Abstract
In the present study, the effects of tamoxifen on pentylenetetrazole (PTZ)-induced repeated seizures and hippocampal neuronal damage in ovariectomized rats were investigated. Thirty seven virgin female Wistar rats were divided to: (1) control, (2) sham-PTZ, (3) sham-PTZ-tamoxifen (sham-PTZ-T), (4) Ovariectomized -PTZ (OVX-PTZ) and (5) OVX-PTZ-tamoxifen (OVX-PTZ-T) groups. The animals of groups 3 and 5 were injected by tamoxifen (10 mg/kg) on 7 consecutive days. After 7 days of tamoxifen injection, they also were then injected by tamoxifen 30 min prior each PTZ injection. PTZ (40 mg/kg) was injected on 6 consecutive days and the animal behaviors were observed for 60 min. The histological methods were then used to determine dark neurons in hippocampus. A significant decrease in the seizure score was seen in OVX-PTZ group compared to Sham-PTZ. The animals of OVX-PTZ-T group had a significant higher seizure score compared to OVX-PTZ group. The dark neurons in DG of OVX group were lower than sham group (p<0.01). The numbers of dark neurons in CA1 area of OVX-PTZ-T group was higher than OVX-PTZ group (p<0.05) compared to control, the numbers of dark neurons in CA3 area showed a significant increase in Sham-PTZ and OVX-PTZ group (p<0.05 and p<0.01 respectively). Dark neurons in OVX-PTZ-T group were higher than OVX-PTZ group (p<0.05). It is concluded that pretreatment of the ovariectomized rats by tamoxifen increased PTZ-induced seizure score and dark neurons. It might be suggested that tamoxifen has agonistic effects for estrogen receptors to change the seizure severity.
Keywords: tamoxifen, seizure, dark neuron, rat, ovariectomized
INTRODUCTION
Estrogen has various effects on the nervous system and it seems to be an essential factor in the normal function of the brain [1, 2]. There is a wide range of estrogen receptors (ERα and ERβ) in numerous regions of the brain including hippocampus. Thus, it is suggested that this hormone affects the structures and functions of hippocampal neurons [3, 4]. This female sexual hormone acts via various mechanisms such as modulation of gene expression, regulation of neurotransmitter release, or direct interactions with neurotransmitter receptors [5]. Thus, estrogen can affect neuronal excitability and seizure susceptibility [6]. But there are controversial results in the case of the effects of estrogen on seizure. In previous studies, both the proconvulsant [7-9] and anticonvulsant effects [10, 11] of estrogen have been shown. We have previously shown that deletion of ovarian hormones attenuate seizure severity in a pentylenetetrazole (PTZ)-induced seizure model [9]. Furthermore, it has been shown that estrogen has neuroprotective effects against seizure-induced neuronal damage [10, 12, 13]. It is suggested that the different effects of estrogens on seizures may depend on various factors such as duration of the treatment, the priority of latency to seizure testing, the mode of administration, the applied dose of estrogen and hormonal status, the region of nervous system or the neurotransmitter system involved, the different seizure inducing models applied, and the sex of the samples [5].
Selective estrogen receptor modulators (SERMs) drugs such as ospemifene, tamoxifen and raloxifene depending on their chemical structure and the specific properties of the target tissues show both agonist/antagonist effects for estrogen actions [14, 15]. Tamoxifen is used for prevention and treatment of all stages of hormone-dependent breast cancer [16]. It is able to act as both estrogen agonist and antagonist depending on the used dose and tissue type. For example the maintenance of bone density and its cardio protective effects are examples of tamoxifen agonistic effects [17]. While, tamoxifen behaves as an antiestrogen in the breast tissue [18] and it also prevents of ovariectomy-induced downregulation of Bcl-2 and up-regulation of Bax expression [19]. In the central nervous system (CNS), tamoxifen was shown to reduce infarct size and neurobehavioral deficits due to middle cerebral artery occlusion in rats [20]. It also attenuates the microglial inflammatory responses and decreased the irradiation-induced brain damage [21]. Tamoxifen was also shown to have the estrogenic effects in hypothalamic differentiation during the neonatal period [22]. Moreover, it enhances choline acetyltransferase mRNA expression in a manner similar to the effects of estrogen in several basal forebrain regions [23]. Tamoxifen also inhibits the release of glutamate to prevent of the neuronal cell death [24]. It has also been suggested that tamoxifen acts as an antihormone in seizure and raises the electroconvulsive threshold in female mice [25].
Dark neurons were noticed to occur in neurosurgical biopsies and it was hypothesized that. They were produced by mechanical stress forces [26-28]. It has been reported that dark neurons may be produced under other conditions such as hypoglycemia, ischemia, and epilepsy [28-30] without any trauma or mechanical forces. Epilepsy produces widespread dark neurons throughout the brain, even under excellent fixation [30, 31].
Brain damage due to various kind of epileptic models such as temporal lobe epilepsy [32-34], partial [35] and febrile seizure [36] has been widely reported.
