Abstract
Aims
Roles of glucagon-like peptide-1 (GLP-1) in extra-pancreatic tissues remain unclear. The aim of this study was to examine determinants of GLP-1 secretory function and possible contribution of GLP-1 to blood pressure (BP) regulation.
Methods and Results
We recruited 128 subjects who received annual examinations and 75g-oral glucose tolerance tests (OGTT) in the Tanno-Sobetsu cohort. Subjects on regular medications for cardiovascular and/or metabolic diseases were excluded, and data for the remaining 103 subjects were used for the univariate and multivariate analyses. Age, plasma glucose (PG), hemoglobin A1c (HbA1c), plasma insulin, and serum lipids were not selected as independent determinants of fasting GLP-1 level by multiple linear regression analysis. However, age and female sex were selected as independent positive determinants of the area under the curve of GLP-1 level during OGTT (AUCGLP-1), an index of GLP-1 secretory function. Multiple linear regression analysis indicated that AUCGLP-1 was an independent negative predictor of systolic BP (SBP), while AUCGLP-1 was not correlated with fasting PG or HbA1c level. In subgroup analyses using the median of AUCGLP-1 to divide the study subjects into high and low GLP-1 response groups, AUCGLP-1 was significantly correlated with both SBP and diastolic BP (r = 0.40 and 0.28, respectively) in the low GLP-1 response group but not in the high GLP-1 response group.
Conclusions
The results of the present study suggest that GLP-1 secretory function is involved in prevention of BP elevation and that the GLP-1 response to oral glucose rather increases with aging perhaps as an adaptive phenomenon.
Introduction
Glucagon-like peptide 1 (GLP-1), one of the incretins, is secreted from L-cells in the small intestine after meals, contributing to enhancement of post-prandial insulin secretion, suppression of glucagon secretion and deceleration of gastric emptying [1], [2]. Both increase in vagal tone and activation of L-cells by dietary nutrients participate in triggering GLP-1 secretion into the blood stream. GLP-1 is rapidly inactivated by dipeptidyl peptidase-4 (DPP-4) and is eliminated mainly from the kidney. Post-prandial level of GLP-1 is reduced in patients with type 2 diabetes [3], [4], and thus DPP-4 inhibitors and GLP-1 analogues have been widely used for control of plasma glucose (PG) levels in diabetic patients. However, the physiological functions of GLP-1 in extra-pancreatic tissues have not been fully characterized, though regulation of bone metabolism, progenitor cell proliferation in the brain, lipogenesis in adipose tissue and angiogenesis in the heart have been proposed [1], [2]. It is also unclear how basal (i.e., pre-prandial) GLP-1 level is regulated and how GLP-1 secretory capacity is regulated in healthy subjects.
In the present study, we first examined whether basal (fasting) GLP-1 level and GLP-1 secretory function are determined by any of the demographic or metabolic parameters in apparently healthy subjects who participated in annual health examinations. Second, we examined the possibility that GLP-1 secretory capacity is involved in blood pressure (BP) regulation. The rationale for this hypothesis is two-fold. First, earlier studies [5], [6] have demonstrated that GLP-1 enhances urinary sodium excretion. Second, GLP-1 and its analogues lowered BP in Dahl salt–sensitive rats [7] and in patients with type 2 diabetes [8]–[11]. Apparently healthy subjects in the Tanno-Sobetsu cohort [12], [13] were recruited to the present study, and we examined relationships between fasting plasma GLP-1 level, plasma GLP-1 response to oral glucose loading, and demographic and metabolic parameters. Results of the analysis suggested that endogenous GLP-1 plays a role in BP regulation and that GLP-1 response to glucose loading rather increases with aging perhaps as an adaptive response.
Methods
The protocol of this study was approved by the Ethics Committee of Sapporo Medical University and we conducted this study according to the principles expressed in the Declaration of Helsink. Written informed consent was obtained from all subjects who participated in the Tanno-Sobetsu Study [12], [13].
Study Subjects
We recruited participants in the Tanno-Sobetsu Study [12], [13], a study with a population-based prospective cohort design, to the present analyses. In the Tanno-Sobetsu Study, residents of two towns, Tanno and Sobetsu, in Japan were recruited for annual or biannual medical examination, including standard blood and urine tests and electrocardiogram. Medical history, including use of medications, was taken and recorded by registered nurses. Parts of plasma samples were frozen and stored for later analyses. If the annual examination showed that fasting PG was 100 ∼ 125 (mg/dl) and/or glycohemoglobin A1c (HbA1c in national glycohemoglobin standardization program [NGSP] scale) was 5.6 ∼ 6.4%, the subject was invited to undergo an oral glucose tolerance test (OGTT) scheduled one month after the annual examination. From 2009 ∼ 2011, 881 subjects received annual examinations in Sobetsu and 315 of them received invitation to OGTT. Totally 128 subjects underwent OGTTs and were recruited to the present study. They did not differ from subjects who did not respond to invitation to OGTT (n = 187) in demographic parameters (i.e., age, sex, BP, fasting PG, HbA1c, serum lipids and renal function indices) (data not shown). Based on history taken by the nurses, 25 of 128 subjects were excluded due to clinical diagnosis of diabetes mellitus and/or regular medications for cardiovascular or metabolic diseases. The remaining 103 subjects constituted the study population for the present analyses.
