Abstract
Animal models of Alzheimer disease (AD) are used to study the mechanisms underlying AD pathogenesis, genetic interactions with genes of interest, and environmental risk factors that cause sporadic AD as well as to test the therapeutic effects of AD drug-candidates on neuropathology and cognitive function. To attain a comparative view on the AD models developed, representative AD lines were selected and summarized with respect to transgenic constructs and AD-related pathology. In addition, age-dependent plaque deposition data available in the literature for six representative AD models such as Tg2576, PDAPP, TgAPP23, Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD mice were reevaluated using a photographic plaque reference scale method that was introduced recently. Tg2576, PDAPP, and TgAPP23 mice, which carry the amyloid precursor protein (APP) transgene, produced initially slow, but progressively accelerated plaque deposition as they aged, resulting in logistic plaque deposition. In contrast, Tg-APPswe/PS1dE9 and 3xTg-AD mice, which carry both APP and PS1 transgenes, developed abruptly accelerated plaque formation from the beginning, resulting in logarithmic plaque deposition. 5XFAD mice, which also carry both the APP and PS1 transgenes, developed a logarithmic deposition beginning at 2 months. This comparative analysis suggests that AD models may be classified into two distinct plaque deposition groups, and that early plaque models such as APPswe/PS1dE9, 3xTg-AD and 5XFAD might be useful to study the biochemical aspects of APP metabolism, whereas late plaque models such as Tg2576, PDAPP, and TgAPP23 might be useful to study more physiological and environmental aspects of AD pathogenesis, which occur on a longer time scale.
Keywords: Alzheimer disease, plaque deposition, APP models, APP and PS1 models, comparison of plaque levels
INTRODUCTION
Alzheimer's disease (AD) is a progressive neurodegenerative disease characterized by neuropathological changes such as amyloid plaque deposition, neurofibrillary tangles (NFTs), neuronal loss, dystrophic neurites, and gliosis, as well as behavioral phenotypes such as learning and memory impairment, anxiety, depression, and other psychological symptoms [1, 2]. AD is a typical age-dependent neurodegenerative disease that affects 5% of individuals >65 years, 20% of those >85 years, and more than one-third of those >90 years [3]. Therefore, in the absence of proper preventative and therapeutic efforts, its prevalence will continue to increase as life expectancy increases.
Early onset familial AD (FAD) accounts for only a small percentage of AD cases. At least 232 different mutations in genes for amyloid precursor protein (APP), presenilin 1 (PS1 or PSEN1) or presenilin 2 (PS2 or PSEN2) (33, 185, and 13 mutations in, respectively, APP, PS1 and PS2) have been identified in FAD [4]. In vitro and in vivo studies suggest that amyloid-β (Aβ) peptides are the primary causative agents in AD pathogenesis [5, 6]. Aβ is produced as a result of the sequential cleavage of APP by β-secretase and γ-secretase. This Aβ-producing amyloidogenic pathway is active when APP has mutations at the cleavage sites by β-secretase or γ-secretase [7, 8]. In contrast, α-secretase cleaves APP within the Aβ domain, precluding the generation of Aβ in normal APP metabolism. Presenilins are the core part of the protein complex known as γ-secretase [9]. FAD mutations in presenilins increase production of amyloidogenic Aβ peptides in both transfected cells and transgenic mice, supporting the Aβ amyloid hypothesis [10]. The apolipoprotein E (APOE) gene is believed to be a genetic risk factor. The APOE gene occurs in >3 different alleles including E2, E3, and E4. The E4 allele has been identified as a risk factor for AD in late-onset families [11]. Individuals with one copy (15% of total population) or two copies (2% of total population) of the E4 allele have an increased chance of developing AD by, respectively, 3 times and 10-20 times, compared to those not carrying any E4 alleles [11, 12].
Most AD cases are late-onset sporadic. Although the exact cause of most sporadic AD cases is not known, several risk factors have been proposed. Clinical and epidemiological studies suggest that aging, stress, gender (female), acid-forming food containing high dietary fat or total energy, dioxins, aluminum, lead, and viral infections could act as risk factors for AD [13], although it remains to be seen if dioxins, aluminum, lead, and viral infections can be salient non-genetic risk factors for people in developed countries.
Animal models of AD play an important role in all areas of AD studies. Proper AD models are needed to study the mechanisms underlying AD pathogenesis, the genetic interactions of the genes of interest, and environmental risk factors that cause sporadic AD, as well as to test the therapeutic effects of AD drug-candidates on neuropathology and cognitive function. First, animal models of AD need to show histopathology such as plaques, NFTs, neuronal loss, and behavioral pathology including cognitive deficits, thus AD models need to show face validity. Second, animal models of AD need to fulfill a substantial similarity between mechanisms underlying changes in behavioral, pathophysiological, and neuronal components in the model and those in AD, thus AD models need to show construct validity. Third, animal models of AD need to provide predictive value of AD pathogenesis, allowing extrapolation of the effects of an experimental manipulation, thus AD models need to show predictive validity. Good predictive value may help unravel a new mechanism and identify putative therapeutics [14-16]. Ideal animal models of AD may have homology to the neurobehavioral pathology and underlying mechanisms of human AD, which include the full blown etiology, pathology, syndrome, and specific mechanisms underlying age-dependent neurobehavioral changes in AD. Several dozens of AD models have been developed to mimic the genetic cause of human AD by generating transgenic mice that overexpress mutant forms of human APP, presenilins, and/or tau protein in the brain. The list of transgenic AD models produced thus far can be found in many excellent reviews [17-21] and at the web site of the Alzheimer Research Forum [http://www.alzforum.org/res/com/tra/default.asp]. Many of the transgenic AD models developed display Aβ accumulation, plaque pathogenesis, gliosis, neuronal loss, tau pathology, and/or cognitive impairments, but no single transgenic AD model recapitulates all aspects of AD pathology. Despite that ideal AD models have not been developed, currently available AD models drive our interest to move from model building to model evaluation and use. AD models have proven useful with respect to investigating mechanisms underlying a certain aspect of AD pathology, evaluating the predictive value of AD pathogenesis, and developing a strategy and drugs to treat AD. In the use of AD models, some scientists tend to seek early-plaque models at the expense of aging effects. However, familial and sporadic AD all occur in an age-dependent manner (Fig. 1), an important feature that can be simulated by current AD models and that should not be compromised with early plaque deposition in the choice of an AD animal model for certain types of research.
