Abstract
Objectives
To determine whether high blood pressure (BP) levels are associated with faster decline in specific cognitive domains.
Design
Prospective longitudinal cohort.
Setting
Uniform Data Set of the National Institutes of Health, National Institute on Aging Alzheimer's Disease Centers.
Participants
One thousand three hundred eighty-five participants with a diagnosis of mild cognitive impairment (MCI) and measured BP values at baseline and two annual follow-up visits.
Measurements
Neuropsychological test scores and Clinical Dementia Rating Sum of Boxes (CDR Sum) score.
Results
Participants with MCI with two or three annual occasions of high BP values (systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg) had significantly faster decline on neuropsychological measures of visuomotor sequencing, set shifting, and naming than those who were normotensive on all three occasions. High systolic BP values were associated as well with faster decline on the CDR Sum score.
Conclusion
Hypertension is associated with faster cognitive decline in persons at risk for dementia.
Keywords: cerebrovascular disease, dementia, hypertension, mild cognitive impairment, neuropsychology
There is controversy as to whether hypertension is a risk factor for cognitive impairment and decline, with some studies finding a positive association1–7 and others not.8–12 recent evidence-based review13 of published studies conducted by an expert panel under the auspices of the National Institutes of Health (NIH) concluded that the evidence of such an association is weak, in part because of the heterogeneity in definitions of mild cognitive impairment (MCI) and hypertension and differences in hypertension ascertainment methods (e.g., measured blood pressure (BP) vs reliance on self-report). In the United States, nearly 70% of persons aged 60 and older have hypertension,14 and it is estimated that 15 million to 18 million persons in this age group will develop dementia by 2050.15 Therefore, determining whether an association exists between high BP and cognitive function is important for targeting potential neuroprotective strategies. The results of clinical drug trials with antihypertensive agents have been mixed concerning their efficacy in preventing cognitive decline and dementia onset,13,16 although encouraging findings are available from a recent clinical trial employing lifestyle changes. One study17 found that prehypertensive and hypertensive adults receiving treatment with diet and aerobic exercise over a 4-month period showed greater improvements in executive functioning, memory, and psychomotor speed than those exposed to a diet intervention alone or a placebo condition of standard care. Systolic and diastolic BP decreased significantly over the study period for the intervention group but not the control group.
The current study examined whether there is an association between high BP and decline in cognitive status in individuals with MCI over a 2-year period. The importance of the effect of adequate BP control on cognitive performance was demonstrated in a cross-sectional study of communityresiding older adults.18 Irrespective of a prior diagnosis of hypertension, persons with high BP (systolic ≥ 140 mmHg or diastolic ≥ 90 mmHg) at the time of neuropsychological testing performed worse than normotensive individuals on measures of visual memory, motor speed, and visuomotor integration. Persons with a prior diagnosis and high BP levels were most vulnerable to poor performance.
BP levels were examined at annual follow-up visits, rather than at a single baseline visit, to determine whether BP was routinely normotensive or high and whether this, in turn, affected the trajectory of cognitive changes. Rather than a single measure of overall cognitive status, participants in the current study underwent multiple tests examining attention, memory, language, and executive functioning. In older adults without dementia and those with MCI and Alzheimer's disease, hypertension is associated with a cognitive phenotype characterized by poorer attention and executive functioning and slower processing speed.10,19–25 Thus, it was expected that these same areas would be most sensitive to the chronic effects of high BP.
Information was collected as part of the Uniform Data Set (UDS), a standardized assessment and data protocol maintained by the National Alzheimer's Coordinating Center, with 31 participating NIH, National Institute on Aging (NIA) Alzheimer's Disease Centers (ADCs) nationwide.26,27 It was hypothesized that individuals with MCI with high BP readings, according to published guidelines for hypertension,28 on more than one occasion would exhibit faster overall cognitive decline than those with normotensive levels on all occasions, with greater vulnerability of attention, executive functioning, and speeded performance.
Methods
Participants
Information from the UDS as of January 2011 was used. Recruitment strategies vary across the ADCs, and participants may come from clinics or the community.27 Criteria used by all ADCs for diagnosing MCI follow guidelines set forth by an expert panel.29 These include clinical judgment that a person is not cognitively normal and does not meet diagnostic criteria for dementia, has preserved or only minimally impaired functional abilities, and has evidence of cognitive impairment or decline based on self- or informant report and objective cognitive tests.