Measurements
Medical examinations were performed in the early morning after an overnight fast. In physical examinations, systolic BP (SBP) and diastolic BP (DBP) were measured twice after a 5-min rest on a seat and the values were averaged. Body mass index (BMI, kg/m2) was calculated as weight (kg)/height2 (meters). Urine was sampled for determination of albumin and creatinine (Cr) levels. Peripheral venous blood was drawn for determination of high-density lipoprotein cholesterol (HDL-C, mg/dL), total cholesterol (TCHO, mg/dL), fasting PG, triglyceride (TG), serum Cr, high-sensitivity C-reactive protein (hs-CRP) and brain natriuretic peptide (BNP). Low-density lipoprotein cholesterol (LDL-C, mg/dL) was calculated by the Friedewald formula (TCHO - HDL - TG/5). Estimated glomerular filtration rate (eGFR) was calculated from data on serum Cr, age and sex by use of equations for Japanese [14]. HbA1c was determined by using latex coagulation method and expressed in NGSP scale. OGTT was performed, and PG, immunoreactive insulin (IRI) and GLP-1 were measured before and at 60 and 120 min after drinking Trelan-G™ (75 g glucose in 225 ml water). PG and IRI levels were measured by the hexokinase method and enzyme immunoassay, respectively. Blood samples for GLP-1 assay were collected in tubes containing DPP-4 inhibitor (BD P700, Becton, Dickinson and Co.). Plasma intact GLP-1 level was determined by an ELISA kit (GLP-1 Active ELISA kit, EGLP-35k, Millipore Inc.), which measures intact GLP-1 without cross-reacting with the inactive form of GLP-1 (9–36). As indices of insulin sensitivity, homeostasis model assessment of insulin resistance (HOMA-IR) and Matsuda-DeFronzo index were calculated as previously reported [15], [16].
Statistical Analysis
Numeric variables are expressed as means ± SD. Analysis of variance was used for testing significant differences between group means. As an index of GLP-1 secretory function, area under the curve (AUC) of GLP-1 in the OGTT (AUCGLP-1) was calculated by use of the trapezoidal rule. Similarly, AUCs of PG (AUCPG) and IRI (AUCIRI) in the OGTT were also calculated. Relationships between parameters were examined by use of simple and multiple linear regression analyses. In multiple linear regression analysis, we prepared several models by using all or different combinations of parameters as independent variables for calculation of both regression coefficients and Akaike Information Criterion (AIC). Among the candidate models, we selected the best-fit model using Akaike's Information Criterion (AIC) for each dependent variable. Differences in time courses of PG and IRI in the OGTT were tested by two-way repeated measures analysis of variance and Bonferroni post hoc test for multiple comparisons. Statistical analyses were carried out using JMP (version7 SAS Institute, Cary, NC, USA). Difference was considered to be statistically significant if p was less than 0.05.
Results
Demographic Characteristics of Study Subjects
Demographic and clinical parameters in the study subjects are shown in Table 1. Women had lower SBP and DBP, higher HDL-C and lower TG level than those in men. While fasting PG and fasting GLP-1 were comparable in men and women, AUCPG was smaller and AUCGLP-1 tended to be higher in women than in men. The other parameters were comparable in men and women.
Table 1. Demographic and clinical parameters in study subjects.
| All (n = 103) | Men (n = 43) | Women (n = 60) | |
| Age (years) | 65±9 | 67±8 | 64±10 |
| BMI (kg/m2) | 24.0±3.8 | 24.5±2.8 | 23.7±4.4 |
| SBP (mmHg) | 140±20 | 146±21 | 135±17* |
| DBP (mmHg) | 79±11 | 83±10 | 76±11* |
| Fasting PG (mg/dl) | 96.6±9.7 | 97.6±9.1 | 95.8±10.2 |
| AUCPG (a.u.) | 1647±335 | 1773±323 | 1557±311* |
| Fasting IRI (µIU/ml) | 6.2±4.6 | 6.9±5.7 | 5.7±3.5 |
| AUCIRI (a.u.) | 579±397 | 629±447 | 543±337 |
| Fasting GLP-1 (pmol/l) | 3.33±1.81 | 3.27±1.76 | 3.37±1.87 |
| AUCGLP-1 (a.u.) | 892±544 | 725±465 | 1012±568 |
| LDL-C (mg/dl) | 131±30 | 130±31 | 131±29 |
| HDL-C (mg/dl) | 66±22 | 58±16 | 71±25* |
| Triglyceride (mg/dl) | 118±62 | 132±74 | 107±50* |
| HbA1c (%) | 5.7±0.2 | 5.7±0.2 | 5.7±0.2 |
| S-Cr (mg/dl) | 0.71±0.14 | 0.81±0.14 | 0.63±0.09* |
| eGFR (mL/min/1.73 m2) | 72.2±12.5 | 72.7±13.9 | 71.9±11.5 |
| U-Alb/U-Cr (mg/gCr) | 17.0±54.2 | 25.8±84.3 | 11.0±8.6 |
| BNP (pg/ml) | 20.9±16.4 | 17.6±15.9 | 23.3±16.5 |
| hs-CRP (mg/dl) | 0.08±0.10 | 0.10±0.12 | 0.07±0.09 |
| Matsuda-DeFronzo index | 8.1±4.6 | 7.5±4.9 | 8.5±4.3 |
| HOMA-IR | 1.5±1.2 | 1.7±1.5 | 1.4±0.9 |
| HOMA-β | 67.5±43.3 | 71.4±49.6 | 64.7±38.3 |
Data are presented as means ± SD.
p<0.05 vs. Men.
SBP, systolic blood pressure; DBP, diastolic blood pressure; PG, plasma glucose; IRI, immunoreactive insulin; AUCGLP-1, area under the curve of GLP-1 level during 75 g oral glucose tolerance test; S-Cr, serum creatinine; U-Alb/U-Cr, urinary albumin concentration-to-urinary creatinine concentration ratio; hs-CRP, high-sensitivity C-reactive protein; a.u., arbitrary unit.
Of the 103 subjects, 52 subjects (50.5%) had SBP>140 mmHg and/or DBP>90 mmHg. In the OGTT, impaired glucose tolerance, impaired fasting PG, and diabetic pattern were observed in 33, 2 and 2 subjects, respectively. HbA1c levels in the two subjects showing a diabetic pattern in the OGTT were 5.7% and 6.1%.