Fig. 1.
Gene, age and other environmental factors on the development of Alzheimer disease (AD). Age-dependent pathogenesis of AD is an important common factor in early-onset familial AD (FAD; carrying mutations at APP, PS1 or PS2), late-onset FAD (carrying ApoE4 allele), and sporadic AD. In all cases of AD, aging or age-related accumulation of undefined factors appears to trigger AD pathogenesis. Environmental risk factors may include besides aging, stress, gender (female), acid-forming food containing high dietary fat or total energy, dioxins, aluminum, lead, and viral infections.
In the present review, we classify AD animal models as APP models, CTF models, Aβ models, presenilin models, APP+PS1 double transgenic models, tau models, and a triple (APP+PS1+tau) transgenic model, with presenting a summary of key features relevant to AD pathology (Table 1).
Table 1.
Transgenic Alzheimer disease (AD) models were classified with respect to transgenic constructs and AD-related pathology

Aβ42 accumulation, plaque depositions, neurofibrillary tangles (NTFs), neuronal loss, and behavioral impairments for each line are summarized with references. APP, amyloid Precursor Protein; PS or PSEN, presenilins; CTF, carboxyl terminal fragment; PDGF-β, platelet-derived growth factor β-chain; PrP, prion Protein; Thy-1, Thymocyte differentiation antigen 1.
APP MODELS
APP models are transgenic mice that express mutant forms of human APP under the control of strong brain-driving promoters, which include platelet-derived growth factor β-chain (PDGFβ), prion protein (PrP), and thymocyte differentiation antigen 1 (Thy-1) genes. APP is a transmembrane protein that contains Aβ42. Mutations in APP around the cleavage sites by β-secretase or γ-secretase increase Aβ42 secretion in the brain. Indeed, overexpression of mutant forms of human APP in the brains of mice results in Aβ42 accumulation, plaque deposition, Aβ-triggered pathology, and cognitive impairment, which have some similarities to those in human AD. The mouse lines PDAPP, Tg2576, TgAPP23, TgCRND8, J20, and TgAPP(Sw,V717F) were all produced using this strategy.
PDAPP
Mice express the human APP695/751/770 with the mutation V717F at the γ-cleavage site under the regulatory control of the PDGFβ promoter. PDAPP mice develop increased expression of APP mRNA levels and plaque deposition in the brain at 6-9 months [22] and develop behavioral defects at 13-15 months of age [23].
Tg2576
Mice express the human APP695 with the Swedish double mutations (K670N/M671L) at the β-secretase cleavage site under control of the hamster PrP promoter. Tg2576 mice show accumulation of Aβ40 and Aβ42 at 6-9 months and plaque deposition beginning at 9 months of age [24, 25]. Tg2576 mice develop cognitive impairment beginning at 6 months of age [26]. Tg2576 mice are widely used partly due to the initial generosity of Dr. K Hsiao to users and later commercial availability from Taconic Inc.
TgAPP23
Mice express the human APP751 containing the Swedish double mutations (K670N/M671L) at the β-secretase cleavage site driven by the mouse Thy-1 promoter. TgAPP23 mice show typical plaques at 6 months [27] and neuritic and synaptic degeneration as well as tau hyperphosphorylation in aged brains [28]. TgAPP23 mice develop cognitive decline at 10 months [29].
J20
Transgenic lines express the human APP695/751/770 containing the Swedish double mutations (K670N/M671L) at the β-secretase cleavage site and the V717F mutation at the γ-secretase cleavage site. The transgene is driven by the PDGFβ promoter [30]. J20 mice develop plaque deposition at 5-7 months and cognitive deficit at 6-7 months [30, 31].
TgCRND8
Mice express the human APP695 containing the Swedish mutations (K670N/M671L) at the β-secretase cleavage site and the V717F mutation at the γ-secretase cleavage site. The transgene is driven by the hamster PrP promoter [32]. TgCRND8 mice develop plaque deposition and cognitive deficits at 3 months [32].
TgAPP(Sw,V717F)
Mice express the human APP751 containing the Swedish mutations (K670N/M671L) at the β-secretase cleavage site and the V717F mutation at the γ-secretase cleavage site driven by the PDGFβ promoter. TgAPP (Sw,V717F) mice show behavioral deficits at 11-14 months in the absence of Aβ42 accumulation and plaque deposition [33].
CTF MODELS
TgCTF104
Mice express the carboxyl terminal fragment of APP (CTF104 of APP591-695) under control of the human NF-L neurofilament promoter [34]. TgCTF104 mice develop plaque deposition at 8-10 months, severe cognitive deficits at 8 months, and neuronal loss at 18-22 months [34].