The aim of the present study was to investigate the effects of tamoxifen on pentylenetetrazole-induced seizure score and dark neuron production in ovariectomized rats.
MATERIALS AND METHODS
Animals and grouping
The experiments were carried out on 37 virgin female Wistar rats weighing 180-220 g. The animals were confined at random in metal cages, and maintained at the animal house under controlled conditions (12 hrs light and dark cycles, 21℃ and 50% relative humidity) with laboratory chow and water provided ad libitum. The animals were randomly divided into 5 groups (N=5-8 in each) as follows: 1- control, 2- sham-PTZ, 3- sham-PTZ-tamoxifen (sham-PTZ-T), 4- OVX-PTZ, 5- OVX-PTZ-Tamoxifen (OVX-PTZ-T).
Tamoxifen in a dose of 10 mg/kg [20] was used in this study. It was dissolved in saline plus a drop of tween 20 and administered on 7 consecutive days intraperitoneally (i.p). After 7 days of tamoxifen injection, PTZ (40 mg/kg) was injected intraperitoneally on 6 consecutive days [37] but the animals also were injected by tamoxifen 30 min prior to each PTZ injection. The rats of sham-PTZ and OVX-PTZ groups received normal saline plus tween 20 instead of tamoxifen. The animals of control group received normal saline (3 ml/kg, i.p.) instead of PTZ.
Surgeries
The rats were ovariectomized under ketamine (150 mg/kg, i.p) anesthesia [38]. After confirmation of anesthesia, ventral incision was made and ovaries and ovarian fats were removed. Ovaries were isolated by ligation of the most proximal portion of the oviduct before removal. These procedures were performed on the sham operated animals except, laparotomy was done without removing the ovaries. The animals were reversed to their cages to recover from the anesthesia [39]. After the surgery, the rats lived in animal house for 6 weeks before conducting the experiment for diminution of the endogenous sex hormones.
PTZ-induced repeated seizures
The animals were injected by 40 mg/kg PTZ [37]. After each PTZ injection, the rats were placed in Plexiglas cages separately and observed for 60 min to detect seizure score.
The resultant seizures were classified according to a modified Racine scale as follows: 1 - Mouth and facial movements; 2 - Head nodding; 3 - Forelimb clonus; 4 - Rearing; 5 - Rearing and falling.
Histological studies
Immediately after interventions and assessment of the animal behaviors, all rats were given a high dose of urethane and transcardially perfused with 100 ml of saline followed by 100 ml fixative solution (glutaraldehyde %3+paraformaldehyde %10 in 0.2 mol buffer phosphate at pH=7.4,T=40 C) for one hour [40, 41]. Just after perfusion, the brains were removed, processed according to routine histological methods and embedded in paraffin. Paraffin blocks were cut into serial coronal sections of 5 µm thickness. Next, ten uniforms random sample sections including hippocampus from each animal were chosen and mounted slides for toluidine blue staining.
Dark neurons per unit area (NA) of the CA1, CA3 and DG subdivisions of hippocampus were counted. The sections were examined under a light microscope using a ×40 objective lens (UPlanFI, Japan) and the images were transferred to a computer using a high-resolution camera (BX51, Japan). All sections were digitally photographed and the numbers of dark neurons were counted using a 10,000 µm2 counting frame. The mean numbers of neurons NA in different regions of hippocampus were calculated using the following formula [42, 43]:
Where "ΣQ"is the sum of counted particles appeared in sections, "a/f" is the area associated with each frame, "ΣP" is sum of frame associated points hitting space.
Statistical analysis
Data were expressed as mean±SEM and were analyzed by non-parametric statistical Kruskal-Wallis followed by Mann-Whitney tests. p values less than 0.05 were considered to be statistically significant.
RESULTS
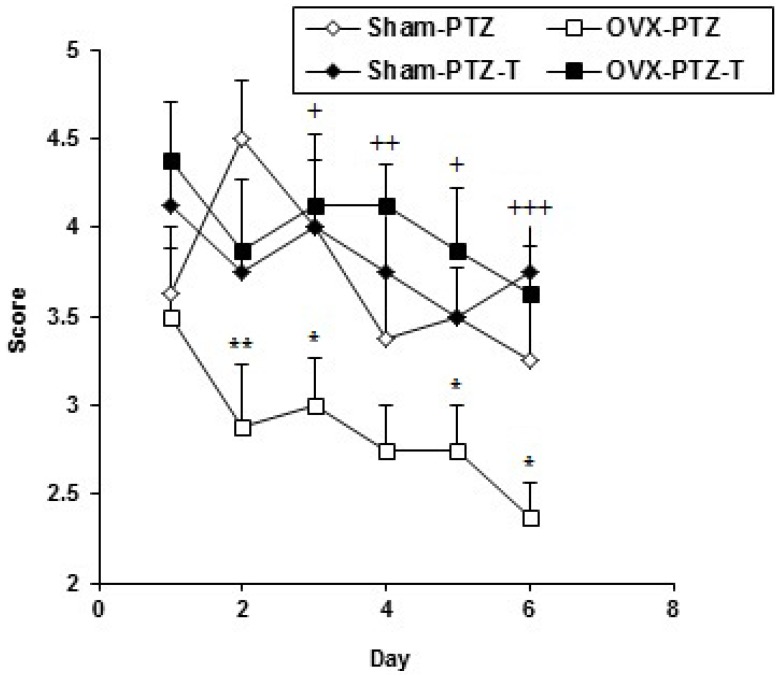
The seizure scores in OVX-PTZ group were significantly lower than sham-PTZ group (p<0.05-p<0.01, Fig. 1). The animals of OVX-PTZ-T had a significant higher seizure score compared to OVX-PTZ group (p<0.05-p<0.001, Fig. 1) however, there was no significant difference between sham-PTZ-T group compared to sham - PTZ group in seizure score (Fig. 1).