Relationships between GLP-1 Secretory Function and Metabolic Parameters
We first examined whether basal (fasting) GLP-1 level correlates with age, SBP, DBP, fasting plasma IRI, fasting PG, serum lipids, HbA1c, HOMA-IR or Matsuda-DeFronzo index. However, none of the parameters were correlated with basal GLP-1 (Table 2) or selected as an independent predictor of basal GLP-1 in multiple linear regression analysis (data not shown).
Table 2. Univariate linear regression analyses for fasting GLP-1 level and AUCGLP-1.
| Fasting GLP-1 (pmol/L) | AUCGLP-1 | |||
| r | p | r | p | |
| Age (years) | −0.068 | 0.49 | 0.18 | 0.073 |
| BMI (kg/m2) | −0.13 | 0.20 | −0.23 | 0.017 |
| SBP (mmHg) | −0.15 | 0.13 | −0.26 | 0.0085 |
| DBP (mmHg) | 0.029 | 0.77 | −0.15 | 0.13 |
| Fasting PG (mg/dl) | −0.025 | 0.80 | −0.049 | 0.62 |
| Fasting IRI (µIU/ml) | 0.024 | 0.81 | −0.12 | 0.22 |
| LDL-C (mg/dl) | −0.082 | 0.41 | −0.14 | 0.15 |
| HDL-C (mg/dl) | −0.036 | 0.71 | 0.16 | 0.12 |
| Triglyceride (mg/dl) | −0.027 | 0.79 | −0.042 | 0.68 |
| HbA1c (%) | 0.12 | 0.24 | 0.15 | 0.13 |
| S-Cr (mg/dl) | −0.092 | 0.36 | −0.091 | 0.36 |
| eGFR (ml/min/1.73 m2) | 0.096 | 0.34 | −0.16 | 0.11 |
| U-Alb/U-Cr (mg/gCr) | −0.076 | 0.45 | −0.13 | 0.21 |
| BNP (pg/ml) | −0.17 | 0.087 | 0.14 | 0.17 |
| hs-CRP (mg/dl) | 0.020 | 0.95 | −0.041 | 0.68 |
| Matsuda-DeFronzo index | −0.077 | 0.44 | 0.020 | 0.84 |
| HOMA-IR | 0.020 | 0.84 | −0.12 | 0.23 |
| HOMA-β | 0.018 | 0.86 | −0.12 | 0.24 |
SBP, systolic blood pressure; DBP, diastolic blood pressure; PG, plasma glucose; IRI, immunoreactive insulin; AUCGLP-1, area under the curve of GLP-1 level during 75 g oral glucose tolerance test; S-Cr, serum creatinine; U-Alb/U-Cr, urinary albumin concentration-to-urinary creatinine concentration ratio; hs-CRP, high-sensitivity C-reactive protein; a.u., arbitrary unit.
In univariate linear regression analysis, AUCGLP-1, an index of secretory function of GLP-1, correlated with BMI and SBP and tended to correlate with age (Table 2). However, in multiple linear regression analysis, sex, age and SBP were selected as independent determinants as shown in Table 3. No significant association was detected between AUCGLP-1 and HbA1c, fasting TG or cholesterol levels or eGFR, and addition of any of these parameters to age, sex and SBP did not improve prediction of AUCGLP-1. AUCGLP-1 was negatively correlated with AUCPG (r = −0.28, p = 0.004) but not with AUCIRI (r = 0.013, p = 0.896).
Table 3. Multiple linear regression analysis for AUCGLP-1 and independent variables.
| B | SE | β | t | p | |
| Sex | 118.0 | 53.1 | 0.215 | 2.22 | 0.029 |
| Age (years) | 12.82 | 6.18 | 0.217 | 2.08 | 0.041 |
| BMI (kg/m2) | −21.39 | 13.4 | −0.150 | −1.60 | 0.113 |
| SBP (mmHg) | −6.221 | 2.66 | −0.226 | −2.34 | 0.022 |
| eGFR (ml/min/1.73 m2) | −1.814 | 4.36 | −0.042 | −0.42 | 0.679 |
B, partial regression coefficient; SE, standard error; β, standardized partial regression coefficient.
The square of the coefficient of multiple correlation (R2) in this model = 0.19.
In multiple regression analyses for fasting PG level and for HbA1c level, neither fasting GLP-1 nor AUCGLP-1 was selected as a significant determinant (data not shown).
Relationship between BP and GLP-1
Fasting GLP-1 level was not correlated with SBP or DBP (Table 2). In contrast, AUCGLP-1 was inversely correlated with SBP (r = −0.26, p = 0.0085) as shown in Figure 1, though such a correlation was not detected for DBP. Table 4 and Table 5 present results of multiple linear regression analyses for SBP and DBP with clinical variables. AUCGLP-1 and age were selected as independent determinants of SBP, though sex was the only variable associated with DBP in the present dataset. Replacement of fasting IRI with AUCIRI in Tables 4 and 5 did not improve R2 values in regression for SBP or DBP (R2 data not shown).
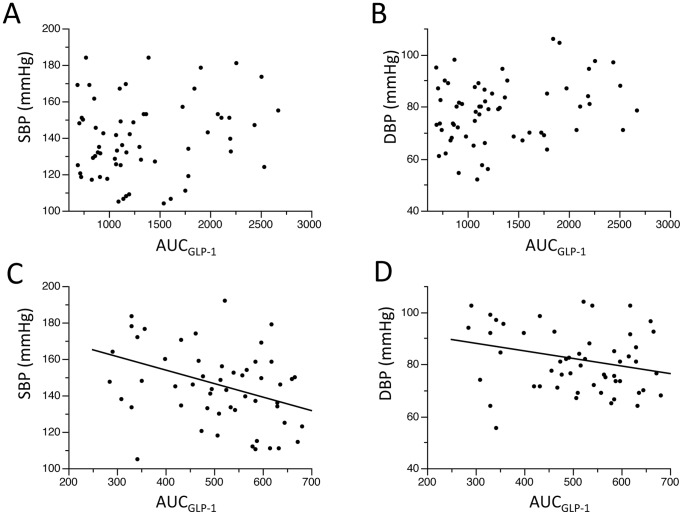
Figure 1. Relationship between blood pressure and AUCGLP-1.