Inclusion criteria required that participants have a diagnosis of MCI from the clinicians at each center, no reported history of a remote or recent stroke or transient ischemic attack, and BP values available at baseline and at least two annual follow-ups. All participants signed consent forms that the institutional review boards at their study sites approved. The outcome measures used in the analyses are listed below.
Clinical dementia rating sum of boxes (CDR Sum).30 The CDR was administered using a structured interview format with the participant and his or her informant to assess the participant's current cognitive and functional status. Memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care are each rated for their level of impairment. The CDR Sum provides a composite of the overall level of impairment.
Neuropsychological Measures
Cognitive test scores were based on the core battery of measures collected by the ADCs.27 Four cognitive domains were evaluated: attention, language, memory, and executive functioning. Attention was assessed according to the maximum number of correct trials for Digits Forward31 and the number of seconds needed to sequence numbers using a pencil (Trail-Making Test (TMT) Part A).32 Language was examined according to the 30-item version of the Boston Naming Test.33 The evaluation of memory included verbal episodic memory (immediate and delayed story recall)31 and semantic memory (timed generation of animal names in 60 s).34 Finally, executive functioning was measured using set shifting tasks involving mental manipulation of digits31 and rapid alternation of numbers/letters and symbols (TMT B and Digit Symbol).32,35
BP Readings
Systolic and diastolic BP readings were obtained at all three yearly visits. The UDS guidelines require that BP be measured with the individual seated.
Statistical Analysis
Longitudinal linear regression analyses were conducted to determine whether there was a difference in cognitive change over time between those with high and normotensive BP readings (PROC MIXED, SAS, SAS Institute, Inc., Cary, NC). All participants had three observations, spaced approximately 1 year apart. Three years was chosen as the follow-up period because this was the largest number of visits that most participants had in common. Only 52% of participants with three visits had cognitive testing or measured BP values at the fourth visit, because of pending visits or incomplete data. It was therefore reasoned that a more-homogenous analytical data set with all members having the same number of visits was optimal. A repeated-measures analysis with a compound symmetry correlation matrix was used, equivalent to an analysis in which subjects are included as a random effect.
Separate models were first run to evaluate the main effects of time (a continuous variable coded 1, 2, and 3) and BP (normotensive or high, see below) on cognitive tests. The primary goal was to determine whether individuals with MCI with high BP worsened more rapidly than those with normal BP. To test this, an interaction term between time as a continuous variable coded as occasion 1, 2, and 3 and a dichotomous variable for high versus normotensive BP was next included in the model. Normotensive BP was defined as readings on all three occasions of systolic BP less than 140 mmHg and diastolic BP less than 90 mmHg.28 High BP was defined as Stage 1 hypertension and higher (systolic BP ≥ 140 mmHg or diastolic BP ≥ 90 mmHg) on two or three occasions.28 Analyses were also conducted examining the effect of one occasion of high BP on cognitive decline.
There were 11 dependent variables: CDR Sum, MMSE36 total score, number o seconds to complete TMT A and B, maximum number of correct trials for Digits Forward and Digits Backward, number of story units recalled immediately after hearing a story and after a delay, number of completed pairings within 90 seconds on the Digit Symbol subtest, number of correct responses on the Boston Naming Test, and number of animal names generated in 60 seconds. For TMT A and B, analyses were conducted of the original variable and the log-transformed variable, because the latter satisfied the normality assumption, and the former did not. Results were concordant, and the untransformed results are reported for ease of interpretation.
Variables that could confound the relationship between BP and cognitive function were entered a priori in all of the statistical models. These covariates were age (continuous); race (white; nonwhite); sex; education (no high school degree vs ≥ high school); and presence vs absence of a self-reported history of baseline diabetes mellitus, heart disease (e.g., myocardial infarction, atrial fibrillation, or congestive heart failure), or depression within the last 2 years. A self-reported history of hypertension within the last 2 years was also included in the models to assess the influence of actual BP readings on performance, irrespective of whether there was a report of hypertension.
Results
Cognitive Decline over 2 years
Data from 1,385 participants with a baseline diagnosis of MCI were available for analysis. Two hundred four (15%) participants had high readings on all three occasions, and 323 (23%) had high readings on two of the three occasions involving Time 1 and Time 2 or Time 1 and Time 3. Preliminary analyses revealed that the individuals with three high readings had patterns of change over time on the cognitive tests similar to the patterns of those with two high readings, so these two groups were combined for analysis. Three hundred seventy-three participants (27%) had one high reading, and 485 (35%) had no high readings. The latter participants served as the reference group for those with two or more high readings and for those with one high reading.