TgβCTF99
Mice express the β-secretase-cut carboxyl terminal fragment (βCTF99) with the V717F mutation under control of the PDGFβ promoter. TgβCTF99 mice display no plaque deposition but develop cognitive deficits at 11-14 months and neuronal loss in the hippocampus and cerebral cortex at 16-18 months [35].
Aβ MODELS
BRI-Aβ42
Mice express Aβ1-42 that is fused to the C-terminus of the BRI protein at the furin cleavage site under control of the mouse PrP promoter [36]. Mice carrying BRI-Aβ42A, but not BRI-Aβ40, develop amyloid deposits at 3 months and neuropathology at 12 months [36].
PS1 AND PS2 TRANSGENIC MODELS
PS1 and PS2 transgenic lines
(Tg-PS1 M146L/or M146V and Tg-pNSE-hPS2) carrying FAD mutant forms of PS1 and PS2 (M146L or M146V), respectively, under control of the PDGFβ and NSE promoter show enhanced levels of Aβ42, supporting the hypothesis that the presenilin mutations cause AD pathogenesis through a gain of deleterious function [9, 10, 37]. However, transgenic mice carrying FAD mutant forms of presenilins or gene-targeted mice carrying FAD-mutant PS1 (PS1P264L/P264L) do not develop plaques, although when crossed with plaque-forming APP lines (eg., Tg2576), the presenilin FAD mutations produce elevated levels of Aβ42 and cause earlier and more extensive plaque deposition [38-41].
APP+PS TRANSGENIC MODELS
APP and PS1 bigenic or monogenic transgenic mice carrying the transgene for both mutant APP and mutant PS1 show increased Aβ42 production and more extensive plaque deposition, which was reviewed well by Hall and Roberson [20].
Tg-APPswe/PS1dE9
Mice express the human APP with the Swedish mutations (K670N/M671L) at the β-secretase cleavage site and PS1 (PS1dE9), which result in plaque deposition in the brain starting at 6 months of age [42-44]. Despite the plaque deposition and behavioral deficits at 6-8 months of age [45, 46], Tg-APPswe/PS1dE9 mice do not develop NFTs or neuronal loss. Tg-APPswe/PS1dE9 mice became popular partly due to the early availability from JAX (Table 1).
5XFAD
Mice express the human APP with the Swedish mutations (K670N/M671L) at the β-secretase cleavage site and two FAD-associated mutations (I716V/V717I) at the γ-secretase cleavage site, and human PS1 with the M146V and L286V mutations under control of the mouse Thy-1 promoter [47]. 5XFAD mice develop plaque deposition at 2 months and cognitive deficits at 4-6 months [47]. 5XFAD mice are now available from JAX (Table 1).
TAU MODELS
Transgenic mice overexpressing mutant tau produce tau pathology, but not Aβ deposits.
JNPL3
Mice express the human tau with the most common mutation (P301L), which causes the fronto-temporal dementia and parkinsonism-linked to chromosome 17 (FTDP-17 mutation) in human, under control of the mouse PrP promoter. JNPL3 mice develop NFTs at 6.5 months and progressive motor disturbance in hemizygous animals [48]. When JNPL3 are crossed with Tg2576, the resulting double mutant (tau/APP) progeny and the Tg2576 parental mice develop Aβ deposits at the same age; however, the tau/APP double mutants exhibit enhanced NFT pathology in the limbic system and olfactory cortex compared to that of the JNPL3 parent strain [49].
TauV337M
Mice express the human tau with the V337M mutation under control of the PDGFβ promoter. TauV337M mice develop NFT and hippocampal neuronal loss at 11 months [50].
TRIPLE (APP+PS+TAU) TRANSGENIC MODEL
3xTg-AD
Mice express the human APP695(Swe), PS1(M146V), and Tau(P301L) under control of the mouse Thy-1 promoter. 3xTg-AD mice develop plaques beginning at 6 months of age and NFTs at 12 months. 3xTg-AD mice display synaptic dysfunction, including long-term potentiation deficits before plaque and tangle pathology [51]. 3xTg-AD mice show memory deficits at 4.5 months, which are correlated with the accumulation of intraneuronal Aβ in the hippocampus and amygdala [52].
REVALUATION OF PLAQUE DEPOSITION PATTERNS IN THE BRAIN OF SIX REPRESENTATIVE AD MODELS USING PHOTOGRAPHIC PLAQUE REFERENCE PANELS
The plaque models Tg2576, PDAPP, TgAPP23, Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD are widely used in many laboratories as they display robust age-dependent plaque deposition in the brain, but their relative plaque deposition patterns and features have not been clearly analyzed due to technical difficulties. In this review, we attempted to re-evaluate plaque deposition levels in each line using a photographic plaque reference scale method [44] and analyzed the resulting data.
