Abstract
Background
Childhood abuse leads to greater morbidity and mortality in adulthood. Dysregulated physiological stress responses may underlie the greater health risk among abused individuals.
Purpose
This study evaluated the impact of childhood abuse on inflammatory responses to naturalistically occurring daily stressors.
Methods
In this cross-sectional study of 130 older adults, recent daily stressors and childhood abuse history were evaluated using the Daily Inventory of Stressful Events and the Childhood Trauma Questionnaire. Blood samples provided data on circulating interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and C-reactive protein (CRP).
Results
Childhood abuse history moderated IL-6 levels, but not TNF-α and CRP responses to daily stressors. Individuals with a childhood abuse history who experienced multiple stressors in the past 24 hours had IL-6 levels 2.35 times greater than those of participants who reported multiple daily stressors but no early abuse history.
Conclusion
Childhood abuse substantially enhances IL-6 responses to daily stressors in adulthood.
Keywords: childhood abuse, daily stress, chronic stress, inflammation, caregiving
In large epidemiological studies, childhood abuse has been associated with greater odds of developing age-related physical diseases in adulthood [1]. The biological embedding hypothesis suggests that childhood abuse may lead to sustained physiological changes that put the individuals at increased risk for poor health outcomes later in life [2]. The emergence of a proinflammatory immune phenotype might account for the greater prevalence of chronic diseases among abused individuals [3].
Individuals with a history of childhood abuse display dysregulated neuroendocrine and immune responses in adulthood. They exhibited increased ACTH responses to acute stressors, blunted response to the dexamethasone-CRH suppression test, decreased cortisol awakening responses, and impaired control over latent viruses [4, 5]. Furthermore, among individuals with a history of basal cell carcinoma, those with a history of childhood abuse had poorer cellular immune responses in the tumor in response to recent life events, compared to participants without an abuse history [6].
Childhood abuse may amplify proinflammatory cytokine responses later in life. Adult individuals exposed to childhood abuse had elevated basal CRP levels and greater IL-6 responses to a laboratory stressor, compared to their non-abused counterparts [7-10]. Furthermore, the impact of family dementia caregiving stress on TNF-α production was enhanced among older adults with a history of childhood abuse [11]. Moreover, childhood abuse promoted the development of glucocorticoid insensitivity and larger inflammatory responses over time among healthy adolescents [12].
We have recently reported the presence of multiple daily stressors in the past 24 hours was associated with elevated IL-6 and CRP levels among older adults [13]. The goal of this study was to examine whether a childhood abuse history would amplify inflammatory responses to naturalistically occurring daily stressors.
Methods
Participants
Participants were part of a larger study on stress and health [11, 13]. Caregivers were recruited from diagnostic clinics, local support groups, and ads in the Alzheimer Association Newsletters. Noncaregiving participants were recruited through ads inlocal newspapers, church groups, and senior citizens’ organizations. The study sample comprised 53 family dementia caregivers and 77 noncaregiving controls. Caregivers were caring for a spouse or a parent with dementia for at least 5 hours per week. Noncaregiving controls were excluded if they were involved in any caregiving activity in the past year. Participants aged 45-90 with a body mass index (BMI) ≤ 40 were included in the study. Individuals with immune-related diseases (e.g., acute infection, diabetes, recent cancer) or using immune-related medication were excluded (e.g., statins, antibiotics). The institutional review board approved this study, and each participant provided informed consent.
Protocol
In this cross-sectional study, study visits were scheduled between 8 to 10 AM to account for diurnal changes on circulating cytokines. After signing the consent form, the participants had blood drawn and body measurements taken for BMI calculation. They also filled out self-reported questionnaires, and completed a semi-structured interview.