Table 1 shows the baseline demographic and clinical features of the participants broken down according to the number of occasions with high BP readings. Participants with two or three high readings were significantly older than those with no high readings or one high reading. These latter two groups, in turn, did not significantly differ in age. Mean systolic and diastolic BP readings differed significantly between all three groups, as did the percentage of persons with a self-reported history of hypertension at baseline. Thirty-nine percent of those with no high readings, 53% of those with one high reading, and 65% of those with two or three high readings had a history of hypertension within the past 2 years. There were no significant differences between the groups in distribution of education, sex, or race, or in self-reported history of diabetes mellitus, heart disease, or depression at baseline.
Table 1. Baseline Characteristics of Patients According to the Number of Occasions with High Blood Pressure (BP) Readings.
| Characteristic | No Elevations, n = 485 | 1 Occasion, n = 373 | 2–3 Occasions, n = 527 | P-Value* |
|---|---|---|---|---|
| Age, mean ± SD | 72.9 ± 9.9a | 73.0 ± 8.6b | 74.7 ± 8.3a,b | .01 |
| BP, mean ± SD | ||||
| Systolic | 120 ± 11a | 133 ± 15a | 147 ± 17a | .001 |
| Diastolic | 70 (8)a | 75 (9)a | 79 (10)a | .001 |
| Education ≥ highschool, n (%) | 483 (94) | 347 (93) | 525 (91) | .19 |
| Male, n (%) | 242 (50) | 172 (46) | 265 (50) | .41 |
| Caucasian, n (%) | 417 (86) | 320 (86) | 434 (83) | .20 |
| Self-reported bas eline conditions, n (%)† | ||||
| Cardiac disease | 138 (29) | 94 (27) | 144 (27) | .55 |
| Diabetes mellitus | 53 (11) | 49 (13) | 55 (10) | .43 |
| Hypertension | 189 (39)a | 198 (53)a | 342 (65)a | .88 |
| Depression | 175 (35) | 129 (35) | 184 (35) | |
SD = standard deviation.
High BP: systolic ≥ 140 mmHg or diastolic ≥ 90 mmHg.28
A common superscript indicates a significant difference between groups.
P-value for any differences between three groups.
Within last 2 years.
Table 2 shows the unadjusted mean raw scores for the CDR Sum and the neuropsychological measures broken down according to number of occasions with high BP readings. The results of linear regression analyses, adjusted for potential confounders, are shown in Table 3. There were significant main effects of high BP on the number of seconds needed to complete TMT A and B. Those with high readings on two or three occasions were significantly slower than those with normotensive readings. As expected, performance worsened significantly over the 2 years for all outcome measures (time variable), with the exception of delayed paragraph recall.
Table 2.
Unadjusted Clinical Dementia Rating and Neuropsychological Test Scores at Baseline and Follow-Up According to Number of Occasions with High Blood Pressure Readings
| Test | 0 Occasions | 1 Occasion | 2–3 Occasions |
|---|---|---|---|
| Mean ± Standard Deviation | |||
| Clinical Dementia Rating sum (maximum 18) | |||
| Year 1 | 1.2 ± 1.0 | 1.4 ± 1.2 | 1.2 ± 1.2 |
| Year 2 | 1.6 ± 1.5 | 1.8 ± 1.6 | 1.8 ± 1.7 |
| Year 3 | 2.1 ± 2.4 | 2.3 ± 2.1 | 2.4 ± 2.3 |