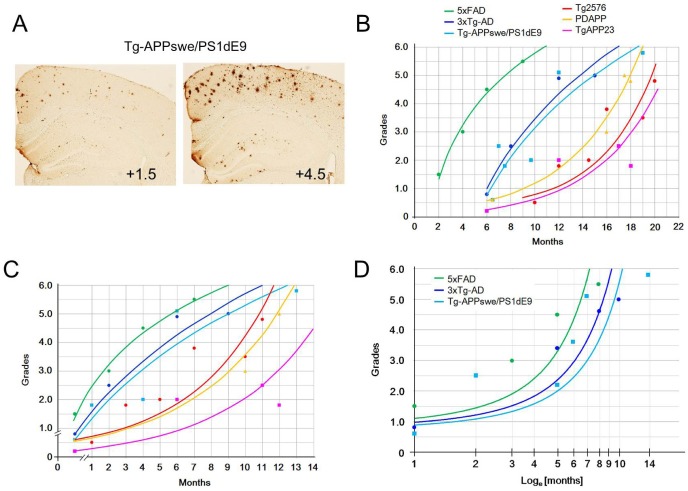
We searched for studies describing plaque deposition in brain sections of AD models and re-evaluated plaque levels in each line using plaque reference panels. Despite that the plaque deposition information contained in the photographic images published in the literature was available at low resolution in most cases, immunostaining of brain sections was not carried out in the same condition, and animals were reared in different laboratories probably under different microenvironments, plaque depositions evaluated for each AD model plotted against age resulted in either logistic or logarithmic curves (Fig. 2A-C). The age-dependent plaque deposition patterns of Tg2576, PDAPP, and TgAPP23 similarly produced logistic curves, whereas the temporal plaque deposition patterns of Tg-APPswe/PS1dE9 and 3xTg-AD produced logarithmic curves. The temporal plaque deposition in 5XFAD mice (Fig. 2B) was left-shifted (that is, time-shifted) and was basically similar to the patterns of Tg-APPswe/PS1dE9 and 3xTg-AD mice. In fact, Tg2576, PDAPP, and TgAPP23 belonged to APP models, whereas Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD belonged to lines carrying both APP and PS1 transgenes in an individual. This interpretation was supported by previous reports describing the plaque loading data between Tg2576 and bi-transgenic mice genetically produced by crossing Tg2576 with Tg-PS1P264L/P264L mice [38] and the plaque loading data between Tg2576 and 5XFAD [47]. The present analysis was primarily based on existing image data available in the literature; thus, it may be an estimate. Nevertheless, the results of this analysis indicate that Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD mice, which carry the APP and PS1 transgenes, produce rapid and logarithmic accumulation of plaques, whereas Tg2576, PDAPP, and TgAPP23 mice, carrying the APP transgene, produce slow and logistic accumulation of plaques in the brain (Fig. 2B, C).
Fig. 2.
Temporal plaque deposition patterns in the brains of six representative AD models. (A) Examples of quantifying plaque deposition levels in the brain of Tg-APPswe/PS1dE9 mice using the six-scale photographic plaque reference panels [44]. (B) Age-dependent plaque deposition in the brains of Tg2576, PDAPP, TgAPP23, Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD mice are plotted with age. Photomicrographs showing plaque deposition in the parietal cortices for each line were searched for in the literature and their plaque deposition levels were re-evaluated using the six-scale photographic plaque reference panels. Plaque deposition levels for each line was reassessed using both the Tg2676 panels and Tg-APPswe/PS1dE9 panels [44], and plaque scores were averaged [25, 27, 44, 45, 47, 55-61]. (C) Age-dependent plaque deposition was re-evaluated using the six-scale reference panels in the brains of Tg2576, PDAPP, TgAPP23, Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD mice and were aligned over the month showing first plaque deposition. Rapid and abrupt plaque accumulation was evident in the AD models that carry both APP and PS1 transgenes compared to the lines that carried the APP transgene. (D) Plaque deposition levels in Tg-APPswe/PS1dE9, 3xTg-AD, and 5xFAD mice plotted over log values of time (loge[months]) produced new plaque deposition patterns that were similar in shape to the un-converted plaque deposition profiles displayed by Tg2576, PDAPP, and TgAPP23 mice.
Considering that the transgenic cassettes carrying hAPP in Tg-APPswe/PS1dE9 and 3xTg-AD mice are similar to the hAPP transgenic cassettes carried by Tg2576, PDAPP, and TgAPP23 mice, accelerated plaque deposition in Tg-APPswe/PS1dE9 and 3xTg-AD is likely driven by the added action of enhanced γ-secretase activity onto the mutant form of human APP. Plaque depositions in Tg-APPswe/PS1dE9 and 3xTg-AD appear to have some time-accelerated process aspects, and there are 5-8 months of aging difference when plaque levels in each group reach to level +3.0 (Fig. 2B, C). Re-plotting the plaque deposition levels in Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD over the log values of time (loge[months]) resulted in new plaque deposition profiles (Fig. 2D) which are increasing rapidly after 3 months compared to the slopes of the unconverted plaque depositions in Tg2576, PDAPP, and TgAPP23 mice (Fig. 2B, C). Thus, the plaque deposition pressure in Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD appears to occur too strongly to be cleaned up even by young healthy brains. On the other hands, presumably 5-8 months of aging is a long period that involves numerous changes besides altered APP metabolism. Therefore, a simple time-accelerated process of APP metabolism may not be proper to explain the rapid plaque deposition in Tg-APPswe/PS1dE9 and 3xTg-AD.
Consistent with this interpretation, it was reported that the plaque deposition pattern in Tg2576 mice was qualitatively different from that in Tg-APPswe/PS1dE9 mice. The brains of Tg2576 mice started to accumulate predominantly small plaques, whereas the brains of Tg-APPswe/PS1dE9 mice began to deposit relatively large plaques [44]. Because accelerated APP metabolism has been proven in the brains of Tg-APPswe/PS1dE9, 3xTg-AD, and 5XFAD, these early plaque deposition models might be useful to study biochemical aspects of APP metabolism and the resulting plaque deposition, whereas Tg2576, PDAPP, and TgAPP23 might be useful for more physiological and environmental aspects of AD pathogenesis, which occur over a longer time scale. As we reported previously, indeed accelerated plaque pathology by behavioral stress in Tg-APPswe/PS1dE9 [53] was less prominent than that in Tg2576 [54]. The aging factor in AD pathogenesis should not be compromised by taking advantage of accelerated plaque deposition, but it should be seriously considered as an important part of AD pathogenesis in choosing AD models. Many AD studies can be successfully performed if provided with a proper AD model after a careful consideration of the plaque deposition profile. AD models displaying more delayed and slow plaque deposition might model the brains of late-onset sporadic AD more closely.