AUCGLP-1 was weakly correlated with SBP (r = −0.26, p = 0.0085, Panel A). However, a significant correlation was not observed for the AUCGLP-1– DBP relationship (Panel B). SBP, systolic blood pressure; DBP, diastolic blood pressure; AUCGLP-1, area under the curve of GLP-1 level in the oral glucose tolerance test.
Table 4. Multiple linear regression analysis for SBP and independent variables.
| B | SE | β | t | p | |
| Sex | −3.26 | 2.01 | −0.164 | −1.62 | 0.108 |
| Age (years) | 0.500 | 0.240 | 0.233 | 2.08 | 0.040 |
| BMI (kg/m2) | 0.0383 | 0.568 | 0.00738 | 0.067 | 0.946 |
| AUCGLP-1 (a.u.) | −0.00868 | 0.00366 | −0.239 | −2.37 | 0.020 |
| Fasting IRI (µU/ml) | 0.518 | 0.468 | 0.120 | 1.11 | 0.270 |
| eGFR (ml/min/1.73 m2) | 0.0124 | 0.166 | 0.00801 | 0.074 | 0.941 |
B, partial regression coefficient; SE, standard error; β, standardized partial regression coefficient; a.u., arbitrary unit.
The square of the coefficient of multiple correlation (R2) in this model = 0.17.
Table 5. Multiple linear regression analysis for DBP and independent variables.
| B | SE | β | t | p | |
| Sex | −3.20 | 1.19 | −0.279 | −2.70 | 0.008 |
| Age (years) | −0.0644 | 0.142 | −0.0523 | −0.455 | 0.650 |
| BMI (kg/m2) | 0.194 | 0.335 | 0.0652 | 0.579 | 0.564 |
| AUCGLP-1 (a.u.) | −0.000767 | 0.00216 | −0.0368 | −0.355 | 0.723 |
| Fasting IRI (µU/ml) | 0.242 | 0.276 | 0.0972 | 0.876 | 0.383 |
| eGFR (ml/min/1.73 m2) | 0.0309 | 0.0982 | 0.0348 | 0.315 | 0.754 |
B, partial regression coefficient; SE, standard error; β, standardized partial regression coefficient; a.u., arbitrary unit.
The square of the coefficient of multiple correlation (R2) in this model = 0.12.
Subgroup Analyses
To examine possible differences in demographic features between the subjects with high AUCGLP-1 and those with low AUCGLP-1, we divided the study subjects into two groups by the median of AUCGLP-1. As shown in Table 6, the low AUCGLP-1 group was younger, included a larger proportion of men and had lower baseline GLP-1 level, larger BMI and higher levels of LDL-C and eGFR than those in the high AUCGLP-1 group. HbA1c and indices of insulin sensitivities (i.e., Matsuda-DeFronzo index and HOMA-IR) were not significantly different between the two groups, though both indices tended to indicate lower insulin sensitivity in the low AUCGLP-1 group. Interestingly, both SBP and DBP were significantly correlated with AUCGLP-1 in the low AUCGLP-1 group (Figure 2), whereas such a correlation was not detected in the high AUCGLP-1 group.
Table 6. Demographic and clinical parameters in low AUCGLP-1 group and high AUCGLP-1 group.
| Low AUCGLP-1 Group (n = 51) | High AUCGLP-1 Group (n = 52) | |
| Men, n (%) | 30 (59)* | 13 (25) |
| Age (year) | 63±10* | 67±8 |
| BMI (kg/m2) | 25.5±3.9* | 22.6±3.1 |
| SBP (mmHg) | 146±20* | 134±18 |
| DBP (mmHg) | 82±12* | 76±11 |
| Fasting PG (mg/dl) | 97.1±9.9 | 96.0±9.6 |
| AUCPG (a.u.) | 1736±359* | 1561±286 |
| Fasting IRI (µIU/ml) | 7.0±5.6 | 5.5±3.0 |
| AUCIRI (a.u.) | 616±450 | 542±338 |
| Fasting GLP-1 (pmol/l) | 2.75±0.76* | 3.89±2.32 |
| AUCGLP-1 (a.u.) | 512±112* | 1265±541 |
| LDL-C (mg/dl) | 139±28* | 123±29 |
| HDL-C (mg/dl) | 62±15 | 69±27 |
| Triglyceride (mg/dl) | 118±55 | 117±70 |
| HbA1c (%) | 5.7±0.2 | 5.7±0.2 |
| S-Cr (mg/dl) | 0.72±0.15 | 0.70±0.14 |
| eGFR (mL/min/1.73 m2) | 75.3±13.7* | 69.2±10.5 |
| U-Alb/U-Cr (mg/gCr) | 25.6±77.0 | 8.81±7.2 |
| BNP (pg/ml) | 17.8±12.3 | 23.9±19.4 |
| hs-CRP (mg/dl) | 0.10±0.11 | 0.07±0.09 |
| Matsuda-DeFronzo index | 7.9±5.2 | 8.3±3.9 |
| HOMA-IR | 1.7±1.5 | 1.3±0.8 |
| HOMA-β | 74.2±52.3 | 60.9±31.3 |
Data are presented as mean ± SD.
p<0.05 vs. High AUCGLP-1 Group.
SBP, systolic blood pressure; DBP, diastolic blood pressure; PG, plasma glucose; IRI, immunoreactive insulin; AUCGLP-1, area under the curve of GLP-1 level during 75 g oral glucose tolerance test; S-Cr, serum creatinine; U-Alb/U-Cr, urinary albumin concentration-to-urinary creatinine concentration ratio; hs-CRP, high-sensitivity C-reactive protein; a.u., arbitrary unit.