| Mini-Mental State Examination (maximum 30) | |||
| Year 1 | 27.8 ± 1.8 | 27.6 ± 1.8 | 27.7 ± 1.7 |
| Year 2 | 27.0 ± 2.7 | 26.7 ± 2.9 | 26.6 ± 3.0 |
| Year 3 | 26.1 ± 4.2 | 26.0 ± 3.4 | 25.7 ± 4.1 |
| Digit Span Forward total number of trials correct (maximum 12) | |||
| Year 1 | 7.9 ± 2.0 | 7.8 ± 2.1 | 7.9 ± 2.1 |
| Year 2 | 7.8 ± 2.0 | 7.8 ± 2.0 | 7.8 ± 2.2 |
| Year 3 | 7.7 ± 2.1 | 7.7 ± 2.1 | 7.6 ± 2.1 |
| Digit Span Backward total number of trials correct (maximum 12) | |||
| Year 1 | 5.9 ± 2.1 | 6.0 ± 2.1 | 6.0 ± 2.1 |
| Year 2 | 5.8 ± 2.1 | 5.9 ± 2.0 | 5.8 ± 2.0 |
| Year 3 | 5.7 ± 2.2 | 5.9 ± 2.1 | 5.5 ± 2.1 |
| Logical Memory Immediate Recall (maximum 25) | |||
| Year 1 | 9.9 ± 4.5 | 9.4 ± 4.2 | 9.4 ± 4.5 |
| Year 2 | 9.7 ± 4.5 | 9.4 ± 4.6 | 9.2 ± 4.6 |
| Year 3 | 9.4 ± 4.8 | 9.0 ± 4.6 | 8.8 ± 5.0 |
| Logical Memory Delayed Recall (maximum 25) | |||
| Year 1 | 7.6 ± 4.9 | 7.0 ± 4.8 | 6.9 ± 5.0 |
| Year 2 | 7.5 ± 5.2 | 7.2 ± 5.1 | 6.8 ± 5.1 |
| Year 3 | 7.5 ± 5.4 | 6.8 ± 5.3 | 6.6 ± 5.5 |
| Trail-Making Test Part A (maximum 150 s)a | |||
| Year 1 | 43.5 ± 19.6 | 42.7 ± 18.4 | 42.3 ± 18.8 |
| Year 2 | 44.9 ± 21.7 | 42.0 ± 18.7 | 43.5 ± 20.8 |
| Year 3 | 45.1 ± 22.3 | 44.0 ± 20.8 | 46.1 ± 23.1 |
| Trail-Making Test Part B (maximum 300 s)a | |||
| Year 1 | 117.7 ± 56.4 | 115.1 ± 55.0 | 121.1 ± 54.0 |
| Year 2 | 118.8 ± 56.7 | 112.2 ± 51.2 | 130.3 ± 62.8 |
| Year 3 | 119.8 ± 59.3 | 117.2 ± 57.4 | 131.4 ± 62.2 |
| Digit Symbol (maximum 93) | |||
| Year 1 | 38.1 ± 11.8 | 38.8 ± 11.4 | 37.5 ± 11.4 |
| Year 2 | 37.5 ± 12.6 | 38.4 ± 11.9 | 36.5 ± 12.0 |
| Year 3 | 36.2 ± 13.7 | 36.2 ± 12.2 | 35.0 ± 12.8 |
| Animals (maximum unlimited) | |||
| Year 1 | 16.1 ± 5.2 | 16.0 ± 4.5 | 15.9 ± 4.8 |
| Year 2 | 15.8 ± 5.2 | 15.9 ± 4.6 | 15.6 ± 5.1 |
| Year 3 | 15.2 ± 5.8 | 14.8 ± 5.0 | 14.9 ± 6.0 |
| Boston Naming Test (maximum 30) | |||
| Year 1 | 24.8 ± 4.9 | 24.9 ± 4.5 | 25.1 ± 4.3 |
| Year 2 | 24.9 ± 4.9 | 24.8 ± 4.6 | 24.5 ± 4.8 |
| Year 3 | 24.4 ± 5.3 | 24.5 ± 5.0 | 24.0 ± 5.4 |
Higher scores denote poorer performance.
Table 3. Regression Coefficients for Participants with Mild Cognitive Impairment (MCI) with Two or Three High Blood Pressure (BP) Readings Versus No High BP Readings over Three Yearly Visits.
| Test | History of Hypertension at First Visit | Change per Year | P-Value for Interactiona | ||
|---|---|---|---|---|---|
| 2 or 3 High Readings and No High Readings Combined | 2 or 3 High Readings | No High Readings | |||
| CDR Sum | 0.05 | 0.51c | 0.55 | 0.47 | .10 |
| Mini-Mental State Examination | 0.06 | −0.76c | −0.81 | −0.71 | .26 |
| Digit Span Forward | −0.15 | −0.13c | −0.15 | −0.10 | .35 |
| Digit Span Backward | −0.09 | −0.16c | −0.20 | −0.10 | .08 |
| Logical Memory Immediate Recall | −0.05 | −0.29c | −0.36 | −0.27 | .66 |
| Logical Memory Delayed Recall | −0.02 | −0.08 | −0.10 | −0.07 | .76 |
| TMT A | 3.48c | 1.72c | 2.31 | 1.06 | .03 |
| TMT B | 8.76b | 5.77c | 7.81 | 3.61 | .02 |
| Digit Symbol | −0.71 | −1.30c | −1.35 | −1.26 | .73 |
| Animals | 0.09 | −0.49c | −0.52 | −0.47 | .76 |
| Boston Naming Test | 0.19 | −0.37c | −0.50 | −0.23 | .01 |
High BP: systolic ≥ 140 mmHg or diastolic ≥ 90 mmHg.
