Abstract
The inflammation that accompanies acute injury has dual functions: bactericidal action and repair. Bactericidal functions protect damaged tissue from infection, and repair functions are initiated to aid in the recovery of damaged tissue. Brain injury is somewhat different from injuries in other tissues in two respects. First, many cases of brain injury are not accompanied by infection: there is no chance of pathogens to enter in ischemia or even in traumatic injury if the skull is intact. Second, neurons are rarely regenerated once damaged. This raises the question of whether bactericidal inflammation really occurs in the injured brain; if so, how is this type of inflammation controlled? Many brain inflammation studies have been conducted using cultured microglia (brain macrophages). Even where animal models have been used, the behavior of microglia and neurons has typically been analyzed at or after the time of neuronal death, a time window that excludes the inflammatory response, which begins immediately after the injury. Therefore, to understand the patterns and roles of brain inflammation in the injured brain, it is necessary to analyze the behavior of all cell types in the injured brain immediately after the onset of injury. Based on our experience with both in vitro and in vivo experimental models of brain inflammation, we concluded that not only microglia, but also astrocytes, blood inflammatory cells, and even neurons participate and/or regulate brain inflammation in the injured brain. Furthermore, brain inflammation played by these cells protects neurons and repairs damaged microenvironment but not induces neuronal damage.
Keywords: brain inflammation, microglia, repair
INTRODUCTION
Brain inflammation has been the therapeutic target of efforts to treat several brain diseases, including ischemia, trauma, and certain neurodegenerative diseases. The brain inflammation that accompanies acute brain injury has been thought to cause secondary injury. However, this idea raises a question: if brain inflammation is toxic and causes secondary injury, which in turn induces brain inflammation causing tertiary brain damage, how is brain injury stopped? According to this scenario, the entire brain would ultimately be damaged, which fortunately does not occur in injured brain.
The brain is a special organ in the context of injury insofar as neurons, once damaged, do not regenerate. Therefore, it is physiologically important that neurons in the injured brain be protected. It is well known that inflammation has dual functions: protecting tissue from infection (bactericidal inflammation), and restoring damaged tissues (repair function) [1, 2]. However, the injured brain is generally not open to the outside environment. In ischemic injury, brain damage occurs due to insufficient blood supply; thus, there is no chance of infection. Even in traumatic injury, pathogens cannot enter the brain if the skull is intact. These observations raise a question whether cytotoxic bactericidal inflammation occurs even in the absence of infection, particularly in the brain where neurons are rarely regenerated. Recently, we have reported that secondary neuronal death does not commonly occur in brain and spinal cord injury models [3-5]. In contusion-induced spinal cord injury, acute neuronal death is followed by secondary neuronal death that occurs at 12-24 h after the injury [3]. However, in ATP-injected brain, acute neuronal death occurs right after the injection, but secondary neuronal death does not occur [4]. In LPS-injected brain, slow neuronal death occurs at 1-3 d after the injection [5]. However, brain inflammation that occurs in these three different injury models is a commonly complicated process involving microglia, monocytes, neutrophils, astrocytes, and neurons. Furthermore, these cells struggle to protect precious neurons in the brain rather than to cause secondary injury. In this review, we describe the patterns of brain inflammation characteristic of each type of cell in the injured brain, and how brain inflammation is regulated to inhibit cytotoxic inflammation.
Cells that participate in brain inflammation
It has long been accepted that the blood-brain barrier keeps blood inflammatory cells (monocytes and neutrophils) from getting into the brain. Thus, the prevailing view has been that microglia-resident brain macrophages-are the only cells that mediate brain inflammation. Recently, however, it has become known that neutrophils and monocytes infiltrate the injured brain and contribute to inflammation. In human autopsy samples of spinal cord injury, neutrophils were found 1-3 days after injury, and monocytes from 3 days to several weeks after injury [6]. Animal models of spinal cord and brain injury also show infiltration of neutrophils and monocytes in injury sites [3-5, 7-9]. However, neutrophils and monocytes are still often mistaken for microglia because of the markers that have been used to detect microglia in the brain. OX-42, an antibody designed to detect CD11b, has been the most often used marker of microglia in the brain. In the intact brain, microglia are the only cells that express CD11b. However, in the injured brain, neutrophils and monocytes, which also express CD11b, infiltrate the brain [10, 11]. Therefore, anti-CD11b antibodies cannot distinguish microglia from neutrophils and monocytes, leading to the misconception that rounded neutrophils and monocytes present in the injured brain are activated microglia. Therefore, other markers are required to discriminate microglia from neutrophils and monocytes. Myeloperoxidase (MPO) can be used as a marker for neutrophils since it is highly expressed in neutrophils but is largely undetectable in microglia and monocytes [4, 5, 9, 12, 13]. Another hallmark of neutrophils is the morphology of their nuclei: unlike microglia and monocytes, neutrophils are typically polymorphonuclear. Antibodies specific for Iba-1 can distinguish neutrophils from microglia and monocytes since Iba-1 is expressed in microglia and monocytes but not in neutrophils [5, 14]. Discriminating microglia from monocytes is more challenging; however, CD45 is helpful in this regard because it is more highly expressed in monocytes than microglia [4, 5, 15]. Therefore, Iba-1/CD45 double-positive, round cells are monocytes, whereas Iba-1-positive/CD45-negative cells bearing processes (short or long) are microglia.