Figure 2. Blood pressure - AUCGLP-1 relationships in the high and low AUCGLP-1 groups.
In the high AUCGLP-1 group, neither SBP nor DBP correlated with AUCGLP-1 (Panels A and B). In contrast, there were significant correlations between AUCGLP-1 and SBP (r = −0.40, p = 0.0032, Panel C) and between AUCGLP-1 and DBP (r = −0.28 p = 0.0448, Panel D) in the low AUCGLP-1 group. SBP, systolic blood pressure; DBP, diastolic blood pressure; AUCGLP-1, area under the curve of GLP-1 level in the oral glucose tolerance test.
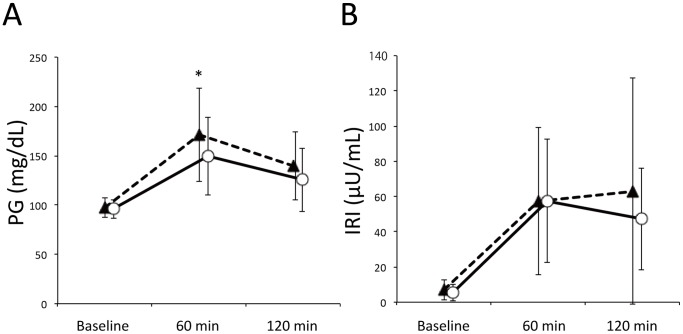
Time courses of PG and IRI levels during OGTT are shown in Figure 3. Levels of fasting PG were similar in the two groups (97.1±9.9 vs. 96.0±9.6 mg/dl). However, PG level at 60 min after oral glucose loading was significantly higher in the low AUCGLP-1 group than in the high AUCGLP-1 group (170.9±47.2 vs. 149.3±39.5 mg/dl). In contrast, IRI levels were similar before and after glucose loading in the high and low AUCGLP-1 groups. AUCPG was significantly larger in the low AUCGLP-1 group than in the high AUCGLP-1 group, though AUCIRI values were similar in the two groups (Table 6).
Figure 3. Time courses of PG and IRI in the OGTT.
Broken lines and solid lines indicate the low AUCGLP-1 group and high AUCGLP-1 group, respectively. The low AUCGLP-1 group showed significantly higher PG level at 60 min after glucose loading than in the high AUCGLP-1 group (Panel A). *p = 0.039. There was no inter-group difference in time courses of plasma IRI during the OGTT (Panel B). PG, plasma glucose; IRI, immunoreactive insulin.
Discussion
Determinants of Baseline Plasma GLP-1 Level and GLP-1 Secretory Function
Since GLP-1 has extra-pancreatic targets of actions [1], [2], not only post-prandial but also baseline (fasting) level of GLP-1 potentially influence functions of extra-pancreatic tissues. However, a few studies have provided information on factors regulating baseline plasma GLP-1 level. Jørgensen et al. [17] reported that growth hormone deficiency induced an increase in fasting GLP-1 level. Pannacciulli et al. [18] showed that fasting GLP-1 level was positively correlated with rates of energy expenditure and negatively correlated with respiratory quotient, indicating a possible link between fatty acid oxidation and GLP-1 level. Since the level of daily energy expenditure potentially modifies insulin sensitivity, we assumed that insulin sensitivity indexes or lipid levels correlate with GLP-1 level. However, we did not find a significant correlation of baseline GLP-1 level with Matsuda-DeFronzo index, HOMA-IR, serum TG, or HDL-C. Also, no significant correlation was found between baseline GLP-1 and BMI, being consistent with results of earlier studies showing that fasting GLP-1 level in obese subjects was unchanged after weight reduction [19], [20]. In contrast to fasting GLP-1 level, post-prandial GLP-1 level was reduced after weight loss in a study by Adam et al. [19]. Nevertheless, the present observations indicate that insulin sensitivity or BMI are not contributory to physiological regulation of fasting GLP-1 level.
To our knowledge, there has been no study that systematically examined determinants of GLP-1 secretory function in non-diabetic subjects. Using AUCGLP-1 during OGTT, we examined the associations of GLP-1 secretory function with metabolic parameters and found that sex and age are independent determinants of AUCGLP-1 (Table 3). The results indicate that response of GLP-1 secretion to glucose loading is larger in women than in men. However, in an earlier study by Carroll et al. [21], time courses of GLP-1 during a 60-min postprandial period were similar in men and women. The number of subjects in their study was small (19 men and 20 women) and they used a 510 kcal test meal to evoke responses of GLP-1 and other hormones. Thus, absence of a sex difference in GLP-1 secretory function in the study by Carroll et al. [21] might have been a type II error or the sex difference in GLP-1 response in the present study might be specific to the situation of loading glucose only. This issue needs further investigation.
In contrast to our expectation, age was indicated to be a positive predictor of AUCGLP-1 in the present analysis (Table 3). We have no clear explanation for the mechanism underlying the positive age-AUCGLP-1 relationship. Since neither serum Cr nor eGFR was selected as an independent determinant of AUCGLP-1 in multiple linear regression analysis (Table 3), age-related decline in renal clearance of GLP-1 cannot explain the age-AUCGLP-1 relationship. One possible explanation is slower degradation of GLP-1 by DPP-4 in elderly subjects. However, this possibility is not supported by recent observations [22]–[24]. Korosi et al. [22] and Tahara et al. [23] showed that plasma DPP-4 activities were similar in young and elderly subjects. Damholt et al. [24] reported that plasma half-life of intravenously injected liraglutide, a GLP-1 analogue, was similar in young and elderly subjects, indicating comparable levels of DPP-4 activity in the two age groups. Another possible explanation for higher AUCGLP-1 in elderly subjects is the age-dependent increase in sensitivity of L-cells to glucose. However, that possibility is counterintuitive and currently lacks any supporting evidence.