Fig. 1.
Comparison of seizure score of Sham-PTZ, Sham-PTZ-T, OVX-PTZ and OVX-PTZ-T groups. Data are presented as mean±SEM (n=8 in each group). The animals of both Sham-PTZ-T and OVX-PTZ-T groups were injected by tamoxifen (10 mg/kg) on 7 consecutive days. They also were then injected by tamoxifen 30 min prior each PTZ injection. PTZ (40 mg/kg) was injected on 6 consecutive days and the seizure score was recorded. The animals of both Sham-PTZ and OVX-PTZ groups received saline instead of tamoxifen. **p<0.01 and *p<0.05 compared to Sham-PTZ, †p<0.05, ††p<0.01 and †††p<0.001 compared to OVX-PTZ group.
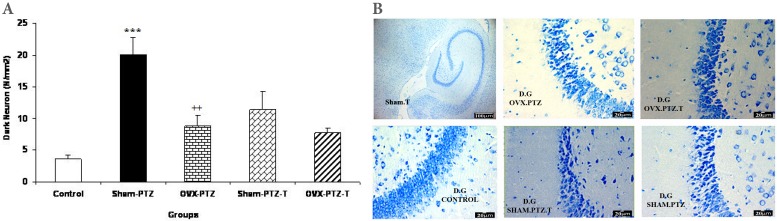
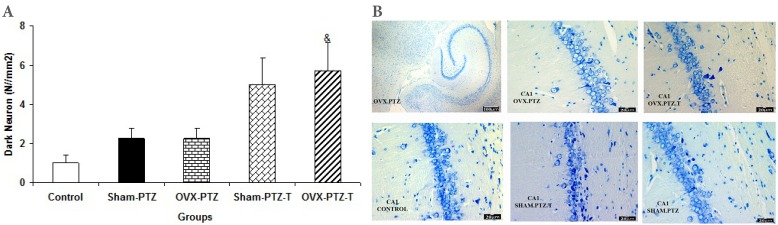
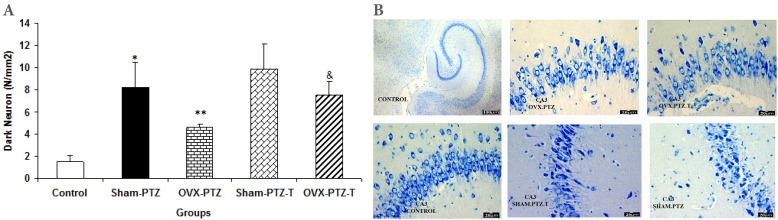
The results of histological study showed that the mean of dark neuron numbers per unit area in DG of sham-PTZ group were higher than that of control group (p<0.001, Fig. 2). The produced dark neuron numbers in DG of OVX-PTZ group were lower than that of sham-PTZ group (p<0.01, Fig. 2). There was no significant difference in dark neurons of DG area between sham-PTZ -T and OVX-PTZ -T groups compared to sham-PTZ and OVX-PTZ groups respectively (Fig. 2). The mean of dark neuron numbers per unit area in CA1 of hippocampal subdivision in OVX-PTZ -T group was higher than that of OVX-PTZ group (p<0.05, Fig. 3). The produced dark neuron numbers per unit area of CA3 showed a significant increase in both Sham-PTZ and OVX-PTZ groups compared to control (p<0.05 and p<0.01 respectively, Fig. 4). The produced dark neurons in OVX-PTZ-T group were also higher than that of OVX-PTZ group (p<0.05, Fig. 4).
Fig. 2.
(A) Comparison of dark neuron numbers per area in DG between control, Sham-PTZ, Sham-PTZ-T, OVX-PTZ and OVX-PTZ-T groups, the brain of control group was removed without PTZ injection. ***p<0.001 compared to control, ††p<0.01 compared to Sham-PTZ group. (B) Photomicrograph shows the dark neuron in DG area of rat hippocampus in the control, Sham-PTZ, Sham-PTZ-T, OVX-PTZ and OVX-PTZ-T groups. Toluidine blue staining, Arrow=dark neuron.
Fig. 3.