ACKNOWLEDGEMENTS
This research was supported by a grant (2012R1A2A1A03010177) from the Ministry of Science, ICT and Future Planning, Republic of Korea.
References
- 1.Prince M, Jackson J. World Alzheimer report: 2009. London: Alzheimer's Disease International; 2009. [Google Scholar]
- 2.Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wimo A, Prince M. World Alzheimer report 2010: the global economic impact of dementia. London: Alzheimer's Disease International; 2011. [Google Scholar]
- 4.Cruts M, Theuns J, Van Broeckhoven C. Locus-specific mutation databases for neurodegenerative brain diseases. Hum Mutat. 2012;33:1340–1344. doi: 10.1002/humu.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Wang DS, Dickson DW, Malter JS. beta-Amyloid degradation and Alzheimer's disease. J Biomed Biotechnol. 2006;2006:58406. doi: 10.1155/JBB/2006/58406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haass C, Lemere CA, Capell A, Citron M, Seubert P, Schenk D, Lannfelt L, Selkoe DJ. The Swedish mutation causes early-onset Alzheimer's disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995;1:1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- 8.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 9.Brunkan AL, Goate AM. Presenilin function and gamma-secretase activity. J Neurochem. 2005;93:769–792. doi: 10.1111/j.1471-4159.2005.03099.x. [DOI] [PubMed] [Google Scholar]
- 10.Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer's disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med. 1997;3:67–72. doi: 10.1038/nm0197-67. [DOI] [PubMed] [Google Scholar]
- 11.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y. Roles of apolipoprotein E4 (ApoE4) in the pathogenesis of Alzheimer's disease: lessons from ApoE mouse models. Biochem Soc Trans. 2011;39:924–932. doi: 10.1042/BST0390924. [DOI] [PubMed] [Google Scholar]
- 13.Grant WB, Campbell A, Itzhaki RF, Savory J. The significance of environmental factors in the etiology of Alzheimer's disease. J Alzheimers Dis. 2002;4:179–189. doi: 10.3233/jad-2002-4308. [DOI] [PubMed] [Google Scholar]
- 14.Held JR. Appropriate animal models. Ann N Y Acad Sci. 1983;406:13–19. doi: 10.1111/j.1749-6632.1983.tb53481.x. [DOI] [PubMed] [Google Scholar]
- 15.Mineur YS, McLoughlin D, Crusio WE, Sluyter F. Genetic mouse models of Alzheimer's disease. Neural Plast. 2005;12:299–310. doi: 10.1155/NP.2005.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Dam D, De Deyn PP. Drug discovery in dementia: the role of rodent models. Nat Rev Drug Discov. 2006;5:956–970. doi: 10.1038/nrd2075. [DOI] [PubMed] [Google Scholar]
- 17.Higgins GA, Jacobsen H. Transgenic mouse models of Alzheimer's disease: phenotype and application. Behav Pharmacol. 2003;14:419–438. doi: 10.1097/01.fbp.0000088420.18414.ff. [DOI] [PubMed] [Google Scholar]
- 18.Eriksen JL, Janus CG. Plaques, tangles, and memory loss in mouse models of neurodegeneration. Behav Genet. 2007;37:79–100. doi: 10.1007/s10519-006-9118-z. [DOI] [PubMed] [Google Scholar]
- 19.Elder GA, Gama Sosa MA, De Gasperi R. Transgenic mouse models of Alzheimer's disease. Mt Sinai J Med. 2010;77:69–81. doi: 10.1002/msj.20159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall AM, Roberson ED. Mouse models of Alzheimer's disease. Brain Res Bull. 2012;88:3–12. doi: 10.1016/j.brainresbull.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lalonde R, Fukuchi K, Strazielle C. APP transgenic mice for modelling behavioural and psychological symptoms of dementia (BPSD) Neurosci Biobehav Rev. 2012;36:1357–1375. doi: 10.1016/j.neubiorev.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Muckestar L, Paganini L, Penniman E, Power M, Schenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J. Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature. 1995;373:523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Chen KS, Knox J, Inglis J, Bernard A, Martin SJ, Justice A, McConlogue L, Games D, Freedman SB, Morris RG. A learning deficit related to age and beta-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000;408:975–979. doi: 10.1038/35050103. [DOI] [PubMed] [Google Scholar]
- 24.Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G. Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- 25.Kawarabayashi T, Younkin LH, Saido TC, Shoji M, Ashe KH, Younkin SG. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Westerman MA, Cooper-Blacketer D, Mariash A, Kotilinek L, Kawarabayashi T, Younkin LH, Carlson GA, Younkin SG, Ashe KH. The relationship between Abeta and memory in the Tg2576 mouse model of Alzheimer's disease. J Neurosci. 2002;22:1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sturchler-Pierrat C, Abramowski D, Duke M, Wiederhold KH, Mistl C, Rothacher S, Ledermann B, Bürki K, Frey P, Paganetti PA, Waridel C, Calhoun ME, Jucker M, Probst A, Staufenbiel M, Sommer B. Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proc Natl Acad Sci U S A. 1997;94:13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturchler-Pierrat C, Staufenbiel M. Pathogenic mechanisms of Alzheimer's disease analyzed in the APP23 transgenic mouse model. Ann N Y Acad Sci. 2000;920:134–139. doi: 10.1111/j.1749-6632.2000.tb06915.x. [DOI] [PubMed] [Google Scholar]