GLP-1 Secretory Function and PG Level after Glucose Loading
We could not find a significant relationship between AUCGLP-1 and HbA1c in the present study subjects, 32% of whom showed impaired glucose tolerance. However, as shown in Figure 3, PG levels after glucose loading were higher in the low AUCGLP-1 group than in the high AUCGLP-1 groups. On the other hand, time courses of IRI during the OGTT were similar in the two groups, indicating that insulin sensitivity was reduced in association with decrease in GLP-1 secretory capacity. Two indices of insulin sensitivity (Matsuda-DeFronzo index and HOMA-IR) tended to show lower insulin sensitivity in the low AUCGLP-1 group (Table 6), though the differences did not reach statistical significance. Furthermore, an association of insulin resistance and reduced response of plasma GLP-1 level to glucose loading has been observed in subjects with type 2 diabetes as well [3], [4]. Taken together, the present results indicate that reduced GLP-1 secretory function is associated with insulin resistance and post-prandial hyperglycemia even before insulin secretion is compromised.
The reason for the association between reduced GLP-1 secretion and insulin resistance is unclear. It is unlikely that change in plasma somatostatin level is involved in the association. Somatostatin, which suppresses GLP-1 secretion, was reported to be reduced in obese subjects [25] and in a rat model of metabolic syndrome [26]. In contrast, insulin has been shown to enhance GLP-1 secretion in an ERK-dependent manner, and this response of GLP-1 to insulin was attenuated by chronic hyperinsulinemia in human NCI-H716L cells and mouse models in vitro and in vivo [27]. Thus, there is the possibility that chronic hyperinsulinemia mediates association of insulin resistance and reduction in GLP-1 secretion in diabetic patients. However, in the present study subjects, in whom fasting IRI levels were mostly within the normal range, a significant correlation between fasting IRI and AUCGLP-1 was not detected (Table 2).
Relationship between Endogenous GLP-1 and BP
GLP-1 has extra-pancreatic actions relevant to BP regulation [1], [2], [28]–[30]. The GLP-1 receptor has been shown to localize in endothelial cells and vascular smooth muscle cells [1], [2], and activation of the GLP-1 receptor induces nitric oxide production in endothelial cells and suppresses proliferation of vascular smooth muscle cells [28]–[30]. GLP-1 is also involved in sodium handling in the kidney. Gutzwiller et al. [5], [6] showed that GLP-1 increased sodium excretion in the proximal renal tubule in both healthy subjects and obese insulin-resistant subjects. Such a natriuretic action of a GLP-1 receptor agonist was not detected in GLP-1 receptor knockout mice [31]. BP tended to be higher in the GLP-1 receptor knockout mice than in wild-type mice, though the difference was not statistically significant. Furthermore, a recent study by Kim et al. [32] showed that activation of the GLP-1 receptor in the atria promotes secretion of atrial natriuretic peptide, leading to blood pressure reduction in mice. Collectively, the findings support the notion that the GLP-1 receptor in the vasculature, kidney and atria participates in BP regulation.
Consistent with the vasoprotective actions of GLP-1, GLP-1 infusion has been reported to improve flow-mediated vasodilatation in patients with type 2 diabetes [33]. In addition, Tesauro et al. [34] recently reported that loss of insulin-mediated enhancement of endothelial-dependent and -independent vasodilation in patients with metabolic syndrome was restored by infusion of GLP-1. This effect of GLP-1 was mimicked by vitamin C and the combination of GLP-1 and vitamin C did not further improve vasodilatory response, suggesting that the pathologic mechanism of reactive oxygen species production is a target of GLP-1. Furthermore, GLP-1 analogues and DPP-4 inhibitors have been shown to reduce BP (prior to reduction of body weight) in diabetic and non-diabetic patients with hypertension, [2], [8]–[11], [35]. In contrast to hypertensive subjects, normotensive subjects have been reported to be insensitive to the BP-lowering effect of GLP-1 [33], [36], [37]. GLP-1 infusion did not significantly change BP or heart rate unless hypoglycemia was induced [33], [36], [37]. However, the present study showed that an index of GLP-1 secretory function, AUCGLP-1, negatively correlated with SBP (Figure 1) and the association of AUCGLP-1 with SBP was independent of BMI, plasma IRI and age (Table 3 and Table 4). Furthermore, the relationship between BP and AUCGLP-1 was clearer in the group with low AUCGLP-1 (Figure 2). Taken together, the findings suggest that a slight decline in GLP-1 secretory function allows BP to elevate. In other words, preserved GLP-1 secretory function, leading to physiological GLP-1-mediated vasodilatation and natriuresis, may play a role in prevention of BP elevation.
Acute administration of GLP-1 or a GLP-1 analogue, exenatide, does not decrease BP in healthy subjects [36], [38]. Thus, the inverse correlation between AUCGLP-1 and SBP (Figures 1 and 2) is unlikely to be mediated by acute effects of GLP-1 on the vasculature and/or the sympathetic nervous system and perhaps reflects chronic vasoprotective and natriuretic actions of endogenous GLP-1 [39]–[41].
Level of daily sodium intake is an established determinant of BP, and our recent study confirmed that estimated sodium intake correlated with BP in subjects in the Tanno-Sobetsu cohort [42]. Sodium intake levels estimated from sex, body weight, urinary sodium and Cr data by use of the equation for Japanese [43] were 13.6±3.8 g/day in men and 12.5±3.5 g/day in women in this cohort. It would have been interesting if natriuresis could have been assessed for examination of the relationship between level of natriuresis and AUCGLP-1. However, it is not clear whether estimated sodium intake is sensitive enough for analysis of a modest change in natriuresis by its regulatory factors, and collection of 24-h urine samples from a general population is difficult. Thus, we did not attempt to directly examine relationship between natriuresis and AUCGLP-1 in the present study.