In addition, astrocytes and even neurons also participate in brain inflammation [16]. Astrocytes produce several anti-inflammatory factors [17-20] and chemokines that recruit monocytes [21], and neurons regulate inflammation both negatively and positively depending on the situation [22-24]. Therefore, brain inflammation is a complicated process in which resident microglia, neutrophils, monocytes, astrocytes, and neurons all play a role.
Microglia
Microglia in vivo and in vitro
Morphological differences in microglia in vivo and in vitro
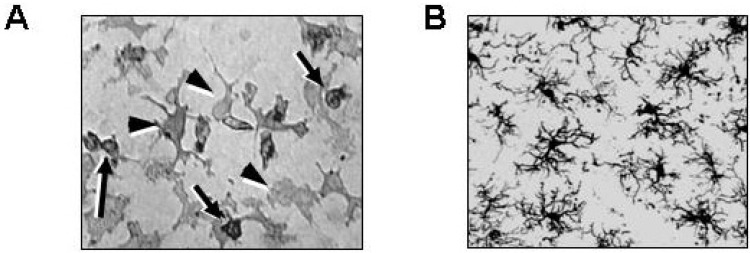
Since microglia were first cultured from the neonatal rat brain [25], their properties and functions have mainly been studied in culture. However, microglia in vitro and in vivo are quite different in several aspects. First, they are morphologically different. When cultured with astrocytes, microglia identified immunohistochemically using Iba-1 and/or CD11b antibodies show two different morphologies: one is round and loosely attached (Fig. 1A, arrows); the other is flat and irregular, and tightly attached (Fig. 1A, arrowheads). Usually, only round cells were considered to be microglia since it is difficult to recognize microglia that are tightly attached to astrocytes without staining with anti-Iba-1 and/or anti-CD11b antibodies. Round microglia flatten in response to lipopolysaccharide (LPS) and interferon (IFN)-γ, or become flattened with a few processes in response to gangliosides [26].
Fig. 1.
Microglia in culture and in the brain. (A) Microglia cultured from neonatal rat brains were stained with anti-CD11b antibodies. Microglia show two different morphologies: round (arrows) and irregular-shaped (arrowheads). (B) Brain sections obtained from 8-week-old rats were stained with anti-Iba-1 antibodies. Highly ramified microglia are detectable in the cortex.
In the intact brain, however, microglia are highly ramified, a morphology that is obviously different from that of microglia in culture (Fig. 1B). In response to brain injury, the processes of Iba-1-stained microglia become short and thick, and the number of processes decreases; but unlike neutrophils and monocytes, these cells never adopt a rounded, ball-like morphology (Fig. 2A, white arrows and white arrowheads at 3 h) [3-5, 8, 9].
Fig. 2.
Time-dependent behavior of Iba-1-, and CD45-positive cells in ATP-injected rat brain substantia nigra. Serial sections obtained 3 hours to 83 days after ATP injection ("*" denotes injection sites) were immunostained for Iba-1 (A) and CD45 (B). The lower panels in (A) represent higher magnification images of the indicated areas in the upper panels. Black arrowheads at 3 h indicate dead cells. White arrows and white arrowheads in (A) indicate thick and short process-bearing Iba-1-positive cells. Black arrows in (A) at 2 d represent round Iba-1-positive cells. CD45-positive cells were found around blood vessels (B, inset). Scale bars: 200 µm (A upper panel, B) and 100 µm (A lower panel). (Adapted from Jeong et al., PLoS One, 2010).
Limited microglial activation in the injured brain
The in vitro and in vivo functions of microglia are also different. When cultured microglia are activated with several kinds of inflammatory stimulators (e.g., LPS, IFN-γ, gangliosides, thrombin), they highly express inducible nitric oxide synthase (iNOS) and proinflammatory cytokines [27-31]. As a result of this pattern of expression, neurons co-cultured with microglia undergo death when challenged with inflammatory stimulators [32]. However, in the brain, microglial behavior is totally different from that in culture. In the intact brain, microglia actively survey the brain, identifying abnormal and/or damaged structures, such as non-functional synapses, and phagocytosing them [33-35]. Microglia also rapidly respond to local injury. Interestingly, unlike the concept (that is generally taken for granted) that neurons are the most vulnerable cells in the brain, microglia and neurons die in the damage core at the same time [3-5], and rather earlier than neurons in the penumbra regions where secondary neuronal death occurs [3, 5, 36]. Microglia die acutely through necrosis in the ATP-injected brain (Fig. 2A, black arrowheads at 3 h) [4], and undergo apoptosis in the LPS-injected brain and following contusion-induced spinal cord injury [3, 5]. Although the cell death mechanism of activated microglia in the brain is not well established, we previously reported that prostaglandins produced by enhanced cyclooxygenase (COX)-2 expression in the injured brain may contribute [37].