Measures
Self-reported questionnaires
The Childhood Trauma Questionnaire (CTQ) is a 28-item self-report inventory that assesses abuse and neglect during chilhood [14]. It assessed emotional (e.g. “people in my family said hurtful or insulting things to me”), physical (e.g. “People in my family hit me so hard that it left me with bruises or marks”), and sexual (e.g. someone tried to touch me in a sexual way, or tried to make me touch them”) abuse when the person was growing up as a child and a teenager. The high-sensitivity cut-off scores were used to identify individuals reporting any form of emotional, physical, or sexual abuse. The neglect subscales were not used in this study because they were confounded by the prevailing socio-economic conditions that occurred during this older cohort’s childhood. The inventory was acceptable to older adult participants.
The Center for Epidemiological Scale-Depression (CESD) assessed depressive symptoms in the past two weeks [15]. The Beck Anxiety Inventory (BAI) evaluated anxiety symptoms in the past two weeks [16]. The Pittsburgh Sleep Quality Index (PSQI) assessed sleep quality and sleep disturbances in the past month [17]. Smoking (i.e, number of cigarettes) and alcohol use (i.e. number of alcoholic beverages) in the past week were evaluated using a standard questionnaire. The Community Healthy Activities Model Program for Seniors (CHAMPS) Questionnaires provided an estimate of the weekly caloric expenditure in physical activity [18]. The Older Adult Resources and Services (OARS) Functional Assessment evaluated chronic medical conditions and medication use [19].
Semi-structured interview
The Daily Inventory of Stressful Events (DISE) assessed the occurrence of daily stressors in the past 24 hours [20]. This instrument was chosen because of its flexibility and sensitivity in the assessment of idiosyncratic daily stressors such as the ones experienced by family dementia caregivers. Because our prior data showed that the occurrence of multiple daily stressors in the past 24 hours was associated with elevated IL-6 and CRP levels, the DISE data were coded as a dichotomous variable distinguishing the presence of zero or one stressors and the occurrence of multiple stressors in the past 24 hours [13].
IL-6, TNF-α, and CRP assays
Serum IL-6 and TNF-α levels were determined using Quantikine High Sensitivity Immunoassay kits (R&D). Data for 10 caregivers and 10 controls were lost because of technical problems with the assay. The high sensitivity C-reactive protein (CRP) assay was performed using chemiluminescence methodology with the Immulite 1000 (Siemens Medical Solutions, Los Angeles, Ca.), as described previously [13].
Statistical analyses
Base 10 logarithmic transformations were applied to variables with a skewed distribution. Chi-square and analysis of variance tests assessed group differences between individuals with and without a history of childhood abuse. Hierarchical linear regression models were fitted with caregiving status, daily stressors, and history of abuse as independent variables, and IL-6, TNF-α and CRP as dependent variables. Interaction terms among daily stressors, caregiving status, and childhood abuse evaluated the moderating effect of early life adversity on inflammatory stress responses. The covariates included the models were age, sex, gender, ethnicity, marital status, education, and BMI. Given the significant group difference, sleep disturbances was also included as a covariate in models evaluating the impact of childhood abuse. A two-sided .05 alpha level was used for the study.
Results
Occurrence of Childhood Abuse
Fifty-seven participants (43.8%) reported at least one type of emotional, physical, or sexual abuse during childhood. Table 1 describes the sociodemographic and psychological characteristics of the study participants. Individuals with a history of childhood abuse were marginally younger than participants without an abuse history. The groups did not differ on any other sociodemographic characteristics.
Table 1.
Sociodemographic and psychological characteristics of the study participants as function of childhood abuse history.
| Non-Abused N= 73 |
Abused N= 57 |
p-value | |
|---|---|---|---|
| Age, mean (SD) | 67.20 (13.74) | 62.47 (12.07) | .06 |
| Sex (N, % women) | 61 (83.5%) | 46 (80.7%) | .67 |
| Ethnicity (% Caucasians) | 59 (80.8%) | 48 (84.2%) | .62 |
| Caregiving status (% of caregivers) | 29 (39.7%) | 24 (42.1%) | .78 |
| BMI, mean (SD) | 26.3 (5.09) | 27.76 (4.66) | .10 |
| Marital status (% married) | 37 (50.7%) | 30 (52.6%) | .83 |
| Education, n (%) | .89 | ||
| At least some High School | 22 (30.1%) | 19 (33.3%) | |
| At least some College | 28 (38.4%) | 22 (38.6%) | |
| Graduate or professional school | 23 (31.5%) | 16 (28.1%) | |
| Depressive symptoms, mean (SD) | 7.90 (7.02) | 9.92 (9.69) | .26 |
| Anxious symptoms, mean (SD) | 4.14 (.54) | 5.89 (.61) | .09 |
| Sleep disturbances, mean (SD) | 4.87 (2.53) | 6.00 (2.99) | .04* |
denotes a statistically significant group difference.