- 29.Hellweg R, Lohmann P, Huber R, Kühl A, Riepe MW. Spatial navigation in complex and radial mazes in APP23 animals and neurotrophin signaling as a biological marker of early impairment. Learn Mem. 2006;13:63–71. doi: 10.1101/lm.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of abeta 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palop JJ, Jones B, Kekonius L, Chin J, Yu GQ, Raber J, Masliah E, Mucke L. Neuronal depletion of calcium-dependent proteins in the dentate gyrus is tightly linked to Alzheimer's disease-related cognitive deficits. Proc Natl Acad Sci U S A. 2003;100:9572–9577. doi: 10.1073/pnas.1133381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, Pearson J, Strome R, Zuker N, Loukides J, French J, Turner S, Lozza G, Grilli M, Kunicki S, Morissette C, Paquette J, Gervais F, Bergeron C, Fraser PE, Carlson GA, George-Hyslop PS, Westaway D. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 33.Lee KW, Lee SH, Kim H, Song JS, Yang SD, Paik SG, Han PL. Progressive cognitive impairment and anxiety induction in the absence of plaque deposition in C57BL/6 inbred mice expressing transgenic amyloid precursor protein. J Neurosci Res. 2004;76:572–580. doi: 10.1002/jnr.20127. [DOI] [PubMed] [Google Scholar]
- 34.Nalbantoglu J, Tirado-Santiago G, Lahsaïni A, Poirier J, Goncalves O, Verge G, Momoli F, Welner SA, Massicotte G, Julien JP, Shapiro ML. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- 35.Lee KW, Im JY, Song JS, Lee SH, Lee HJ, Ha HY, Koh JY, Gwag BJ, Yang SD, Paik SG, Han PL. Progressive neuronal loss and behavioral impairments of transgenic C57BL/6 inbred mice expressing the carboxy terminus of amyloid precursor protein. Neurobiol Dis. 2006;22:10–24. doi: 10.1016/j.nbd.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 36.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D, Morgan D, Gordon MN, Holcomb L, Refolo L, Zenk B, Hardy J, Younkin S. Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383:710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- 38.Flood DG, Reaume AG, Dorfman KS, Lin YG, Lang DM, Trusko SP, Savage MJ, Annaert WG, De Strooper B, Siman R, Scott RW. FAD mutant PS-1 gene-targeted mice: increased Aβ42 and Aβ deposition without APP overproduction. Neurobiol Aging. 2002;23:335–348. doi: 10.1016/s0197-4580(01)00330-x. [DOI] [PubMed] [Google Scholar]
- 39.Chui DH, Tanahashi H, Ozawa K, Ikeda S, Checler F, Ueda O, Suzuki H, Araki W, Inoue H, Shirotani K, Takahashi K, Gallyas F, Tabira T. Transgenic mice with Alzheimer presenilin 1 mutations show accelerated neurodegeneration without amyloid plaque formation. Nat Med. 1999;5:560–564. doi: 10.1038/8438. [DOI] [PubMed] [Google Scholar]
- 40.Mohmmad Abdul H, Sultana R, Keller JN, St Clair DK, Markesbery WR, Butterfield DA. Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid beta-peptide (1-42), HO and kainic acid: implications for Alzheimer's disease. J Neurochem. 2006;96:1322–1335. doi: 10.1111/j.1471-4159.2005.03647.x. [DOI] [PubMed] [Google Scholar]
- 41.Toda T, Noda Y, Ito G, Maeda M, Shimizu T. Presenilin-2 mutation causes early amyloid accumulation and memory impairment in a transgenic mouse model of Alzheimer's disease. J Biomed Biotechnol. 2011;2011:617974. doi: 10.1155/2011/617974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jankowsky JL, Slunt HH, Ratovitski T, Jenkins NA, Copeland NG, Borchelt DR. Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomol Eng. 2001;17:157–165. doi: 10.1016/s1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- 43.Jankowsky JL, Xu G, Fromholt D, Gonzales V, Borchelt DR. Environmental enrichment exacerbates amyloid plaque formation in a transgenic mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2003;62:1220–1227. doi: 10.1093/jnen/62.12.1220. [DOI] [PubMed] [Google Scholar]
- 44.Kim TK, Lee JE, Park SK, Lee KW, Seo JS, Im JY, Kim ST, Lee JY, Kim YH, Lee JK, Han PL. Analysis of differential plaque depositions in the brains of Tg2576 and Tg-APPswe/PS1dE9 transgenic mouse models of Alzheimer disease. Exp Mol Med. 2012;44:492–502. doi: 10.3858/emm.2012.44.8.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savonenko A, Xu GM, Melnikova T, Morton JL, Gonzales V, Wong MP, Price DL, Tang F, Markowska AL, Borchelt DR. Episodic-like memory deficits in the APPswe/PS1dE9 mouse model of Alzheimer's disease: relationships to beta-amyloid deposition and neurotransmitter abnormalities. Neurobiol Dis. 2005;18:602–617. doi: 10.1016/j.nbd.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 46.Kim TK, Han HE, Kim H, Lee JE, Choi D, Park WJ, Han PL. Expression of the plant viral protease NIa in the brain of a mouse model of Alzheimer's disease mitigates Aβ pathology and improves cognitive function. Exp Mol Med. 2012;44:740–748. doi: 10.3858/emm.2012.44.12.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lewis J, McGowan E, Rockwood J, Melrose H, Nacharaju P, Van Slegtenhorst M, Gwinn-Hardy K, Paul Murphy M, Baker M, Yu X, Duff K, Hardy J, Corral A, Lin WL, Yen SH, Dickson DW, Davies P, Hutton M. Neurofibrillary tangles, amyotrophy and progressive motor disturbance in mice expressing mutant (P301L) tau protein. Nat Genet. 2000;25:402–405. doi: 10.1038/78078. [DOI] [PubMed] [Google Scholar]