Microglia adjacent to the damaged area rapidly extend their processes to injury sites, surrounding and isolating damaged tissue within a few minutes (Fig. 2A, white arrows at 3 h) [4, 34]. Microglia also change their morphology-their processes become thicker and the number of processes decreases-becoming what is termed activated microglia (Fig. 2A, white arrow heads). However, microglia never show a round morphology like they exhibit in culture. Although Iba-1-positive round cells are detected beginning 2 d after damage (Fig. 2A, black arrows at 2 d), these cells also highly express CD45, a marker of monocytes, suggesting that these Iba-1-positive round cells are monocytes (Fig. 2B) [4, 8].
The morphologically activated resident microglia in the region adjacent to the damage core (Fig. 2A, white arrows and white arrowheads) are CD45-negative [4]. These cells produce some inflammatory mediators, including interleukin (IL)-1β [4], but not cytotoxic inflammatory mediators, even in LPS- and ATP-damaged brains, the ischemic brain, and contusion-induced injured spinal cords [3, 4, 5, 36]. Accordingly, neurons in areas where microglia are morphologically activated are healthy [3, 4, 38], suggesting that activated microglia are not toxic to neurons. Taken together, these findings strongly suggest that the most important function of activated microglia is to isolate damaged sites as soon as possible, thereby preventing propagation of the disrupted microenvironment to areas outside the original damage area. In addition, activated microglia express some chemokines and growth factors that support survival of surrounding neurons. Thus, microglia prevent secondary neuronal damage rather than inducing secondary injury [4].
Microglia barely proliferate in the injured brain
One additional difference in microglial behavior between in vitro and in vivo is proliferation. In culture, microglia proliferate in the presence of astrocytes and/or neurons. It has been reported that astrocytes produce microglial mitogens, including GM-CSF (granulocyte monocyte colony-stimulating factor) [39, 40]. In the injured brain, it has been reported that microglia are BrdU-positive, which is evidence of proliferation [41, 42]. However, the number of Iba-1-positive round, but not ramified, cells increases explosively between 3 and 7 days (Fig. 2A, 3 d vs. 7 d) [4, 8], which is impossible to account for by proliferation of microglia. Several lines of experimental evidence show why this is the case. First, Ki67, a marker of proliferation, is mainly detectable in Olig2 (oligodendrocyte transcription factor 2)-positive cells and vimentin/GFAP (glial fibrillary acidic protein)-positive cells at approximately 3 days after injury, but is not found in either round or ramified Iba-1-positive cells at any time [8]. Second, Iba-1-positive round cells that highly express CD45 appear around blood vessels (Fig. 2B, 2 d in inset), indicating that these cells are from the blood [3, 4, 8]. Third, depletion of blood white cells by irradiation diminishes infiltration of microglia [5]. Therefore, Iba-1-positive round cells that fill the damage core are monocytes from the blood rather than proliferated resident microglia.
In the injured brain, a number of energy-consuming transporters are engaged to remove glutamate and potassium flooding from damaged cells, increasing energy requirements; however, the blood supply is insufficient in the injured brain owing to blood vessel damage. In this situation, it makes no physiological sense to induce microglial proliferation, which requires considerable amounts of energy and metabolites. Furthermore, there is a virtually endless source of monocytes in the blood. These observations indicate that, in the injured brain, the damage core is filled by monocytes infiltrating from the blood rather than by proliferation of resident microglia. In the damaged brain, the proliferation of other cell types (e.g., oligodendrocytes, astrocytes, endothelial cells) appears to start as part of a healing process that commences after monocytes remove cell debris and dead cells in the damage core [8].
Why is brain inflammation different in vivo and in vitro?
Different activators in vitro and in vivo?
Microglial activation should be tightly regulated in vivo to protect neurons in the injured brain [16]. Thus, it is curious why microglial behavior is so different in vivo and in vitro. One explanation for this difference is the nature of in vitro and in vivo inflammatory stimulators. In vitro, the phenotypes and functions of activated microglia are usually examined by inducing microglial activation with LPS [27], which activates MAPK (mitogen-activated protein kinase) and STAT (signal transducer and activator of transcription) pathways-the most important signaling pathways involved in inducing inflammation [27-30, 43]. However, LPS rarely gets into the brain to activate microglia since the brain-damaged environment is usually sterile, as noted above. In the damaged brain, microglia are activated by numerous endogenous microglial activators released by damaged cells and/or circulating in blood, including plasma membrane components (gangliosides); DAMPs (danger-associated molecular patterns), such as ATP and HMGB1; and proteases released from damaged cells [24, 28, 44, 45]. Some blood proteins that are involved in coagulation and fibrinolysis, including thrombin, prothrombin, plasmin and tissue plasminogen activators, can enter the brain and activate microglia [29-31, 46]. However, these endogenous activators may not be as strong as LPS [21, 27-29]. Notably, beta-amyloid and ATP, which are known to activate microglia, are very weak activators compared to LPS [19, 21].