(A) Comparison of dark neuron numbers per area in CA1areas among control, Sham-PTZ, Sham-PTZ-T, OVX-PTZ and OVX-PTZ-T groups, the brain of control group was removed without PTZ injection. &P<0.05 compared to OVX-PTZ group. (B) Photomicrograph shows the dark neuron in CA1 area of rat hippocampus in the control, Sham-PTZ, Sham-PTZ-T, OVX-PTZ and OVX-PTZ-T groups, Toluidine blue staining, Arrow=dark neuron.
Fig. 4.
(A) Comparison of dark neuron numbers per area in CA3 area among control, Sham-PTZ, Sham-PTZ-T, OVX-PTZ and OVX-PTZ-T groups, the brain of control group was removed without PTZ injection. *p<0.05 and **p<0.01 compared to control, &p<0.05 compared to OVX-PTZ group. (B) Photomicrograph shows the dark neuron in CA3 area of rat hippocampus in the control, Sham-PTZ, Sham-PTZ-T, OVX-PTZ and OVX-PTZ-T groups, Toluidine blue staining, Arrow=dark neuron.
DISCUSSION
Animal and human studies have shown a clear relationship between seizures and pathological conditions in CNS [36, 44, 45]. Progressive functional and structural abnormalities in CNS, due to repeated seizures have been well documented [46-48]. It has been shown that seizure may lead to morphological changes such as production of dark neurons, in brain tissue.
Dark neurons, previously, were considered as histological artifacts in neurosurgical biopsies [27, 49] but later, the dark neurons were seen after brain trauma [26, 27]. Recently, it has been well documented that dark neurons are also produced without any trauma or mechanical forces [28, 50]. Dark neurons have basophilic appearance and morphological changes and might be seen after hypoglycemia, ischemia, stress and epilepsy [28-30, 51, 52]. Epilepsy has also been introduced as an important cause of dark neuron production [30, 31, 53]. The results of present study showed that PTZ induced repeated seizures were resulted in dark neuron production in hippocampal regions which confirmed our previous studies [43, 54]. Several studies have also confirmed hippocampal damages created by seizures [34-36].
In the present study, ovariectomy modified the PTZ-induced seizures. The results showed that the ovariectomized rats experienced seizures in lower scores than sham-operated ones. We have previously also showed that deletion of ovarian hormones lowered the score in repeated seizure model [37]. Latencies to the first minimal clonic seizure (MCS) as well the first generalized tonic-clonic seizures (GTCS) were also higher in OVX rats than sham operated ones when a high dose of PTZ was administered [9]. All of this findings support the proconvulsant effects of estrogen which has been repeatedly reported [7, 55, 56]. In contrast, it was shown that estrogen treatment may decrease the pilocarpine-induced temporal lobe epilepsy in animal model [57].
It has also been shown that estradiol and other female sex hormones have neuroprotective properties against seizure-induced neuronal damage [10, 12, 13]. However, the dark neuron numbers were higher in the sham group in comparison to the OVX group in the present study. It seems that the interactions between the sex hormones, seizure severity, and seizure-induced neurodegeneration is quite complex. In their study, Velísková et al. showed that estrogen postponed the kainic acid-induced clonic seizures. Estrogen also reduced kainic acid-neuronal damage in CA3 subfield and in the hilus of the dentate gyrus [10]. Hoffman et al. showed that 7 days after ovariectomy, administration of progesterone pellets (silastic capsules containing crystalline progesterone) had anticonvulsant effects, whereas estrogen pellets (0.1 or 0.5 mg/21 days) had little beneficial effect on seizure behavior. They also indicated that these two ovarian hormones reduced kainic acid-induced neuronal damage in the hippocampus [12]. Thus, in the present study, we would have predicted that loss of ovarian hormones should enhance PTZ-induced neuronal damage. In fact, the opposite effects were observed - for example loss of ovarian steroid hormones reduced hippocampal neuronal damage from PTZ-induced seizures. This observation might be due to higher intensity of seizure in the presence of ovarian hormones. Schauwecker et al. indicated that ovariectomy and chemically-induced ovarian failure resulted in a reduction of seizure-induced neurodegeneration; whereas, the administration of estrogen pellets immediately after ovariectomy, prevented the death of hippocampal neurons from kainate-induced seizures [58].
It is believed that the different effects of estrogens on seizures may depend on various factors such as the duration of the treatment, the priority of latency to seizure testing, the mode of administration, the applied dose of estrogen and hormonal status, the region of nervous system or the neurotransmitter system involved, the different seizure inducing models applied, and the sex of the samples [5].