History of Childhood Abuse, Affective Disturbances, Sleep Quality, and Daily Stressors
Adjusting for caregiving status and sociodemographic characteristics, history of childhood abuse was not associated with depressive symptoms, β = .07 (SE = .07), p = .37, R2 = .006, but was marginally associated with anxiety symptoms, β = .11, (SE= .07), p = .09, R2 = .02. Furthermore, individuals with a history of abuse reported more sleep disturbances than their non-abused counterparts, β = 1.07 (SE = .47), p = .02, R2 = .052.
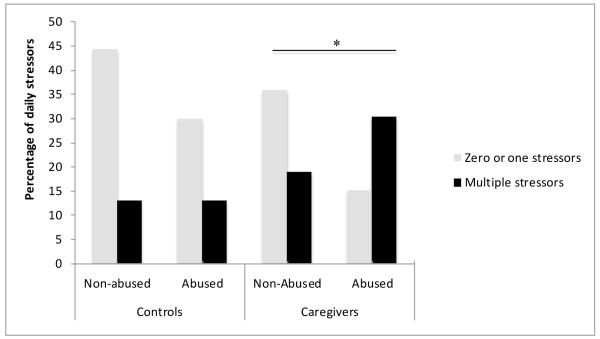
As reported previously, caregivers were more likely to report multiple stressors in the past 24 hours, compared to noncaregiving control participants, χ2(1) = 7.32, p = .007. Individuals who reported a history of childhood abuse were more likely to report multiple daily stressors in the past 24 hours compared to participants without such history of abuse, χ2(1) = 4.41, p = .04. Further analysis revealed that the greater occurrence of daily stressors associated with childhood abuse history was observed among caregivers, χ2(1) = 5.44, p = .02, but not among noncaregiving controls, χ2(1) = .48, p = .49. The impact of caregiving status and childhood abuse on the occurrence of daily stressors is depicted in Figure 1.
Figure 1.
Occurrence of stressors in the past 24 hours as a function of caregiving status and childhood adversity. Data represents the total percentage of daily stressors within each group (caregiving and controls). * indicates a significant difference between abused and non-abused participants in the caregiving, but not the control group.
History of Childhood Abuse and Inflammatory Responses to Daily Stressors
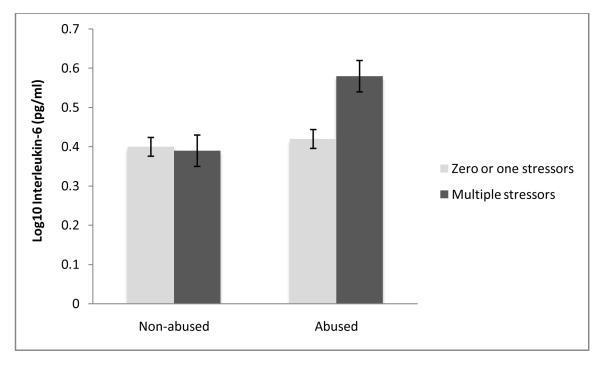
Adjusting for age, sex, ethnicity, education, BMI, marital status, sleep disturbances and caregiving status, a childhood abuse history, β = .09 (SE = .03), p = .01, R2 = .055, and the occurrence of multiple stressors in the past 24 hours, β = .08 (SE = .04), p = .03, R2 = .04, were associated with greater circulating IL-6 levels, compared to the absence of an abuse history and the report of zero or one stressors in the past 24 hours. There was a significant interaction between childhood abuse history and daily stressors, β = .17, (SE = .07), p = .02, R2 = .039. As illustrated in Figure 2, childhood abuse was associated with larger IL-6 responses to the occurrence of multiple daily stressors. Using the raw, non-transformed data, abused individuals who experienced multiple daily stressors had IL-6 levels 2.35 times greater than those of their non-abused counterparts who also reported multiple stressors in the past 24 hours. The three-way interaction among childhood abuse history, daily stressors, and caregiving status was not significantly related to IL-6, β = −.19, (SE = .15), p = .21, R2 = .012.