Another reason for the observed difference in activation behavior in vitro and in vivo is the difference in the duration of microglial activation. In vitro, stimuli used to activate microglia remain in the culture dish until being removed. However, in vivo, microglial activators produced by damage are removed by the continuously flowing cerebrospinal fluid (CSF), which turns over about four times a day in the case of humans [47]. Therefore, harmful factors released from damaged cells or infiltrating from blood do not remain in the injured brain for a long time.
Astrocytes and neurons inhibit inflammation in the brain
A major determinant of the difference between in vitro and in vivo inflammation may be the absence and presence of cell-cell interactions, respectively. It has been reported that the interaction of microglia with astrocytes and neurons is an important mechanism for inhibiting excessive microglial activation. Neurons inhibit microglial inflammatory responses through the expression of ligands such as CD200 and fractalkine, whose receptors are expressed in microglia [22, 23]. Consistent with this, brain inflammation is significantly enhanced in fractalkine receptor-knockout mice [23]. Therefore, neurons inhibit microglial activation in the intact brain.
Astrocytes produce soluble factors that inhibit microglial activation [16-20]; suggested candidate molecules include transforming growth factor (TGF)-β and prostaglandins [17, 48, 49]. Uncharacterized soluble factor(s) released from astrocytes induce the expression of antioxidant enzymes such as HO-1 (heme oxygenase-1) that inhibit microglial activation [19]. Interestingly, in injury states, astrocytes rapidly produce anti-inflammatory molecules [20], which could be a mechanism for inhibition of microglial activation in the injured brain. In addition, astrocytes support neuronal survival in several ways, including providing neurons with growth factors and nutrients, and maintaining homeostasis of extracellular fluid by taking up glutamate and potassium. Recently, we found that, in spinal cord injury, the functional loss and/or death of astrocytes precedes secondary neuronal death [3]. On the basis of these findings, we speculate that the loss of astrocyte function, and not brain inflammation, could be a cause of secondary brain injury.
Monocytes in the injured brain
As is the case for microglia, the role of monocytes in the injured brain is a matter of controversy. Monocyte activation is classified as classical and alternative (in other words, bactericidal and reparative) [for review, see 1]. When monocytes are exposed to classical activators such as IFN-γ, they produce significant amounts of cytotoxic inflammatory mediators, including reactive oxygen species (ROS) and TNF-α. However, monocytes activated by IL-4 and IL-13 express repair-related genes, including mannose receptors [1]. Some studies have reported that monocytes alternatively activated in vitro change to classically activated cells about 7 days after transplantation in the contusion-injured spinal cord [50]. However, it is unlikely that monocytes are classically activated and/or change their phenotype from alternative to classical activation since debris and dead cells disappear and a cavity is formed after monocyte infiltration [51], indicating that monocytes are alternatively activated and phagocytose dead cells and debris. Furthermore, damage does not increase further during or after monocyte infiltration [3], an outcome that would not be possible if monocytes were classically activated and produced cytotoxic mediators. In fact, damage increases between 12 hours and 1 day after contusion-induced spinal cord injury, but does not further increase thereafter [3]. Recently, we also reported that monocytes infiltrating the injured brain and spinal cord express alternative activation markers [3, 4]. Even in LPS-injected brains, microarray analyses have revealed that repair-related genes, including those for proliferation, wound healing and phagocytosis, increase at times corresponding to monocyte infiltration [8]. At least at the immunohistochemistry level, monocytes in the injured brain highly express mannose receptors and lysosomal enzymes, but not classical activation markers [4, 8]. More importantly, the damaged brain is repaired after monocyte infiltration, as evidenced by the fact that astrocytes, blood vessels, oligodendrocytes, myelin, and neurites reappear and fill the damage core [8]. Monocytes appear to produce certain chemokines that recruit astrocytes; thus, astrocytes extend their processes toward monocytes during the recovery period [8]. Therefore, the entry of monocytes into the brain is physiologically relevant for the repair of damaged tissue.
After finishing the roles of repair, infiltrating monocytes disappear from the damaged brain within 1 or 2 months (Fig. 2) via two different pathways. Most monocytes die in the brain about 5 days after infiltration [8]. However, some monocytes differentiate into resident brain microglia [52], providing a source of monocyte-derived microglia to refill areas in the damage core left by the death of original resident microglia [4]. During this differentiation process, monocytes exhibit a change in morphology and decreased CD45 expression [53]; within several months of the injury, CD45 expression becomes barely detectable [4].