Tamoxifen is a mixed estrogen agonist/antagonist, with estrogen antagonistic effects in some tissues such as breast [59] and estrogen agonistic effects in other tissues such as uterine [60] and bone tissues [61]. The results of present study showed that tamoxifen increased the PTZ-induced seizure score in ovariectomized rats however, it wasn't effective when was administered to the sham operated rats. It seems that the effect of tamoxifen is different in the presence and absence of ovarian hormones. Increasing in the uterine weight as well the luminal epithelial thickness has also been reported [62, 63]. This effect was weaker than 17 β-estradiol in ovariectomized rats. Based on the results of present study the estrogenic effects of tamoxifen in the brain might be suggested. Borowicz et al. who showed that tamoxifen at doses of 20-50 mg/kg significantly raised the threshold for electroconvulsions in female mice [25]. It has been also shown that tamoxifen impaired memory functions in experimental animals [64] however, in another study, Kathleen O'Neill et al. have shown that tamoxifen treatment had no effect on memory function and Alzheimer's disease [14]. The results of present study also showed that tamoxifen increased the dark neuron production in CA1 and CA3 areas of hippocampus. Interestingly, our results demonstrated that tamoxifen enhanced both the seizure severity and dark neuron density in ovariectomized rats. In fact, our results confirmed that tamoxifen can mimic the proconvulsant effects of ovarian hormones.
The results of present study showed that treatment of the ovariectomized rats with tamoxifen increased the seizure score and the dark neuron production due to repeated seizures. Regarding these results, the agonistic effects tamoxifen for estrogen receptors to change the seizure severity might be suggested.
ACKNOWLEDGEMENTS
The results described in this paper were from a M.Sc. student thesis. The authors would like to thank the vice chancellor for research, Mashhad University of Medical Sciences for financial support and we also thank Ms. F. Motejadded for her excellent technical assistance.
References
- 1.Arai Y, Sekine Y, Murakami S. Estrogen and apoptosis in the developing sexually dimorphic preoptic area in female rats. Neurosci Res. 1996;25:403–407. doi: 10.1016/0168-0102(96)01070-x. [DOI] [PubMed] [Google Scholar]
- 2.Miranda RC, Sohrabji F, Toran-Allerand D. Interactions of estrogen with the neurotrophins and their receptors during neural development. Horm Behav. 1994;28:367–375. doi: 10.1006/hbeh.1994.1033. [DOI] [PubMed] [Google Scholar]
- 3.Foster TC. Role of estrogen receptor alpha and beta expression and signaling on cognitive function during aging. Hippocampus. 2012;22:656–669. doi: 10.1002/hipo.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolley CS. Estrogen-mediated structural and functional synaptic plasticity in the female rat hippocampus. Horm Behav. 1998;34:140–148. doi: 10.1006/hbeh.1998.1466. [DOI] [PubMed] [Google Scholar]
- 5.Velísková J. Estrogens and epilepsy: why are we so excited? Neuroscientist. 2007;13:77–88. doi: 10.1177/1073858406295827. [DOI] [PubMed] [Google Scholar]
- 6.Stevens SJ, Harden CL. Hormonal therapy for epilepsy. Curr Neurol Neurosci Rep. 2011;11:435–442. doi: 10.1007/s11910-011-0196-9. [DOI] [PubMed] [Google Scholar]
- 7.Nicoletti F, Speciale C, Sortino MA, Summa G, Caruso G, Patti F, Canonico PL. Comparative effects of estradiol benzoate, the antiestrogen clomiphene citrate, and the progestin medroxyprogesterone acetate on kainic acid-induced seizures in male and female rats. Epilepsia. 1985;26:252–257. doi: 10.1111/j.1528-1157.1985.tb05414.x. [DOI] [PubMed] [Google Scholar]
- 8.Kokka N, Sapp DW, Witte U, Olsen RW. Sex differences in sensitivity to pentylenetetrazol but not in GABAA receptor binding. Pharmacol Biochem Behav. 1992;43:441–447. doi: 10.1016/0091-3057(92)90174-e. [DOI] [PubMed] [Google Scholar]
- 9.Hosseini M, Sadeghnia HR, Salehabadi S, Alavi H, Gorji A. The effect of L-arginine and L-NAME on pentylenetetrazole induced seizures in ovariectomized rats, an in vivo study. Seizure. 2009;18:695–698. doi: 10.1016/j.seizure.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 10.Velísková J, Velísek L, Galanopoulou AS, Sperber EF. Neuroprotective effects of estrogens on hippocampal cells in adult female rats after status epilepticus. Epilepsia. 2000;41(Suppl 6):S30–S35. doi: 10.1111/j.1528-1157.2000.tb01553.x. [DOI] [PubMed] [Google Scholar]