Figure 2.
Interleukin-6 response as a function of exposure to stressors in the past 24 hours and childhood adversity. Error bars represent the standard error of the mean.
Adjusting for age, sex, ethnicity, education, BMI, marital status, sleep disturbances and caregiving status, childhood adversity was marginally related to circulating TNF-α, β = .06 (SE = .03), p = .07, R2 = .031, but not daily stressors, β = −.01 (SE = .03), p = .84, R2 < .001. The interaction between daily stressors and abuse history, β = .009 (SE = .07), p = .90, R2 < .001, and the interaction among caregiving status, daily stressors, and abuse history, β = −.04 (SE = .14), p = .77, R2 = .001, did not predict circulating TNF-α levels.
Adjusting for age, sex, ethnicity, education, BMI, marital status, and caregiving status, the occurrence of multiple stressors in the past 24 hours was associated with greater circulating CRP levels, β = .17,(SE = .08), p = .03, R2 = .027. However, childhood abuse history was not related to CRP, β = .07 (SE = .08), p = .39, R2 = .005. The interaction between daily stressors and abuse history, β = .10 (SE = .16), p = .52, R2 = .003, and among caregiving status, daily stressors, and abuse history, β = .24 (SE = .33), p = .47, R2 = .003, did not predict CRP levels.
Childhood Abuse, Health Behaviors, Medication Use, and IL-6 Response to Daily Stressors
After adjusting for health behaviors such as smoking, alcohol consumption, exercise, and self-reported physical diseases, the interaction between daily stressors and childhood abuse history remained a significant predictor of IL-6, β = .17 (SE = .07), p = .02, R2 = .041. Similarly, when non-steroidal anti-inflammatory and antidepressant medication use were included in the model, the interaction of daily stressors and abuse history remained significantly associated with IL-6, β = .15 (SE = .07), p = .04, R2 = .032.
Discussion
Consistent with theoretical models suggesting a biological embedding of early life stress [2, 3], results revealed that childhood abuse history was associated with amplified IL-6 responses to naturalistically occurring daily stressors. The effect of early abuse was substantial; when abused individuals experienced multiple daily stressors they had IL-6 levels 2.35 times higher than those of non-abused participants reporting multiple stressors. Greater IL-6 responses to daily stressors may fuel chronic low grade-inflammation, suggesting a psychobiological mechanism by which childhood abuse lead to enhanced disease risk over time [21, 22].
Data showed that childhood abuse impacted daily stress exposure among caregivers, but not among noncaregiving controls. These results are in line with longitudinal studies showing that childhood abuse is associated with greater exposure to chronic and episodic stressors [24]. In contrast, none of the three-way interactions among daily stress, caregiving status, and childhood abuse significantly predicted inflammatory markers production. However, it is important to acknowledge that this study was underpowered to detect such three-way interactions.
In accord with other studies, TNF-α did not increase in response to daily stressors [23] and this response was not moderated by childhood abuse. In contrast to other studies [10], childhood abuse was not related to CRP in the current sample. Individuals with a childhood abuse history who are currently depressed exhibit higher circulating CRP levels than non-depressed, abused participants [4, 25]. The low proportion of clinically depressed individuals in our sample might have contributed to the lack of a significant relationship between childhood abuse and circulating CRP levels.
The current sample includes older adults with an average age of 65.28 years. Abused individuals were on average 4.73 years younger than the non-abused participants. The fact that we observed stress-related group differences despite the natural age-related increase in IL-6 suggests that the impact of childhood abuse on the IL-6 stress response might actually be underestimated in our sample [26].