Neutrophils
Neutrophils are the first-responder cells that enter damaged tissue to protect the body from infection. Thus, not surprisingly, neutrophils express cytotoxic inflammatory mediators, including iNOS and MPO. Neutrophils also express cytotoxic inflammatory mediators in the injured brain [5]. Fortunately, unlike monocytes, which appear to infiltrate injury sites independent of injury type [3, 5, 9], the degree of neutrophil infiltration differs depending on the extent and type of insult [5, 6, 9]. For example, neutrophils vigorously infiltrate contusion-injured spinal cord but barely infiltrate laceration-injured spinal cord [6]. Neutrophils also exhibit extensive infiltration in the LPS-injected brain but show little infiltration of the ATP-injected brain [4, 5].
There are also regional differences in the extent of neutrophil infiltration and the degree of brain injury. When the same amount of LPS is injected into the rat cortex, hippocampus or substantia nigra, neutrophil infiltration is prominent in the substantia nigra in association with extensive neuronal death; in contrast, neutrophil infiltration is much lower in the cortex and hippocampus and neuronal cell death is not detectable in these areas [9]. It is possible that neurons in the substantia nigra, including dopaminergic neurons, are more vulnerable than neurons in other areas. However, in the substantia nigra, the extent of neuronal death is dependent on the extent of neutrophil infiltration [5]. Therefore, we conclude that bactericidal inflammatory mediators from neutrophils are toxic to neurons.
Neutrophil infiltration also appears to be a mechanism for protecting the brain in injury states although neutrophils are toxic to neurons. Neutrophils are vigorously recruited to the damaged brain only when there is a chance of infection: strong neutrophil infiltration is observed in the LPS-injected brain or contusion-injured spinal cord, but not in the ATP-injected brain or laceration-injured spinal cord [4-6], as noted above. This is because, as a bacteria wall component, injected LPS may be perceived as a sign of infection that recruit neutrophils. Moreover, the spinal cord is exposed to the outside environment when injured by contusion, which enhances the chance of infection or accompanies infection. Therefore, in unavoidable situations, neutrophil infiltration serves to prevent further brain damage.
Neutrophils infiltrating the damaged brain undergo death about 1 day after the injury [5]. Therefore, no MPO-positive and polymorphic nuclei-bearing cells are detectable thereafter. However, monocytes can be misinterpreted as neutrophils, and vice versa, since both types of cells are from the blood and express CD11b [10, 11]. This misinterpretation may create confusion regarding the roles of neutrophils, monocytes, and even microglia, in the brain.
CONCLUSION
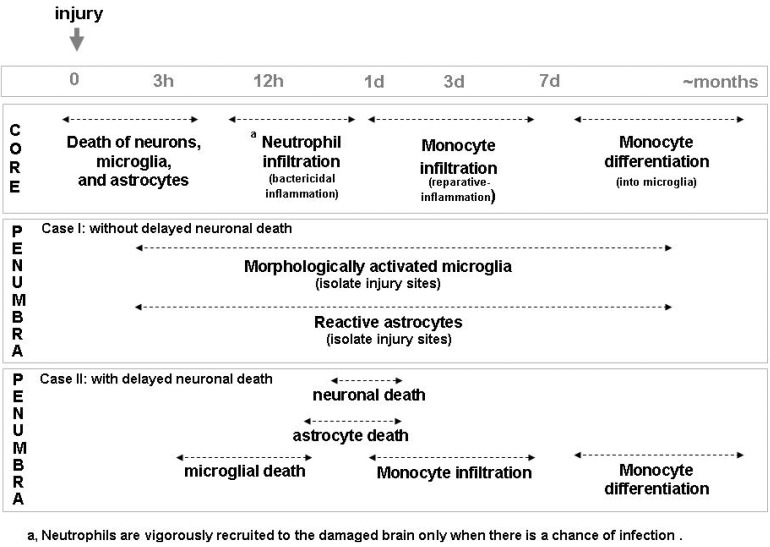
Brain inflammation in vivo is a complicated processes involving several types of cells, including microglia, neutrophils, monocytes, astrocytes, and neurons (Fig. 3). Microglia in the brain continuously survey the brain to identify even tiny structural abnormalities in neurons. In response to injury, microglia around injury sites rapidly isolate damaged areas and prevent propagation of the disrupted injury site microenvironment to surrounding areas (Fig. 3, penumbra case I). Neutrophils then infiltrate, but only in cases where there is a risk of infection. Thereafter, monocytes infiltrate, serving to help repair the damaged brain. Astrocytes and neurons also participate in brain inflammation through down-regulation of cytotoxic inflammation. It is noticeable that in the penumbra region where secondary neuronal death occurs, microglia and astrocytes die earlier than neurons (Fig. 3, penumbra case II). We suggest that the secondary injury that accompanies acute injury may be caused by insufficient support of neurons by these cells. As Dr. W. Streit put it previously [54], the loss of beneficial functions and/or gain of detrimental functions of microglia and astrocytes-to which we would add neutrophils and monocytes-due to aging and genetic mutation increase the risk of brain diseases. Therefore, the collective contribution of all cells of the brain and blood to brain inflammation represents the utmost effort to protect the brain from injury.