- 11.Yamano Y, Sakane S, Takamatsu J, Ohsawa N. Estrogen supplementation for bone dematuration in young epileptic man treated with anticonvulsant therapy; a case report. Endocr J. 1999;46:301–307. doi: 10.1507/endocrj.46.301. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman GE, Moore N, Fiskum G, Murphy AZ. Ovarian steroid modulation of seizure severity and hippocampal cell death after kainic acid treatment. Exp Neurol. 2003;182:124–134. doi: 10.1016/s0014-4886(03)00104-3. [DOI] [PubMed] [Google Scholar]
- 13.Reibel S, André V, Chassagnon S, André G, Marescaux C, Nehlig A, Depaulis A. Neuroprotective effects of chronic estradiol benzoate treatment on hippocampal cell loss induced by status epilepticus in the female rat. Neurosci Lett. 2000;281:79–82. doi: 10.1016/s0304-3940(00)00784-9. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill K, Chen S, Diaz Brinton R. Impact of the selective estrogen receptor modulator, tamoxifen, on neuronal outgrowth and survival following toxic insults associated with aging and Alzheimer's disease. Exp Neurol. 2004;188:268–278. doi: 10.1016/j.expneurol.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Zheng H, Kangas L, Härkönen PL. Comparative study of the short-term effects of a novel selective estrogen receptor modulator, ospemifene, and raloxifene and tamoxifen on rat uterus. J Steroid Biochem Mol Biol. 2004;88:143–156. doi: 10.1016/j.jsbmb.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Teunissen SF, Rosing H, Koornstra RH, Linn SC, Schellens JH, Schinkel AH, Beijnen JH. Development and validation of a quantitative assay for the analysis of tamoxifen with its four main metabolites and the flavonoids daidzein, genistein and glycitein in human serum using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2519–2529. doi: 10.1016/j.jchromb.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Patisaul HB, Aultman EA, Bielsky IF, Young LJ, Wilson ME. Immediate and residual effects of tamoxifen and ethynylestradiol in the female rat hypothalamus. Brain Res. 2003;978:185–193. doi: 10.1016/s0006-8993(03)02807-5. [DOI] [PubMed] [Google Scholar]
- 18.Michalsen BT, Gherezghiher TB, Choi J, Chandrasena RE, Qin Z, Thatcher GR, Bolton JL. Selective estrogen receptor modulator (SERM) lasofoxifene forms reactive quinones similar to estradiol. Chem Res Toxicol. 2012;25:1472–1483. doi: 10.1021/tx300142h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma K, Mehra RD. Long-term administration of estrogen or tamoxifen to ovariectomized rats affords neuroprotection to hippocampal neurons by modulating the expression of Bcl-2 and Bax. Brain Res. 2008;1204:1–15. doi: 10.1016/j.brainres.2008.01.080. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Jin Y, Behr MJ, Feustel PJ, Morrison JP, Kimelberg HK. Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp Neurol. 2005;196:41–46. doi: 10.1016/j.expneurol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Liu JL, Tian DS, Li ZW, Qu WS, Zhan Y, Xie MJ, Yu ZY, Wang W, Wu G. Tamoxifen alleviates irradiation-induced brain injury by attenuating microglial inflammatory response in vitro and in vivo. Brain Res. 2010;1316:101–111. doi: 10.1016/j.brainres.2009.12.055. [DOI] [PubMed] [Google Scholar]
- 22.Pinilla L, Barreiro ML, Gonzalez LC, Tena-Sempere M, Aguilar E. Comparative effects of testosterone propionate, oestradiol benzoate, ICI 182,780, tamoxifen and raloxifene on hypothalamic differentiation in the female rat. J Endocrinol. 2002;172:441–448. doi: 10.1677/joe.0.1720441. [DOI] [PubMed] [Google Scholar]
- 23.McMillan PJ, LeMaster AM, Dorsa DM. Tamoxifen enhances choline acetyltransferase mRNA expression in rat basal forebrain cholinergic neurons. Brain Res Mol Brain Res. 2002;103:140–145. doi: 10.1016/s0169-328x(02)00195-x. [DOI] [PubMed] [Google Scholar]
- 24.Kuo JR, Wang CC, Huang SK, Wang SJ. Tamoxifen depresses glutamate release through inhibition of voltage-dependent Ca2+ entry and protein kinase Cα in rat cerebral cortex nerve terminals. Neurochem Int. 2012;60:105–114. doi: 10.1016/j.neuint.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 25.Borowicz KK, Luszczki J, Matuszek M, Kleinrok Z, Czuczwar SJ. Effects of tamoxifen, mifepristone and cyproterone on the electroconvulsive threshold and pentetrazole-induced convulsions in mice. Pol J Pharmacol. 2002;54:103–109. [PubMed] [Google Scholar]
- 26.Cammermeyer J. Is the solitary dark neuron a manifestation of postmortem trauma to the brain inadequately fixed by perfusion? Histochemistry. 1978;56:97–115. doi: 10.1007/BF00508437. [DOI] [PubMed] [Google Scholar]