Limitations in the Present Study
There are several limitations in this study. First, subjects in the Tanno-Sobetsu cohort received OGTT on a voluntary basis in addition to examinations mandatory for registration, and invitation to undergo an OGTT was based on FPG and HbA1c data indicating possible glucose intolerance. Thus, selection bias for both higher health-oriented subjects and those with lower glucose tolerance is likely to be present in this study. Second, we excluded all subjects on regular medications and aimed to exclude patients with untreated diabetes and cardiac diseases primarily by medical history (i.e., questionnaire). Thus, the possibility of asymptomatic and undiagnosed cardiovascular diseases could not be ruled out. In fact, BP was above normal limits in approximately half of the study subjects, though diagnosis of hypertension cannot be made by a single measurement of BP. Third, the present study has limitations due to observational cross-sectional analysis and we could not critically discuss cause-and-results relationships for significant associations of AUCGLP-1 with age and with BP. Longitudinal analyses are necessary to confirm the present findings, particularly age-related change in GLP-1 secretory function and relationship between BP and GLP-1 secretory function.
Conclusion
Multiple linear regression analyses of data from subjects on no medication in a general population indicated that an index of GLP-1 secretory function, AUCGLP-1, positively correlated with age and negatively correlated with SBP. Endogenous GLP-1 may be involved in BP regulation, and age-dependent increase in GLP-1 secretory response to glucose loading might be an adaptive response. Mechanisms underlying the changes in AUCGLP-1 and the relationship between endogenous GLP-1 and BP regulation remain to be further studied.
Acknowledgments
The authors sincerely thank the nurses and staff in Sobetsu Town Office for their help in recruitment of study subjects and data collection.
Funding Statement
This study was supported by research grants from Scientific Research (H19-cardiovascular-common-021 and H20-cardiovascular-common-013) from the Japanese Ministry of Health, Labor, and Welfare. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Baggio LL, Druncker DJ (2007) Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157. [DOI] [PubMed] [Google Scholar]
- 2. Ravassa S, Zudaire A, Díez J (2012) GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res 94: 316–323. [DOI] [PubMed] [Google Scholar]
- 3. Toft-Nielsen MB, Damholt MB, Madsbad S, Hilsted LM, Hughes TE, et al. (2001) Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab 86: 3717–3723. [DOI] [PubMed] [Google Scholar]
- 4. Vilsboll T, Krarup T, Deacon CF, Madsbad S, Holst JJ 2001) Reduced postprandial concentrations of intact biologically active glucagon-like peptide 1 in type 2 diabetic patients. Diabetes 50: 609–613. [DOI] [PubMed] [Google Scholar]
- 5. Gutzwiller JP, Tschopp S, Bock A, Zehnder CE, Huber AR, et al. (2004) Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab 89: 3055–3061. [DOI] [PubMed] [Google Scholar]
- 6. Gutzwiller JP, Hruz P, Huber A, Hamel C, Zehnder C, et al. (2006) Glucagon-like peptide 1 is involved in sodium and water homeostasis in humans. Digestion 73: 142–150. [DOI] [PubMed] [Google Scholar]
- 7. Yu M, Moreno C, Hoagland K, Dahly A, Ditter K, et al. (2003) Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens 21: 1125–1135. [DOI] [PubMed] [Google Scholar]
- 8. Garber A, Henry R, Ratner R, Garcia-Hernandez P, Rodriguez-Pattzi H, et al. (2009) Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): a randomised, 52-week, phase III, double-blind, parallel-treatment trial. Lancet 373: 473–481. [DOI] [PubMed] [Google Scholar]
- 9. Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, et al. (2009) Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD). Diabetes Care 32: 1224–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blonde L, Russell-Jones D (2009) The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: an overview of the LEAD 1–5 studies. Diabetes, Obesity and Metabolism 11: 26–34. [DOI] [PubMed] [Google Scholar]
- 11. Okerson T, Yan P, Stonehouse A, Brodows R (2010) Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am J Hypertens 23: 334–339. [DOI] [PubMed] [Google Scholar]
- 12. Ohnishi H, Saitoh S, Takagi S, Katoh N, Chiba Y, et al. (2006) Incidence of type 2 diabetes in individuals with central obesity in a rural Japanese population: the Tanno and Sobetsu Study. Diabetic Care 29: 1128–1129. [DOI] [PubMed] [Google Scholar]
- 13. Mitsumata K, Saitoh S, Ohnishi H, Akasaka H, Miura T (2012) Effects of parental hypertension on longitudinal trends in blood pressure and plasma metabolic profile: mixed-effects model analysis. Hypertension 60: 1124–1130. [DOI] [PubMed] [Google Scholar]
- 14. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, et al. (2009) Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992. [DOI] [PubMed] [Google Scholar]
- 15. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 16. Matsuda M, DeFronzo RA (1999) Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22: 1462–1470. [DOI] [PubMed] [Google Scholar]
- 17. Jørgensen JO, Rosenfalck AM, Fisker S, Nyholm B, Fineman MS, et al. (2000) Circulating levels of incretin hormones and amylin in the fasting state and after oral glucose in GH-deficient patients before and after GH replacement: a placebo-controlled study. Eur J Endocrinol 143: 593–599. [DOI] [PubMed] [Google Scholar]
- 18. Pannacciulli N, Bunt JC, Koska J, Bogardus C, Krakoff J (2006) High fasting plasma concentrations of glucagon-like peptide 1 are associated with higher resting energy expenditure and fat oxidation rates in humans. Am J Clin Nutri 84: 556–560. [DOI] [PubMed] [Google Scholar]