Given the cross-sectional and observational design of the study, we cannot rule out the possibility that a third unmeasured variable accounts for the relationship between childhood abuse and adulthood IL-6 stress responses. Furthermore, given that adulthood trauma may impact inflammatory markers, the lack of assessment of cumulative lifetime trauma exposure is a limitation of the study. Replication of these results in the context of longitudinal investigations is therefore warranted.
In conclusion, results from the present study confirm and extend laboratory findings indicating that childhood abuse history amplifies IL-6 responses to stressors. Early life stress may set the path for dysregulated inflammatory stress responses across the lifespan.
Acknowledgments
The study was supported in part by NIH grants AG025732, UL1RR025755, Ohio State University Comprehensive Cancer Center Core Grant CA16058.
Footnotes
Conflict of Interest Statement The authors have no conflict of interest to disclose.
References
- 1.Wegman HL, Stetler C. A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom Med. 2009;71:805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- 2.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2011 doi: 10.1016/j.physbeh.2011.08.019. In Press. [DOI] [PubMed] [Google Scholar]
- 3.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol Bull. 2011 doi: 10.1037/a0024768. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52:671–690. doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- 5.Shirtcliff EA, Coe CL, Pollak SD. Early childhood stress is associated with elevated antibody levels to herpes simplex virus type 1. PNAS. 2009;106:2963–2967. doi: 10.1073/pnas.0806660106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagundes CP, Glaser R, Johnson SL, et al. Basal cell carcinoma: stressful life events and the tumor environment. Arch Gen Psychiatry. doi: 10.1001/archgenpsychiatry.2011.1535. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor SE, Lehman B, Kiefe C, Seeman T. Relationship of early life stress and psychological functioning to adult c-reactive protein in the coronary artery risk development in young adults study. Biol Psychiatry. 2006;60:819–824. doi: 10.1016/j.biopsych.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Pace TWW, Mletzko TC, Alagbe O, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1632. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 9.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology. 2010;35:2617–2623. doi: 10.1038/npp.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. PNAS. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med. 2011;73:16–22. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller GE, Chen E. Harsh family climate in early life presages the emergence of a proinflammatory phenotype in adolescence. Psychol Sci. 2010;21:848–856. doi: 10.1177/0956797610370161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouin JP, Glaser R, Malarkey WB, Beversdorf D, Kiecolt-Glaser J. Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 2011 doi: 10.1037/a0025536. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein DP, Fink L. Childhood Trauma Questionnaire: A retrospective self-report. The Psychological Corporation; San Antonio, Texas: 1998. [Google Scholar]
- 15.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 16.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 18.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33:1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS Multidimensional Functional Assessment Questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- 20.Almeida DM, Wethington E, Kessler RC. The Daily Inventory of Stressful Events: An interview-based approach for measuring daily stressors. Assessment. 2002;9:41–55. doi: 10.1177/1073191102091006. [DOI] [PubMed] [Google Scholar]
- 21.Ellins E, Halcox J, Donald A, et al. Arterial stiffness and inflammatory response to psychophysiological stress. Brain Behav Immun. 2008;22:941–948. doi: 10.1016/j.bbi.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Brydon L, Steptoe A. Stress-induced increases in interleukin-6 and fibrinogen predict ambulatory blood pressure at 3-year follow-up. J Hypertens. 2005;23:1001–1007. doi: 10.1097/01.hjh.0000166841.57474.d0. [DOI] [PubMed] [Google Scholar]
- 23.Steptoe A, Hamer M, Chida Y. The effects of acute psychological stress on circulating inflammatory factors in humans: A review and meta-analysis. Brain Behav Immun. 2007;21:901–912. doi: 10.1016/j.bbi.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 24.Hazel NA, Hammen C, Brennan PA, Najman J. Early childhood adversity and adolescent depression: the mediating role of continued stress. Psychol Med. 2008;38:581–589. doi: 10.1017/S0033291708002857. [DOI] [PubMed] [Google Scholar]
- 25.Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ershler W, Keller E. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Ann Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]