- 27.Ooigawa H, Nawashiro H, Fukui S, Otani N, Osumi A, Toyooka T, Shima K. The fate of Nissl-stained dark neurons following traumatic brain injury in rats: difference between neocortex and hippocampus regarding survival rate. Acta Neuropathol. 2006;112:471–481. doi: 10.1007/s00401-006-0108-2. [DOI] [PubMed] [Google Scholar]
- 28.Kherani ZS, Auer RN. Pharmacologic analysis of the mechanism of dark neuron production in cerebral cortex. Acta Neuropathol. 2008;116:447–452. doi: 10.1007/s00401-008-0386-y. [DOI] [PubMed] [Google Scholar]
- 29.Auer RN, Kalimo H, Olsson Y, Siesjö BK. The temporal evolution of hypoglycemic brain damage. I. Light- and electron-microscopic findings in the rat cerebral cortex. Acta Neuropathol. 1985;67:13–24. doi: 10.1007/BF00688120. [DOI] [PubMed] [Google Scholar]
- 30.Baracskay P, Szepesi Z, Orbán G, Juhász G, Czurkó A. Generalization of seizures parallels the formation of "dark" neurons in the hippocampus and pontine reticular formation after focal-cortical application of 4-aminopyridine (4-AP) in the rat. Brain Res. 2008;1228:217–228. doi: 10.1016/j.brainres.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 31.Gallyas F, Kiglics V, Baracskay P, Juhász G, Czurkó A. The mode of death of epilepsy-induced "dark" neurons is neither necrosis nor apoptosis: an electron-microscopic study. Brain Res. 2008;1239:207–215. doi: 10.1016/j.brainres.2008.08.069. [DOI] [PubMed] [Google Scholar]
- 32.Kälviäinen R, Salmenperä T, Partanen K, Vainio P, Riekkinen P, Sr, Pitkänen A. MRI volumetry and T2 relaxometry of the amygdala in newly diagnosed and chronic temporal lobe epilepsy. Epilepsy Res. 1997;28:39–50. doi: 10.1016/s0920-1211(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 33.Bernasconi N, Bernasconi A, Caramanos Z, Antel SB, Andermann F, Arnold DL. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–469. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 34.Pitkänen A, Tuunanen J, Kälviäinen R, Partanen K, Salmenperä T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- 35.Salmenperä T, Kälviäinen R, Partanen K, Pitkänen A. Hippocampal and amygdaloid damage in partial epilepsy: a cross-sectional MRI study of 241 patients. Epilepsy Res. 2001;46:69–82. doi: 10.1016/s0920-1211(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 36.Toth Z, Yan XX, Haftoglou S, Ribak CE, Baram TZ. Seizure-induced neuronal injury: vulnerability to febrile seizures in an immature rat model. J Neurosci. 1998;18:4285–4294. doi: 10.1523/JNEUROSCI.18-11-04285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ebrahimzadeh Bideskan AR, Hosseini M, Mohammadpour T, Karami R, Khodamoradi M, Nemati Karimooy H, Alavi H. Effects of soy extract on pentylenetetrazol-induced seizures in ovariectomized rats. Zhong Xi Yi Jie He Xue Bao. 2011;9:611–618. doi: 10.3736/jcim20110606. [DOI] [PubMed] [Google Scholar]
- 38.Hosseini M, Sharifi MR, Alaei H, Shafei MN, Karimooy HA. Effects of angiotensin II and captopril on rewarding properties of morphine. Indian J Exp Biol. 2007;45:770–777. [PubMed] [Google Scholar]
- 39.Saffarzadeh F, Eslamizade MJ, Nemati Karimooy HA, Hadjzadeh MA, Khazaei M, Hosseini M. The effect of L-arginine on Morris water maze tasks of ovariectomized rats. Acta Physiol Hung. 2010;97:216–223. doi: 10.1556/APhysiol.97.2010.2.8. [DOI] [PubMed] [Google Scholar]
- 40.Bayat M, Hasanzadeh GR, BarzroodIpour M, Javadi M. The effect of low protein diet on thalamic projections of hippocampus in rat. Neuroanatomy. 2005;4:43–48. [Google Scholar]
- 41.Gost JI, Insausti R, Gonzalo LM. Production and characterization of a monoclonal antibody that selectively marks the astrocyte population in the central nervous system. Rev Med Univ Navarra. 1993;38:9–20. [PubMed] [Google Scholar]
- 42.Rajabzadeh AA, Ebrahimzadeh Bideskan AR, Haghir H, Fazel AR. Morphometrical study of polysialylated neural cell adhesion molecule positive cells in rat pups hippocampus following induction of seizure during pregnancy. Iran Biomed J. 2011;15:157–163. doi: 10.6091/IBJ.998.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karimzadeh F, Hosseini M, Mangeng D, Alavi H, Hassanzadeh GR, Bayat M, Jafarian M, Kazemi H, Gorji A. Anticonvulsant and neuroprotective effects of Pimpinella anisum in rat brain. BMC Complement Altern Med. 2012;12:76. doi: 10.1186/1472-6882-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holmes GL. Seizure-induced neuronal injury: animal data. Neurology. 2002;59:S3–S6. doi: 10.1212/wnl.59.9_suppl_5.s3. [DOI] [PubMed] [Google Scholar]