- 49.Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G, Yen SH, Sahara N, Skipper L, Yager D, Eckman C, Hardy J, Hutton M, McGowan E. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 50.Tanemura K, Murayama M, Akagi T, Hashikawa T, Tominaga T, Ichikawa M, Yamaguchi H, Takashima A. Neurodegeneration with tau accumulation in a transgenic mouse expressing V337M human tau. J Neurosci. 2002;22:133–141. doi: 10.1523/JNEUROSCI.22-01-00133.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 52.Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM. Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron. 2005;45:675–688. doi: 10.1016/j.neuron.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 53.Seo JS, Lee KW, Kim TK, Baek IS, Im JY, Han PL. Behavioral stress causes mitochondrial dysfunction via ABAD up-regulation and aggravates plaque pathology in the brain of a mouse model of Alzheimer disease. Free Radic Biol Med. 2011;50:1526–1535. doi: 10.1016/j.freeradbiomed.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 54.Lee KW, Kim JB, Seo JS, Kim TK, Im JY, Baek IS, Kim KS, Lee JK, Han PL. Behavioral stress accelerates plaque pathogenesis in the brain of Tg2576 mice via generation of metabolic oxidative stress. J Neurochem. 2009;108:165–175. doi: 10.1111/j.1471-4159.2008.05769.x. [DOI] [PubMed] [Google Scholar]
- 55.Jankowsky JL, Slunt HH, Gonzales V, Jenkins NA, Copeland NG, Borchelt DR. APP processing and amyloid deposition in mice haplo-insufficient for presenilin 1. Neurobiol Aging. 2004;25:885–892. doi: 10.1016/j.neurobiolaging.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Hao J, Liu R, Zhang Z, Lei G, Su C, Miao J, Li Z. Soluble Aβ levels correlate with cognitive deficits in the 12-month-old APPswe/PS1dE9 mouse model of Alzheimer's disease. Behav Brain Res. 2011;222:342–350. doi: 10.1016/j.bbr.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 57.Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Yue X, Lu M, Lancaster T, Cao P, Honda S, Staufenbiel M, Harada N, Zhong Z, Shen Y, Li R. Brain estrogen deficiency accelerates Abeta plaque formation in an Alzheimer's disease animal model. Proc Natl Acad Sci U S A. 2005;102:19198–19203. doi: 10.1073/pnas.0505203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oddo S, Caccamo A, Kitazawa M, Tseng BP, LaFerla FM. Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiol Aging. 2003;24:1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Guan H, Liu Y, Daily A, Police S, Kim MH, Oddo S, LaFerla FM, Pauly JR, Murphy MP, Hersh LB. Peripherally expressed neprilysin reduces brain amyloid burden: a novel approach for treating Alzheimer's disease. J Neurosci Res. 2009;87:1462–1473. doi: 10.1002/jnr.21944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caccamo A, Oddo S, Billings LM, Green KN, Martinez-Coria H, Fisher A, LaFerla FM. M1 receptors play a central role in modulating AD-like pathology in transgenic mice. Neuron. 2006;49:671–682. doi: 10.1016/j.neuron.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 62.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Lieberburg I, Motter R, Nguyen M, Soriano F, Vasquez N, Weiss K, Welch B, Seubert P, Schenk D, Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 63.Reilly JF, Games D, Rydel RE, Freedman S, Schenk D, Young WG, Morrison JH, Bloom FE. Amyloid deposition in the hippocampus and entorhinal cortex: quantitative analysis of a transgenic mouse model. Proc Natl Acad Sci U S A. 2003;100:4837–4842. doi: 10.1073/pnas.0330745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Irizarry MC, Soriano F, McNamara M, Page KJ, Schenk D, Games D, Hyman BT. Abeta deposition is associated with neuropil changes, but not with overt neuronal loss in the human amyloid precursor protein V717F (PDAPP) transgenic mouse. J Neurosci. 1997;17:7053–7059. doi: 10.1523/JNEUROSCI.17-18-07053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weiss C, Venkatasubramanian PN, Aguado AS, Power JM, Tom BC, Li L, Chen KS, Disterhoft JF, Wyrwicz AM. Impaired eyeblink conditioning and decreased hippocampal volume in PDAPP V717F mice. Neurobiol Dis. 2002;11:425–433. doi: 10.1006/nbdi.2002.0555. [DOI] [PubMed] [Google Scholar]
- 66.Dodart JC, Meziane H, Mathis C, Bales KR, Paul SM, Ungerer A. Behavioral disturbances in transgenic mice overexpressing the V717F beta-amyloid precursor protein. Behav Neurosci. 1999;113:982–990. doi: 10.1037//0735-7044.113.5.982. [DOI] [PubMed] [Google Scholar]
