJ, Szabo SJ, Murphy KM, Chaplin DD (1999) Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol 162: 2375–2383. [PubMed] [Google Scholar]
- 43. Wilson MS, Taylor MD, Balic A, Finney CA, Lamb JR, et al. (2005) Suppression of allergic airway inflammation by helminth-induced regulatory T cells. J Exp Med 202: 1199–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Taylor MD, van der Werf N, Harris A, Graham AL, Bain O, et al. (2009) Early recruitment of natural CD4+ Foxp3+ Treg cells by infective larvae determines the outcome of filarial infection. Eur J Immunol 39: 192–206. [DOI] [PubMed] [Google Scholar]
- 45. Baumgart M, Tompkins F, Leng J, Hesse M (2006) Naturally occurring CD4+Foxp3+ regulatory T cells are an essential, IL-10-independent part of the immunoregulatory network in Schistosoma mansoni egg-induced inflammation. J Immunol 176: 5374–5387. [DOI] [PubMed] [Google Scholar]
- 46. Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, et al. (2010) Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med 207: 2331–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ilic N, Worthington JJ, Gruden-Movsesijan A, Travis MA, Sofronic-Milosavljevic L, et al. (2011) Trichinella spiralis antigens prime mixed Th1/Th2 response but do not induce de novo generation of Foxp3+ T cells in vitro. Parasite Immunol 33: 572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wohlleben G, Trujillo C, Muller J, Ritze Y, Grunewald S, et al. (2004) Helminth infection modulates the development of allergen-induced airway inflammation. Int Immunol 16: 585–596. [DOI] [PubMed] [Google Scholar]
- 49. Schnoeller C, Rausch S, Pillai S, Avagyan A, Wittig BM, et al. (2008) A helminth immunomodulator reduces allergic and inflammatory responses by induction of IL-10-producing macrophages. J Immunol 180: 4265–4272. [DOI] [PubMed] [Google Scholar]
- 50. Schabussova I, Hufnagl K, Wild C, Nutten S, Zuercher AW, et al. (2011) Distinctive anti-allergy properties of two probiotic bacterial strains in a mouse model of allergic poly-sensitization. Vaccine 29: 1981–1990. [DOI] [PubMed] [Google Scholar]
- 51. Schabussova I, Wiedermann U (2008) Lactic acid bacteria as novel adjuvant systems for prevention and treatment of atopic diseases. Curr Opin Allergy Clin Immunol 8: 557–564. [DOI] [PubMed] [Google Scholar]
- 52. Kilian HD, Nielsen G (1989) Cell-mediated and humoral immune responses to BCG and rubella vaccinations and to recall antigens in onchocerciasis patients. Trop Med Parasitol 40: 445–453. [PubMed] [Google Scholar]
- 53. Rougemont A, Boisson-Pontal ME, Pontal PG, Gridel F, Sangare S (1977) Tuberculin skin tests and B.C.G. vaccination in hyperendemic area of onchocerciasis. Lancet 1: 309. [DOI] [PubMed] [Google Scholar]
- 54. Stewart GR, Boussinesq M, Coulson T, Elson L, Nutman T, et al. (1999) Onchocerciasis modulates the immune response to mycobacterial antigens. Clin Exp Immunol 117: 517–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Elias D, Wolday D, Akuffo H, Petros B, Bronner U, et al. (2001) Effect of deworming on human T cell responses to mycobacterial antigens in helminth-exposed individuals before and after bacille Calmette-Guerin (BCG) vaccination. Clin Exp Immunol 123: 219–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. van Riet E, Adegnika AA, Retra K, Vieira R, Tielens AG, et al. (2007) Cellular and humoral responses to influenza in gabonese children living in rural and semi-urban areas. J Infect Dis 196: 1671–1678. [DOI] [PubMed] [Google Scholar]
- 57. Cooper PJ, Espinel I, Paredes W, Guderian RH, Nutman TB (1998) Impaired tetanus-specific cellular and humoral responses following tetanus vaccination in human onchocerciasis: a possible role for interleukin-10. J Infect Dis 178: 1133–1138. [DOI] [PubMed] [Google Scholar]
- 58. Cooper PJ, Espinel I, Wieseman M, Paredes W, Espinel M, et al. (1999) Human onchocerciasis and tetanus vaccination: impact on the postvaccination antitetanus antibody response. Infect Immun 67: 5951–5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Noland GS, Chowdhury DR, Urban JF Jr, Zavala F, Kumar N (2010) Helminth infection impairs the immunogenicity of a Plasmodium falciparum DNA vaccine, but not irradiated sporozoites, in mice. Vaccine 28: 2917–2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Esen M, Mordmuller B, de Salazar PM, Adegnika AA, Agnandji ST, et al. (2012) Reduced antibody responses against Plasmodium falciparum vaccine candidate antigens in the presence of Trichuris trichiura. Vaccine. [DOI] [PubMed]
- 61. Hartmann W, Haben I, Fleischer B, Breloer M (2011) Pathogenic nematodes suppress humoral responses to third-party antigens in vivo by IL-10-mediated interference with Th cell function. J Immunol 187: 4088–4099. [DOI] [PubMed] [Google Scholar]
- 62. Mond JJ, Lees A, Snapper CM (1995) T cell-independent antigens type 2. Annu Rev Immunol 13: 655–692. [DOI] [PubMed] [Google Scholar]
- 63. Boitelle A, Di Lorenzo C, Scales HE, Devaney E, Kennedy MW, et al. (2005) Contrasting effects of acute and chronic gastro-intestinal helminth infections on a heterologous immune response in a transgenic adoptive transfer model. Int J Parasitol 35: 765–775. [DOI] [PubMed] [Google Scholar]
- 64.Hartmann W, Eschbach ML, Breloer M (2012) Strongyloides ratti infection modulates B and T cell responses to third party antigens. Exp Parasitol. [DOI] [PubMed]
- 65. Joachim A, Ruttkowski B, Daugschies A (2001) Characterisation of stage-specific proteins of Oesophagostomum dentatum by preparative isoelectric focusing and lectin blotting. Parasitol Int 50: 41–45. [DOI] [PubMed] [Google Scholar]
- 66. Joachim A, Ruttkowski B, Daugschies A (1999) Changing surface antigen and carbohydrate patterns during the development of Oesophagostomum dentatum . Parasitology 119 (Pt 5): 491–501. [DOI] [PubMed] [Google Scholar]
- 67. Yan S, Bleuler-Martinez S, Plaza DF, Kunzler M, Aebi M, et al. (2012) Galactosylated fucose epitopes in nematodes: increased expression in a Caenorhabditis mutant associated with altered lectin sensitivity and occurrence in parasitic species. J Biol Chem 287: 28276–28290. [DOI] [PMC free article] [PubMed] [Google Scholar]