- 19. Adam TC, Jocken J, Westerterp-Plantenga MS (2005) Decreased glucagon like peptide 1 release after weight loss in overweight/obese subjects. Obes Res 13: 710–716. [DOI] [PubMed] [Google Scholar]
- 20. De Luis D, Pacheco D, Conde R, Primo D, Aller R, et al. (2012) Basal GLP-1 levels in morbidity obese patients following biliopancreatic diversion surgery. Ann Nutr Metab 61: 70–73. [DOI] [PubMed] [Google Scholar]
- 21. Carroll JF, Kaiser KA, Franks SF, Deere C, Caffrey JL (2007) Influence of BMI and gender on postprandial hormone responses. Obesity 15: 2974–2983. [DOI] [PubMed] [Google Scholar]
- 22. Korosi J, McIntosh CH, Pederson RA, Demuth HU, Habener JF, et al. (2001) Effect of aging and diabetes on the enteroinsular axis. J Gerontol A Biol Sci Med Sci 56: M575–579. [DOI] [PubMed] [Google Scholar]
- 23. Tahara N, Yamagishi S, Takeuchi A, Tahara A, Kaifu K, et al. (2013) Serum levels of advanced glycation end products (AGEs) are independently correlated with circulating levels of dipeptidyl peptidase-4 (DPP-4) in humans. Clin Biochem 46: 300–303. [DOI] [PubMed] [Google Scholar]
- 24. Damholt B, Golor G, Wierich W, Pedersen P, Ekblom M, et al. (2006) An open-label, parallel group study investigating the effects of age and gender on the pharmacokinetics of the once-daily glucagon-like peptide-1 analogue liraglutide. J Clin Pharmacol 46: 635–641. [DOI] [PubMed] [Google Scholar]
- 25. Schusdziarra V (1988) Physiological significance of gastrointestinal somatostatin. Horm Res 29: 75–78. [DOI] [PubMed] [Google Scholar]
- 26. Li W, Shi YH, Yang RL, Cui J, Xiao Y, et al. (2010) Effect of somatostatin analog on high-fat diet-induced metabolic syndrome: involvement of reactive oxygen species. Peptides 31: 625–629. [DOI] [PubMed] [Google Scholar]
- 27. Lim GE, Huang GJ, Flora N, LeRoith D, Rhodes CJ, et al. (2009) Insulin regulates glucagon-like peptide-1 secretion from the enteroendocrine L cell. Endocrinology 150: 580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Goto H, Nomiyama T, Mita T, Yasunari E, Azuma K, et al. (2011) Extendin-4, a glucagon-like peptide-1 receptor agonist, reduces intimal thickening after vascular injury. Biochem Biophys Res Commun 405: 79–84. [DOI] [PubMed] [Google Scholar]
- 29. Hirata Y, Kurobe H, Nishio C, Tanaka K, Fukuda D, et al. (2013) Extendin-4, a glucagon-like peptide-1 receptor agonist, attenuates neointimal hyperplasia after vascular injury. Eur J Pharmacol 699: 106–111. [DOI] [PubMed] [Google Scholar]
- 30. Dong Z, Chai W, Wang W, Zhao L, Fu Z, et al. (2013) Protein kinase A mediates glucagon-like peptide 1-induced nitric oxide production and muscle microvascular recruitment. Am J Physiol Endocrinol Metab 304: E222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, et al. (2012) Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Renal Physiol 303: F963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, et al. (2013) GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med 19: 567–575. [DOI] [PubMed] [Google Scholar]
- 33. Nyström T, Gutniak MK, Zhang Q, Zhang F, Holst JJ, et al. (2004) Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrionol Metab 287: E1209–1215. [DOI] [PubMed] [Google Scholar]
- 34. Tesauro M, Barini A, Schinzari F, Pitocco D, Adamo A, et al. (2013) Effects of GLP-1 on forearm vasodilator function and glucose disposal during hyperinsulinemia in the metabolic syndrome. Diabetes Care 36: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yerram P, Whaley-Connell A (2012) Novel role for the incretins in blood pressure regulation. Curr Opin Nephrol Hypertens 21: 463–468. [DOI] [PubMed] [Google Scholar]
- 36. Bharucha AE, Charkoudian N, Andrews CN, Camilleri M, Sletten D, et al. (2008) Effects of glucagon-like peptide-1, yohimbine and nitrergic modulation on sympathetic and parasympathetic activity in humans. Am J Physiol Regul Integr Comp Physiol 295: R874–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Edwards CM, Todd JF, Ghatei MA, Bloom SR (1998) Subcutaneous glucagon-like peptide-1 (7–36) amide is insulinotropic and can cause hypoglycemia in fasted healthy subjects. Clin Sci 95: 719–724. [DOI] [PubMed] [Google Scholar]
- 38. Mendis B, Simpson E, MacDonald I, Mansell P (2012) Investigation of the haemodynamic effects of exenatide in healthy male subjects. Br J Clin Pharmacol 74: 437–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hattori Y, Jojima T, Tomizawa A, Satoh H, Hattori S, et al. (2010) A glucagon-like peptide-1 (GLP-1) analogue, liraglutide, upregulates nitric oxide production and exerts anti-inflammatory actions in endothelial cells. Diabetologia 53: 2256–2263. [DOI] [PubMed] [Google Scholar]
- 40. Erdogdu O, Nathanson D, Sjöholm A, Nyström T, Zhang Q (2010) Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA-, and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Mol Cell Endocrinol 325: 26–35. [DOI] [PubMed] [Google Scholar]
- 41. Oesburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, et al. (2010) Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 30: 1407–1414. [DOI] [PubMed] [Google Scholar]
- 42. Akasaka H, Ohnishi H, Saitoh S, Shimamoto K, Miura T (2012) Relationship between blood pressure and oxidative stress via sodium intake: the Tanno and Sobetsu Study. [Abstract] J Hypertens 30: e303. [Google Scholar]
- 43. Kawano Y, Tsuchihashi T, Matsuura H, Ando K, Fujita T, et al. (2007) Report of the Working Group for Dietary Salt Reduction of the Japanese Society of Hypertension: (2) Assessment of salt intake in the management of hypertension. Hypertens Res 30: 887–893. [DOI] [PubMed] [Google Scholar]