- 67.Takeuchi A, Irizarry MC, Duff K, Saido TC, Hsiao Ashe K, Hasegawa M, Mann DM, Hyman BT, Iwatsubo T. Age-related amyloid beta deposition in transgenic mice overexpressing both Alzheimer mutant presenilin 1 and amyloid beta precursor protein Swedish mutant is not associated with global neuronal loss. Am J Pathol. 2000;157:331–339. doi: 10.1016/s0002-9440(10)64544-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Irizarry MC, McNamara M, Fedorchak K, Hsiao K, Hyman BT. APPSw transgenic mice develop age-related A beta deposits and neuropil abnormalities, but no neuronal loss in CA1. J Neuropathol Exp Neurol. 1997;56:965–973. doi: 10.1097/00005072-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 69.Pompl PN, Mullan MJ, Bjugstad K, Arendash GW. Adaptation of the circular platform spatial memory task for mice: use in detecting cognitive impairment in the APP(SW) transgenic mouse model for Alzheimer's disease. J Neurosci Methods. 1999;87:87–95. doi: 10.1016/s0165-0270(98)00169-1. [DOI] [PubMed] [Google Scholar]
- 70.Corcoran KA, Lu Y, Turner RS, Maren S. Overexpression of hAPPswe impairs rewarded alternation and contextual fear conditioning in a transgenic mouse model of Alzheimer's disease. Learn Mem. 2002;9:243–252. doi: 10.1101/lm.51002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.King DL, Arendash GW. Behavioral characterization of the Tg2576 transgenic model of Alzheimer's disease through 19 months. Physiol Behav. 2002;75:627–642. doi: 10.1016/s0031-9384(02)00639-x. [DOI] [PubMed] [Google Scholar]
- 72.Van Dam D, D'Hooge R, Staufenbiel M, Van Ginneken C, Van Meir F, De Deyn PP. Age-dependent cognitive decline in the APP23 model precedes amyloid deposition. Eur J Neurosci. 2003;17:388–396. doi: 10.1046/j.1460-9568.2003.02444.x. [DOI] [PubMed] [Google Scholar]
- 73.Calhoun ME, Wiederhold KH, Abramowski D, Phinney AL, Probst A, Sturchler-Pierrat C, Staufenbiel M, Sommer B, Jucker M. Neuron loss in APP transgenic mice. Nature. 1998;395:755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- 74.Maier M, Seabrook TJ, Lazo ND, Jiang L, Das P, Janus C, Lemere CA. Short amyloid-beta (Abeta) immunogens reduce cerebral Abeta load and learning deficits in an Alzheimer's disease mouse model in the absence of an Abeta-specific cellular immune response. J Neurosci. 2006;26:4717–4728. doi: 10.1523/JNEUROSCI.0381-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hyde LA, Kazdoba TM, Grilli M, Lozza G, Brusa R, Zhang Q, Wong GT, McCool MF, Zhang L, Parker EM, Higgins GA. Age-progressing cognitive impairments and neuropathology in transgenic CRND8 mice. Behav Brain Res. 2005;160:344–355. doi: 10.1016/j.bbr.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 76.Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, Schmidt SD, Chishti MA, Horne P, Heslin D, French J, Mount HT, Nixon RA, Mercken M, Bergeron C, Fraser PE, St George-Hyslop P, Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 77.Hwang DY, Chae KR, Kang TS, Hwang JH, Lim CH, Kang HK, Goo JS, Lee MR, Lim HJ, Min SH, Cho JY, Hong JT, Song CW, Paik SG, Cho JS, Kim YK. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. 2002;16:805–813. doi: 10.1096/fj.01-0732com. [DOI] [PubMed] [Google Scholar]
- 78.Malm T, Koistinaho J, Kanninen K. Utilization of APPswe/PS1dE9 transgenic mice in research of Alzheimer's disease: focus on gene therapy and cell-based therapy applications. Int J Alzheimers Dis. 2011;2011:517160. doi: 10.4061/2011/517160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jankowsky JL, Melnikova T, Fadale DJ, Xu GM, Slunt HH, Gonzales V, Younkin LH, Younkin SG, Borchelt DR, Savonenko AV. Environmental enrichment mitigates cognitive deficits in a mouse model of Alzheimer's disease. J Neurosci. 2005;25:5217–5224. doi: 10.1523/JNEUROSCI.5080-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong H, Callaghan D, Wodzinska J, Xu J, Premyslova M, Liu QY, Connelly J, Zhang W. Biochemical and behavioral characterization of the double transgenic mouse model (APPswe/PS1dE9) of Alzheimer's disease. Neurosci Bull. 2011;27:221–232. doi: 10.1007/s12264-011-1015-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Devi L, Ohno M. Phospho-eIF2alpha level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PLoS One. 2010;5:e12974. doi: 10.1371/journal.pone.0012974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohno M, Cole SL, Yasvoina M, Zhao J, Citron M, Berry R, Disterhoft JF, Vassar R. BACE1 gene deletion prevents neuron loss and memory deficits in 5XFAD APP/PS1 transgenic mice. Neurobiol Dis. 2007;26:134–145. doi: 10.1016/j.nbd.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kimura R, Ohno M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiol Dis. 2009;33:229–235. doi: 10.1016/j.nbd.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Devi L, Ohno M. Genetic reductions of beta-site amyloid precursor protein-cleaving enzyme 1 and amyloid-beta ameliorate impairment of conditioned taste aversion memory in 5XFAD Alzheimer's disease model mice. Eur J Neurosci. 2010;31:110–118. doi: 10.1111/j.1460-9568.2009.07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKee AC, Carreras I, Hossain L, Ryu H, Klein WL, Oddo S, LaFerla FM, Jenkins BG, Kowall NW, Dedeoglu A. Ibuprofen reduces Abeta, hyperphosphorylated tau and memory deficits in Alzheimer mice. Brain Res. 2008;1207:225–236. doi: 10.1016/j.brainres.2008.01.095. [DOI] [PMC free article] [PubMed] [Google Scholar]