- 45.Sankar R, Shin DH, Liu H, Mazarati A, Pereira de Vasconcelos A, Wasterlain CG. Patterns of status epilepticus-induced neuronal injury during development and long-term consequences. J Neurosci. 1998;18:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weniger G, Boucsein K, Irle E. Impaired associative memory in temporal lobe epilepsy subjects after lesions of hippocampus, parahippocampal gyrus, and amygdala. Hippocampus. 2004;14:785–796. doi: 10.1002/hipo.10216. [DOI] [PubMed] [Google Scholar]
- 47.Carreño M, Donaire A, Sánchez-Carpintero R. Cognitive disorders associated with epilepsy: diagnosis and treatment. Neurologist. 2008;14:S26–S34. doi: 10.1097/01.nrl.0000340789.15295.8f. [DOI] [PubMed] [Google Scholar]
- 48.Zeman A. When a patient with epilepsy complains about poor memory. Pract Neurol. 2009;9:85–89. doi: 10.1136/jnnp.2009.172205. [DOI] [PubMed] [Google Scholar]
- 49.Jortner BS. The return of the dark neuron. A histological artifact complicating contemporary neurotoxicologic evaluation. Neurotoxicology. 2006;27:628–634. doi: 10.1016/j.neuro.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 50.File SE. Tolerance to the anti-pentylenetetrazole effects of diazepam in the mouse. Psychopharmacology (Berl) 1983;79:284–286. doi: 10.1007/BF00427828. [DOI] [PubMed] [Google Scholar]
- 51.Ishida K, Shimizu H, Hida H, Urakawa S, Ida K, Nishino H. Argyrophilic dark neurons represent various states of neuronal damage in brain insults: some come to die and others survive. Neuroscience. 2004;125:633–644. doi: 10.1016/j.neuroscience.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 52.Czurkó A, Nishino H. 'Collapsed' (argyrophilic, dark) neurons in rat model of transient focal cerebral ischemia. Neurosci Lett. 1993;162:71–74. doi: 10.1016/0304-3940(93)90562-y. [DOI] [PubMed] [Google Scholar]
- 53.Söderfeldt B, Kalimo H, Olsson Y, Siesjö BK. Bicuculline-induced epileptic brain injury. Transient and persistent cell changes in rat cerebral cortex in the early recovery period. Acta Neuropathol. 1983;62:87–95. doi: 10.1007/BF00684924. [DOI] [PubMed] [Google Scholar]
- 54.Karimzadeh F, Alavi H, Mahmoudian AR, Hassanzadeh GR, Hosseini M. The effect of PTZ-induced seizures on neuronal damage in amygdala of rats. Pharmacologyonline. 2009;3:560–566. [Google Scholar]
- 55.Harden CL, Pulver MC, Ravdin L, Jacobs AR. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402–1407. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 56.Gevorkyan ES, Nazaryan KB, Kostanyan AA. Modifying effect of estradiol and progesterone on epileptic activity of the rat brain. Neurosci Behav Physiol. 1989;19:412–415. doi: 10.1007/BF01197874. [DOI] [PubMed] [Google Scholar]
- 57.Pereira M, Jr, Soares JM, Jr, Valente SG, Oliveira PB, Cavalheiro EA, Amado D, Baracat EC. Estrogen effects on pilocarpine-induced temporal lobe epilepsy in rats. Maturitas. 2009;62:190–196. doi: 10.1016/j.maturitas.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 58.Schauwecker PE, Wood RI, Lorenzana A. Neuroprotection against excitotoxic brain injury in mice after ovarian steroid depletion. Brain Res. 2009;1265:37–46. doi: 10.1016/j.brainres.2009.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alkner S, Bendahl PO, Fernö M, Nordenskjöld B, Rydén L South Swedish and South-East Swedish Breast Cancer Groups. Tamoxifen reduces the risk of contralateral breast cancer in premenopausal women: Results from a controlled randomised trial. Eur J Cancer. 2009;45:2496–2502. doi: 10.1016/j.ejca.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 60.Kostrzewska A, Laudañski T, Batra S. Potent inhibition by tamoxifen of spontaneous and agonist-induced contractions of the human myometrium and intramyometrial arteries. Am J Obstet Gynecol. 1997;176:381–386. doi: 10.1016/s0002-9378(97)70503-9. [DOI] [PubMed] [Google Scholar]
- 61.Goulding A, Gold E, Feng W. Tamoxifen in the rat prevents estrogen-deficiency bone loss elicited with the LHRH agonist buserelin. Bone Miner. 1992;18:143–152. doi: 10.1016/0169-6009(92)90854-7. [DOI] [PubMed] [Google Scholar]
- 62.Carthew P, Edwards RE, Nolan BM. Uterotrophic effects of tamoxifen, toremifene, and raloxifene do not predict endometrial cell proliferation in the ovariectomized CD1 mouse. Toxicol Appl Pharmacol. 1999;158:24–32. doi: 10.1006/taap.1999.8679. [DOI] [PubMed] [Google Scholar]
- 63.Landry M, Di Paolo T. Effect of chronic estradiol, tamoxifen or raloxifene treatment on serotonin 5-HT1A receptor. Brain Res Mol Brain Res. 2003;112:82–89. doi: 10.1016/s0169-328x(03)00049-4. [DOI] [PubMed] [Google Scholar]
- 64.Chen D, Wu CF, Shi B, Xu YM. Tamoxifen and toremifene cause impairment of learning and memory function in mice. Pharmacol Biochem Behav. 2002;71:269–276. doi: 10.1016/s0091-3057(01)00656-6. [DOI] [PubMed] [Google Scholar]