The values represent the regression coefficients from the main analysis of the effect of two or more high BP readings on the cognitive tests. The first column represents the effect of hypertension at baseline on the cognitive tests. The second column represents the change per year in the entire population. The third column represents the change per year for both groups (2–3 occasions vs 0 occasions). The last column shows the p-values of the interaction effect. For all tests, change per year is in a worse direction (for Trail-Making Test (TMT) Parts A and B, and Clinical Demential Rating Sum of Boxes (CDR Sum), higher scores indicate worse performance, for all other tests, lower scores indicate worse performance).
The p-value for the interaction term indicates whether the observed difference between change per year for those with two to three occasions versus no occasions of high BP is likely to be because of chance.
P < .05.
P < .001.
Significant interactions were observed between number of high BP readings and time for three cognitive outcomes. Those with high readings on two or three occasions had a greater decline than those with normotensive readings on all occasions in number of seconds to complete TMT A and B and ability to name pictured items. There was a trend (P = .08) as well for a faster decline on the Digits Backward subtest for those with high readings on two or three occasions. Figure 1(A–D) depicts the time trends for individuals with high BP and those with normotensive values for these four tests (using observed baseline values and model-predicted values for years 2 and 3).
Figure 1.
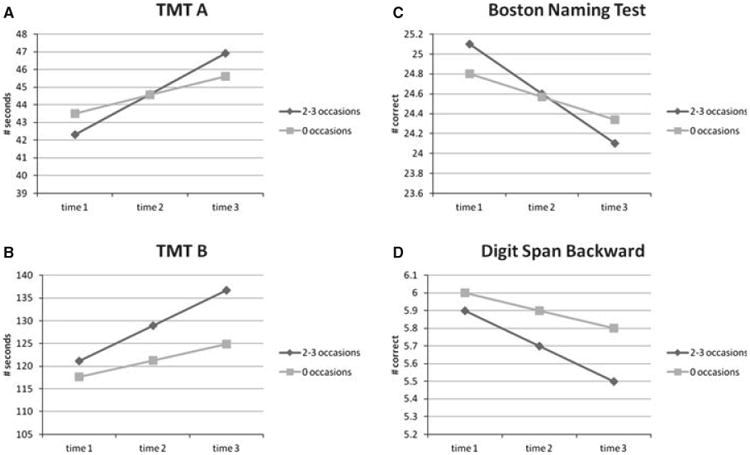
Performance over time as a function of having two or three occasions versus zero occasions of high blood pressure readings: (A) number of seconds to complete Trail-Making Test (TMT) Part A, (B) number of seconds to complete Trail-Making Test Part B, (C) number of correct responses on Boston Naming Test, (D) number of correct trials on the Digit Span Backward subtest.
Less than 2% of the total sample reported experiencing an intervening stroke between the first evaluation and the follow-up occasions. Exclusion of these individuals did not change the results.
The above analyses were also performed comparing those with one occasion of high BP with those who were normotensive on all three occasions. There were no significant interactions for any of the outcomes, indicating comparable changes over time for the groups.
Systolic BP and Outcome
Most high readings were from high systolic rather than diastolic pressure. Sixty-three percent of participants had at least one systolic reading of 140 mmHg or greater, compared with 19% with at least one diastolic reading of 90 mmHg or higher. The percentages of individuals with two or more high readings were 38% and 4%, for systolic and diastolic BP, respectively. Thus, supplemental analyses were conducted focusing on the effect of systolic BP on performance over time. First, the effect of two or more high (≥ 140 mmHg) readings of systolic BP versus never having had high systolic BP was examined. After adjusting for confounders as before, significantly greater worsening over time was observed for those with two or more high readings than for those with no high readings on CDR Sum (P = .03), TMT A (P = .02), TMT B (P = .007), and the Boston Naming Test (P = .005). Average systolic BP readings across the three visits were also examined and compared with average systolic BP of 140 mmHg or greater versus less than 140 mmHg over three visits. Adjusting for potential confounders, significant interactions were found between group and time for TMT B (P = .04) and the Boston Naming Test (P = .02), whereas the interaction with time for TMT A was not significant (P = .06).