Fig. 3.
A proposed model to show behavior of cells in injured brain. In the damage core, not only neurons but microglia and astrocytes die. Then, neutrophils infiltrate the brain when there is a sign of infection. Monocytes infiltrate and function to repair damage sites. In the penumbra region where neuronal death does not occur, microglia and astrocytes are morphologically activated and isolate damage sites (Penumbra case I). In the penumbra region where secondary neuronal death occurs, microglia and astrocytes die rather than earlier than neurons (Penumbra case II).
ACKNOWLEDGEMENTS
This work was supported by a KOSEF NRL Program grant (no. 2-2008025-0) funded by the Korean government (MEST) and a grant (NRF-2012R1A5A2051429) from KOSEF through the Chronic Inflammatory Disease Research Center (CIDRC) at Ajou University.
References
- 1.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. Macrophage plasticity and polarization in tissue repair and remodelling. J Pathol. 2013;229:176–185. doi: 10.1002/path.4133. [DOI] [PubMed] [Google Scholar]
- 3.Min KJ, Jeong HK, Kim B, Hwang DH, Shin HY, Nguyen AT, Kim JH, Jou I, Kim BG, Joe EH. Spatial and temporal correlation in progressive degeneration of neurons and astrocytes in contusion-induced spinal cord injury. J Neuroinflammation. 2012;9:100. doi: 10.1186/1742-2094-9-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jeong HK, Ji KM, Kim B, Kim J, Jou I, Joe EH. Inflammatory responses are not sufficient to cause delayed neuronal death in ATP-induced acute brain injury. PLoS One. 2010;5:e13756. doi: 10.1371/journal.pone.0013756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji KA, Yang MS, Jeong HK, Min KJ, Kang SH, Jou I, Joe EH. Resident microglia die and infiltrated neutrophils and monocytes become major inflammatory cells in lipopolysaccharide-injected brain. Glia. 2007;55:1577–1588. doi: 10.1002/glia.20571. [DOI] [PubMed] [Google Scholar]
- 6.Fleming JC, Norenberg MD, Ramsay DA, Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD, Weaver LC. The cellular inflammatory response in human spinal cords after injury. Brain. 2006;129:3249–3269. doi: 10.1093/brain/awl296. [DOI] [PubMed] [Google Scholar]
- 7.Xu JA, Hsu CY, Liu TH, Hogan EL, Perot PL, Jr, Tai HH. Leukotriene B4 release and polymorphonuclear cell infiltration in spinal cord injury. J Neurochem. 1990;55:907–912. doi: 10.1111/j.1471-4159.1990.tb04577.x. [DOI] [PubMed] [Google Scholar]
- 8.Jeong HK, Ji KM, Kim J, Jou I, Joe EH. Repair of astrocytes, blood vessels, and myelin in the injured brain: possible roles of blood monocytes. Mol Brain. 2013;6:28. doi: 10.1186/1756-6606-6-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji KA, Eu MY, Kang SH, Gwag BJ, Jou I, Joe EH. Differential neutrophil infiltration contributes to regional differences in brain inflammation in the substantia nigra pars compacta and cortex. Glia. 2008;56:1039–1047. doi: 10.1002/glia.20677. [DOI] [PubMed] [Google Scholar]
- 10.Hickstein DD, Ozols J, Williams SA, Baenziger JU, Locksley RM, Roth GJ. Isolation and characterization of the receptor on human neutrophils that mediates cellular adherence. J Biol Chem. 1987;262:5576–5580. [PubMed] [Google Scholar]
- 11.Altieri DC, Edgington TS. A monoclonal antibody reacting with distinct adhesion molecules defines a transition in the functional state of the receptor CD11b/CD18 (Mac-1) J Immunol. 1988;141:2656–2660. [PubMed] [Google Scholar]
- 12.Schultz J, Corlin R, Oddi F, Kaminker K, Jones W. Myeloperoxidase of the leucocyte of normal human blood. 3. Isolation of the peroxidase granule. Arch Biochem Biophys. 1965;111:73–79. doi: 10.1016/0003-9861(65)90324-3. [DOI] [PubMed] [Google Scholar]
- 13.Dunn WB, Hardin JH, Spicer SS. Ultrastructural localization of myeloperoxidase in human neutrophil and rabbit heterophil and eosinophil leukocytes. Blood. 1968;32:935–944. [PubMed] [Google Scholar]
- 14.Tanaka R, Komine-Kobayashi M, Mochizuki H, Yamada M, Furuya T, Migita M, Shimada T, Mizuno Y, Urabe T. Migration of enhanced green fluorescent protein expressing bone marrow-derived microglia/macrophage into the mouse brain following permanent focal ischemia. Neuroscience. 2003;117:531–539. doi: 10.1016/s0306-4522(02)00954-5. [DOI] [PubMed] [Google Scholar]