Discussion
The results support an association between high BP and risk of cognitive decline in MCI. Individuals with high BP readings on two or three annual assessments experienced greater slowing on TMT A and B than those with no high readings on any occasion, as well as a trend for greater worsening on Digit Span Backward. Intervening strokes did not explain these findings. The observation of a faster decline in naming ability was unexpected but could reflect the time demands of this measure, because individuals are penalized if their responses exceed 20 seconds. High systolic BP appeared to account for the results based on the higher percentage of persons with high systolic (63%) versus diastolic (20%) readings, but the importance of diastolic BP should not be dismissed, because its role could not be adequately evaluated due to its low frequency of occurrence. Examination of relationships with actual systolic BP levels, based on the number of occasions with clinically significant high readings or averaged values, also demonstrated vulnerability on tasks requiring rapid performance and set shifting (TMT A and B) and expressive language (naming). Moreover, CDR Sum declined more precipitously in those with two or three occasions of high systolic BP.
These results are consistent with the findings of a previous study that recently demonstrated an association between hypertension and risk for cognitive decline in a Chinese population,3 but unlike that study, which examined the risk of a change in diagnosis from MCI to Alzheimer's disease, the current study examined specific cognitive domains that are especially vulnerable. In addition, unlike the previous study, the participants in the current study were excluded if they had a previous history of stroke or transient ischemic attack. Thus, the results demonstrate the influence of a vascular risk factor on cognitive changes in the absence of overt clinical disease.
The results of studies examining hypertension as a risk factor for cognitive decline and Alzheimer's disease have been mixed, with two recent reviews13,37 concluding that the overall evidence is weak. The current study circumvented some of the methodological problems mentioned in these recent reviews that can hamper interpretation of many studies, including reliance on self-reported hypertension at baseline and reliance on a single BP reading at baseline. Studies that rely solely on self-reports of hypertension may incorrectly classify some participants. Findings from the National Health and Nutrition Examination Surveys, which indicate that, between 2005 and 2006, 7% of the U.S. population had systolic BP readings of 140 mmHg or diastolic BP readings of 90 mmHg or higher. Yet, no healthcare provider had told these individuals that they had high BP. These results highlight the unreliability of self-report data. Overall, only 78% of hypertensive adults were aware that they had this condi-tion.38 Moreover, studies that rely on a single reading at one time point do not take into account whether the status of individuals changes. For example, in the data from the current study, 43% of those who were normotensive at baseline had one or two high BP readings at year 2 and 3 visits. Such changes would result in the misclassification of individuals, weakening associations with cognitive performance and functional outcome.
A limitation of this study is that the follow-up period was short (2 years). Only 52% of the sample had complete cognitive data and BP readings at 3 years. Given the desire to study a homogenous population with equal length of follow-up, using data for the third year would have halved the sample size. An alternative approach of imputing missing data for the third year was deemed inadvisable given the amount of missing data that would need to be imputed. Further follow-up would allow clearer determination of whether the differences between the hypertensive and normotensive individuals persist over time. Two aspects of the findings for the cognitive test results increase confidence in their validity. First, participants with two or three high readings had greater worsening of cognition over time than those with no high readings, whereas those with only one high reading did not. This suggests a doseresponse relationship whereby only those with more-sustained uncontrolled BP showed an effect. Another source of confidence is that the pattern of impairments is consistent with the clinical phenotype of slow processing speed and set shifting difficulty reported in the literature.19–25
Another limitation of this study is that an established database was relied on, as opposed to the study being designed and therefore allowing a mediation model for the association between hypertension and cognitive decline to be tested. There are potentially many mechanisms through which high BP exerts a deleterious effect on the course of cognitive function, including disruption of cerebral white matter integrity and the greater risk for white matter infarcts, greater brain atrophy, and silent strokes.39–42 Brain imaging is not a required data element of the ADCs, but findings of greater cerebrovascular changes in those classified with high BP would clarify the mechanism of the observed relationships. Neuropathological studies have also demonstrated an association between uncontrolled hypertension and neurofibrillary tangles and neuritic plaques.43–45 Studies using in vivo amyloid imaging techniques and studies examining relationships between hypertension and associated measures of chronic inflammation, including serum interleukin-6 and C-reactive protein, would be of interest. Other possible mechanisms, including treatment with antihypertensive medications and cholinesterase inhibitors, may have been operative as well. These potential associations will await longitudinal studies to further elucidate mechanisms for cognitive decline.
Acknowledgments
We thank the ADC participants for their willingness to devote their time to research and the staff members who work tirelessly to make the research possible.
Supported by the Emory Alzheimer's Disease Research Center (NIH-NIA 5 P50 AG025688) (AIL).
Sponsor's Role: This project was accomplished through the auspices of the National Alzheimer's Coordinating Center (NACC), a NIH-NIA funded Center that facilitates collaborative research. NACC provided the data used in this study under cooperative agreement number U01 AG016976.
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Conception and design: Goldstein, Levey, and Steenland. Analysis and interpretation of data: Steenland. Drafting of the manuscript: Goldstein and Steenland. Critical revision of the manuscript for important intellectual content: Goldstein, Levey, and Steenland. Obtaining funding: Levey. Administrative, technical, or material support: Levey. Supervision: Goldstein.
References
- 1.Kivipelto M, Helkala EL, Hanninen T, et al. Midlife vascular risk factors and late-life mild cognitive impairment. A population-based study. Neurology. 2001;56:1683–1689. doi: 10.1212/wnl.56.12.1683. [DOI] [PubMed] [Google Scholar]
- 2.Knopman DS, Mosley TH, Catellier DJ, et al. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: The ARIC MRI Study. Alzheimers Dement. 2009;5:207–214. doi: 10.1016/j.jalz.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Wang YJ, Zhang M, et al. Vascular risk factors promote conversion from mild cognitive impairment to Alzheimer Disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]
- 4.Obisesan TO, Obisesan OA, Martins S, et al. High blood pressure, hypertension, and high pulse pressure are associated with poorer cognitive function in persons aged 60 and older: The Third National Health and Nutrition Examination Survey. J Am Geriatr Soc. 2008;56:501–509. doi: 10.1111/j.1532-5415.2007.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavlik VN, Hyman DJ, Doody R. Cardiovascular risk factors and cognitive function in adults 30–59 years of age (NHANES III) Neuroepidemiology. 2005;24:42–50. doi: 10.1159/000081049. [DOI] [PubMed] [Google Scholar]
- 6.Tzourio C, Dufouil C, Ducimetiere P, et al. Cognitive decline in individuals with high blood pressure: A longitudinal study in the elderly. EVA Study Group. Epidemiology of Vascular Aging. Neurology. 1999;53:1948–1952. doi: 10.1212/wnl.53.9.1948. [DOI] [PubMed] [Google Scholar]
- 7.Waldstein SR, Giggey PP, Thayer JF, et al. Nonlinear relations of blood pressure to cognitive function: The Baltimore Longitudinal Study of Aging. Hypertension. 2005;45:374–379. doi: 10.1161/01.HYP.0000156744.44218.74. [DOI] [PubMed] [Google Scholar]
- 8.Di Carlo A, Lamassa M, Baldereschi M, et al. CIND and MCI in the Italian elderly: Frequency, vascular risk factors, progression to dementia. Neurology. 2007;68:1909–1916. doi: 10.1212/01.wnl.0000263132.99055.0d. [DOI] [PubMed] [Google Scholar]
- 9.Johnson KC, Margolis KL, Espeland MA, et al. A prospective study of the effect of hypertension and baseline blood pressure on cognitive decline and dementia in postmenopausal women: The Women's Health Initiative Memory Study. J Am Geriatr Soc. 2008;56:1449–1458. doi: 10.1111/j.1532-5415.2008.01806.x. [DOI] [PubMed] [Google Scholar]
- 10.Reitz C, Tang MX, Manly J, et al. Hypertension and the risk of mild cognitive impairment. Arch Neurol. 2007;64:1734–1740. doi: 10.1001/archneur.64.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology. 2004;63:1882–1891. doi: 10.1212/01.wnl.0000144281.38555.e3. [DOI] [PubMed] [Google Scholar]
- 12.Tervo S, Kivipelto M, Hanninen T, et al. Incidence and risk factors for mild cognitive impairment: A population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Dis. 2004;17:196–203. doi: 10.1159/000076356. [DOI] [PubMed] [Google Scholar]
- 13.Williams JW, Plassman BL, Burke J, et al. Evidence Report/Technology Assessment No 193 AHRQ Publication No 10-E005. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Preventing Alzheimer's Disease and Cognitive Decline. [PMC free article] [PubMed] [Google Scholar]
- 14.Ong KL, Cheung BMY, Man YB, et al. Prevalence, awareness, treatment, and control of hypertension among United States adults 1999–2004. Hypertension. 2007;49:69–75. doi: 10.1161/01.HYP.0000252676.46043.18. [DOI] [PubMed] [Google Scholar]
- 15.Taking Care: Ethical Caregiving in Our Aging Society. Washington, DC: Department of Health and Human Services; 2005. Department of Health Human Services' Presidents Commission on Bioethics. [Google Scholar]
- 16.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith PJ, Blumenthal JA, Babyak MA, et al. Effects of the dietary approaches to stop hypertension diet, exercise, and caloric restriction on neurocognition in overweight adults with high blood pressure. Hypertension. 2010;55:1331–1338. doi: 10.1161/HYPERTENSIONAHA.109.146795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waldstein SR, Brown JRP, Maier KJ, et al. Diagnosis of hypertension and high blood pressure levels negatively affect cognitive function in older adults. Ann Behav Med. 2005;29:174–180. doi: 10.1207/s15324796abm2903_3. [DOI] [PubMed] [Google Scholar]