- 15.Shah VO, Civin CI, Loken MR. Flow cytometric analysis of human bone marrow. IV. Differential quantitative expression of T-200 common leukocyte antigen during normal hemopoiesis. J Immunol. 1988;140:1861–1867. [PubMed] [Google Scholar]
- 16.Yang MS, Min KJ, Joe E. Multiple mechanisms that prevent excessive brain inflammation. J Neurosci Res. 2007;85:2298–2305. doi: 10.1002/jnr.21254. [DOI] [PubMed] [Google Scholar]
- 17.Vincent VA, Tilders FJ, Van Dam AM. Inhibition of endotoxin-induced nitric oxide synthase production in microglial cells by the presence of astroglial cells: a role for transforming growth factor beta. Glia. 1997;19:190–198. doi: 10.1002/(sici)1098-1136(199703)19:3<190::aid-glia2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Pyo H, Yang MS, Jou I, Joe EH. Wortmannin enhances lipopolysaccharide-induced inducible nitric oxide synthase expression in microglia in the presence of astrocytes in rats. Neurosci Lett. 2003;346:141–144. doi: 10.1016/s0304-3940(03)00505-6. [DOI] [PubMed] [Google Scholar]
- 19.Min KJ, Yang MS, Kim SU, Jou I, Joe EH. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. J Neurosci. 2006;26:1880–1887. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JH, Min KJ, Seol W, Jou I, Joe EH. Astrocytes in injury states rapidly produce anti-inflammatory factors and attenuate microglial inflammatory responses. J Neurochem. 2010;115:1161–1171. doi: 10.1111/j.1471-4159.2010.07004.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim B, Jeong HK, Kim JH, Lee SY, Jou I, Joe EH. Uridine 5'-diphosphate induces chemokine expression in microglia and astrocytes through activation of the P2Y6 receptor. J Immunol. 2011;186:3701–3709. doi: 10.4049/jimmunol.1000212. [DOI] [PubMed] [Google Scholar]
- 22.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- 23.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 24.Kim YS, Kim SS, Cho JJ, Choi DH, Hwang O, Shin DH, Chun HS, Beal MF, Joh TH. Matrix metalloproteinase-3: a novel signaling proteinase from apoptotic neuronal cells that activates microglia. J Neurosci. 2005;25:3701–3711. doi: 10.1523/JNEUROSCI.4346-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JY, Kim HY, Jou I, Park SM. GM1 induces p38 and microtubule dependent ramification of rat primary microglia in vitro. Brain Res. 2008;1244:13–23. doi: 10.1016/j.brainres.2008.09.072. [DOI] [PubMed] [Google Scholar]
- 27.Pyo H, Jou I, Jung S, Hong S, Joe EH. Mitogen-activated protein kinases activated by lipopolysaccharide and beta-amyloid in cultured rat microglia. Neuroreport. 1998;9:871–874. doi: 10.1097/00001756-199803300-00020. [DOI] [PubMed] [Google Scholar]
- 28.Pyo H, Joe E, Jung S, Lee SH, Jou I. Gangliosides activate cultured rat brain microglia. J Biol Chem. 1999;274:34584–34589. doi: 10.1074/jbc.274.49.34584. [DOI] [PubMed] [Google Scholar]
- 29.Ryu J, Pyo H, Jou I, Joe E. Thrombin induces NO release from cultured rat microglia via protein kinase C, mitogen-activated protein kinase, and NF-kappa B. J Biol Chem. 2000;275:29955–29959. doi: 10.1074/jbc.M001220200. [DOI] [PubMed] [Google Scholar]
- 30.Ryu J, Min KJ, Rhim TY, Kim TH, Pyo H, Jin B, Kim SU, Jou I, Kim SS, Joe EH. Prothrombin kringle-2 activates cultured rat brain microglia. J Immunol. 2002;168:5805–5810. doi: 10.4049/jimmunol.168.11.5805. [DOI] [PubMed] [Google Scholar]
- 31.Min KJ, Jou I, Joe E. Plasminogen-induced IL-1beta and TNF-alpha production in microglia is regulated by reactive oxygen species. Biochem Biophys Res Commun. 2003;312:969–974. doi: 10.1016/j.bbrc.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 32.Chao CC, Hu S, Molitor TW, Shaskan EG, Peterson PK. Activated microglia mediate neuronal cell injury via a nitric oxide mechanism. J Immunol. 1992;149:2736–2741. [PubMed] [Google Scholar]
- 33.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 34.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 35.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto H, Kumon Y, Watanabe H, Ohnishi T, Shudou M, Ii C, Takahashi H, Imai Y, Tanaka J. Antibodies to CD11b, CD68, and lectin label neutrophils rather than microglia in traumatic and ischemic brain lesions. J Neurosci Res. 2007;85:994–1009. doi: 10.1002/jnr.21198. [DOI] [PubMed] [Google Scholar]