- 19.Elias PK, Elias MF, D'Agostino RB, et al. NIDDM and blood pressure as risk factors for poor cognitive performance. The Framingham Study. Diabetes Care. 1997;20:1388–1395. doi: 10.2337/diacare.20.9.1388. [DOI] [PubMed] [Google Scholar]
- 20.Elias MF, Orono ME, Robbins MA, et al. A longitudinal study of blood pressure in relation to performance on the Wechsler Adult Intelligence Scale. Health Psychol. 1998;17:486–493. doi: 10.1037//0278-6133.17.6.486. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein FC, Ashley AV, Freedman LJ, et al. Hypertension and cognitive performance in African Americans with Alzheimer's disease. Neurology. 2005;64:899–901. doi: 10.1212/01.WNL.0000152888.26576.37. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein FC, Ashley AV, Endeshaw YW, et al. Effects of hypertension and hypercholesterolemia on cognitive functioning in patients with Alzheimer's disease. Alz Dis Assoc Disord. 2008;22:336–342. doi: 10.1097/wad.0b013e318188e80d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamar M, Libon DJ, Ashley AV, et al. The impact of vascular comorbidity on abstract reasoning and concept formation in Alzheimer's disease. J Int Neuropsychol Soc. 2010;16:77–83. doi: 10.1017/S1355617709990981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saxby BK, Harrington F, McKeith IG, et al. Effects of hypertension on attention, memory, and executive function in older adults. Health Psychol. 2003;22:587–591. doi: 10.1037/0278-6133.22.6.587. [DOI] [PubMed] [Google Scholar]
- 25.Waldstein SR, Jennings JR, Ryan CM, et al. Hypertension and neuropsychological performance in men: Interactive effects of age. Health Psychol. 1996;15:102–109. doi: 10.1037//0278-6133.15.2.102. [DOI] [PubMed] [Google Scholar]
- 26.Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: The Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21:249–258. doi: 10.1097/WAD.0b013e318142774e. [DOI] [PubMed] [Google Scholar]
- 27.Weintraub S, Salmon D, Mercaldo, et al. The Alzheimer's Disease Centers' Uniform Data Set (UDS): The neuropsychologic test battery. Alz Dis Assoc Disord. 2009;23:91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 29.Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- 30.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Wechsler Memory Scale—Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 32.Army Individual Test Battery. Department of Health and Human Services. Washington, DC: War Department, Adjutant General's Office; 1944. [Google Scholar]
- 33.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 34.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd. New York: Oxford University Press; 2006. [Google Scholar]
- 35.Wechsler D. The Wechsler Adult Intelligence Scale – Revised. San Antonio, TX: The Psychological Corporation; 1981. [Google Scholar]
- 36.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 37.Power MC, Weuve J, Gagne JJ, et al. The association between blood pressure and incident Alzheimer Disease: A systematic review and meta-analysis. Epidemiology. 2011;23:646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ostchega Y, Yoon SS, Hughes J, et al. Hypertension Awareness, Treatment, and Control-Continued Disparities in Adults: United States, 2005–2006. Hyattsville, MD: National Center for Health Statistics; 2008. [PubMed] [Google Scholar]
- 39.DeCarli C, Miller BL, Swan GE, et al. Cerebrovascular and brain morphologic correlates of mild cognitive impairment in the National Heart, Lung, and Blood Institute Twin Study. Arch Neurol. 2001;58:643–647. doi: 10.1001/archneur.58.4.643. [DOI] [PubMed] [Google Scholar]
- 40.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: The Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 41.Seshadri S, Wolf PA, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function: The Framingham Offspring Study. Neurology. 2004;63:1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 42.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman LB, Schmeidler J, Lesser GT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology. 2009;72:1720–1726. doi: 10.1212/01.wnl.0000345881.82856.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: The HAAS. Neurobiol Aging. 2000;21:57–62. doi: 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 45.Sparks DL, Scheff SW, Liu H, et al. Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci. 1995;131:162–169. doi: 10.1016/0022-510x(95)00105-b. [DOI] [PubMed] [Google Scholar]