- 37.Yang MS, Ji KA, Jeon SB, Jin BK, Kim SU, Jou I, Joe E. Interleukin-13 enhances cyclooxygenase-2 expression in activated rat brain microglia: implications for death of activated microglia. J Immunol. 2006;177:1323–1329. doi: 10.4049/jimmunol.177.2.1323. [DOI] [PubMed] [Google Scholar]
- 38.Jeong HK, Jou I, Joe EH. Systemic LPS administration induces brain inflammation but not dopaminergic neuronal death in the substantia nigra. Exp Mol Med. 2010;42:823–832. doi: 10.3858/emm.2010.42.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gebicke-Haerter PJ, Bauer J, Schobert A, Northoff H. Lipopolysaccharide-free conditions in primary astrocyte cultures allow growth and isolation of microglial cells. J Neurosci. 1989;9:183–194. doi: 10.1523/JNEUROSCI.09-01-00183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SC, Liu W, Brosnan CF, Dickson DW. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994;12:309–318. doi: 10.1002/glia.440120407. [DOI] [PubMed] [Google Scholar]
- 41.Giordana MT, Attanasio A, Cavalla P, Migheli A, Vigliani MC, Schiffer D. Reactive cell proliferation and microglia following injury to the rat brain. Neuropathol Appl Neurobiol. 1994;20:163–174. doi: 10.1111/j.1365-2990.1994.tb01175.x. [DOI] [PubMed] [Google Scholar]
- 42.Liu J, Bartels M, Lu A, Sharp FR. Microglia/macrophages proliferate in striatum and neocortex but not in hippocampus after brief global ischemia that produces ischemic tolerance in gerbil brain. J Cereb Blood Flow Metab. 2001;21:361–373. doi: 10.1097/00004647-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Kim OS, Park EJ, Joe EH, Jou I. JAK-STAT signaling mediates gangliosides-induced inflammatory responses in brain microglial cells. J Biol Chem. 2002;277:40594–40601. doi: 10.1074/jbc.M203885200. [DOI] [PubMed] [Google Scholar]
- 44.Walz W, Ilschner S, Ohlemeyer C, Banati R, Kettenmann H. Extracellular ATP activates a cation conductance and a K+ conductance in cultured microglial cells from mouse brain. J Neurosci. 1993;13:4403–4411. doi: 10.1523/JNEUROSCI.13-10-04403.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JB, Sig Choi J, Yu YM, Nam K, Piao CS, Kim SW, Lee MH, Han PL, Park JS, Lee JK. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the postischemic brain. J Neurosci. 2006;26:6413–6421. doi: 10.1523/JNEUROSCI.3815-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pineda D, Ampurdanés C, Medina MG, Serratosa J, Tusell JM, Saura J, Planas AM, Navarro P. Tissue plasminogen activator induces microglial inflammation via a noncatalytic molecular mechanism involving activation of mitogen-activated protein kinases and Akt signaling pathways and AnnexinA2 and Galectin-1 receptors. Glia. 2012;60:526–540. doi: 10.1002/glia.22284. [DOI] [PubMed] [Google Scholar]
- 47.Wright EM. Transport processes in the formation of the cerebrospinal fluid. Rev Physiol Biochem Pharmacol. 1978;83:3–34. [PubMed] [Google Scholar]
- 48.Rozenfeld C, Martinez R, Figueiredo RT, Bozza MT, Lima FR, Pires AL, Silva PM, Bonomo A, Lannes-Vieira J, De Souza W, Moura-Neto V. Soluble factors released by Toxoplasma gondii-infected astrocytes down-modulate nitric oxide production by gamma interferon-activated microglia and prevent neuronal degeneration. Infect Immun. 2003;71:2047–2057. doi: 10.1128/IAI.71.4.2047-2057.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park SJ, Lee JH, Kim HY, Choi YH, Park JS, Suh YH, Park SM, Joe EH, Jou I. Astrocytes, but not microglia, rapidly sense H2O2 via STAT6 phosphorylation, resulting in cyclooxygenase-2 expression and prostaglandin release. J Immunol. 2012;188:5132–5141. doi: 10.4049/jimmunol.1101600. [DOI] [PubMed] [Google Scholar]
- 50.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, Young W. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148:453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- 52.Djukic M, Mildner A, Schmidt H, Czesnik D, Brück W, Priller J, Nau R, Prinz M. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain. 2006;129:2394–2403. doi: 10.1093/brain/awl206. [DOI] [PubMed] [Google Scholar]
- 53.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- 54.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119:89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]