Abstract
Purpose
We examined the reproducibility of using the constant infusion paradigm for equilibrium measurement of D2/3 receptors using [11C]-(+)-PHNO PET.
Methods
Six subjects were scanned with a bolus plus constant infusion (Kbol=80 min) of [11C]-(+)-PHNO. Binding potential (BPND) was computed using the equilibrium approach and compared to simplified reference tissue model (SRTM).
Results
Rate of change in the concentration activity curve from 60-90 min was −5 ±13 %/hr in the caudate, putamen, substantia nigra, thalamus and cerebellum, but was 15 ± 15 %/hr in the ventral striatum and pallidum. Test-retest variability was lower in striatal compared to extrastriatal regions (4 ± 8 % vs. −8 ± 22 %, respectively) using the equilibrium approach with comparable results with SRTM. The equilibrium ratio and SRTM yielded reliable BPND estimates (ICC = 0.88, 0.82 respectively).
Conclusions
These studies support the reproducibility of the bolus plus constant infusion paradigm with [11C]-(+)-PHNO PET.
Keywords: [11C]-(+)-PHNO, PET, human, dopamine D2/3 receptor, constant infusion
Introduction
[11C]-(+)-PHNO is a high affinity D2/3 agonist radiotracer used for positron emission tomography (PET) brain imaging[1-4]. Unlike other D2/3 radiotracers that have higher affinity for D2 over D3 receptors (e.g., [11C]raclopride, [18F]fallypride, [11C]FLB, [11C]NPA, and [11C]MNPA), the affinity of [11C]-(+)-PHNO is ∼ 40 times higher for D3 than D2 receptors[5-7]. As an agonist, [11C]-(+)-PHNO binds to receptors in the high affinity state, whereas antagonists bind to both high and low affinity receptors[8, 9]. The high affinity D2/3 receptors are thought to be more functionally relevant than low affinity receptors and more susceptible to extracellular changes in endogenous dopamine, which binds primarily to high-affinity D2/3 receptors[1, 2]. Thus, [11C]-(+)-PHNO may provide a more sensitive measure of functionally relevant D2/3 receptors than antagonist radiotracers.
A preliminary study using a bolus administration of [11C]-(+)-PHNO demonstrated that the radiotracer has good test-retest reliability, with variability between scans less than 12.5% across regions[9]. The bolus plus constant infusion method provides several advantages over the bolus-only method of radiotracer administration [10]. Therefore, in the current study, we examined the feasibility and reproducibility of a constant infusion paradigm for examining D2/3 receptor availability with [11C]-(+)-PHNO PET.
Materials and Methods
Participants
Six physically and mentally healthy nonsmoker men (n=4) and women (n=2), 31 ± 6 years of age (range: 23 to 36 years) participated in the study. Eligibility was determined as follows: subjects were examined by a study physician to exclude major medical issues or neurological disorders; electrocardiogram, serum chemistries, thyroid function studies, complete blood count, urinalysis, and urine toxicology screening were performed; the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders was administered to rule out Axis I psychiatric disorders. Participants had no history of significant medical illness or major head trauma. Females had negative pregnancy tests at intake and on each PET scan day prior to radiotracer injection.
[11C]PHNO PET Imaging
[11C]-(+)-PHNO was prepared as previously described[11]. The specific activity at the end of synthesis was 116 ± 34 MBq/nmol. Each participant had one magnetic resonance imaging (MRI) scan and 2 [11C]-(+)-PHNO PET scans at least 1 week apart (11 ± 6 days) at the Yale PET Center on the High Resolution Research Tomograph (HRRT) (Siemens/CTI, Knoxville, TN, USA). An initial 6-min transmission scan using an orbiting 137Cs point-source was acquired prior to radiotracer injection for attenuation correction. One venous catheter in the forearm antecubital vein was established for administration of [11C]-(+)-PHNO. The injected dose for the test scan was 562 ± 92 MBq with a mass of 0.06 ± 0.01 ug/kg and the retest scan dose was 543 ± 170 MBq with a mass of 0.05 ± 0.02 ug/kg. For the radiotracer injection, [11C]-(+)-PHNO was diluted with sterile saline solution and delivered as a bolus plus constant infusion (Kbol=80 min) by a syringe pump (Harvard PHD 22/2000, Harvard Apparatus, Holliston, Massachusetts, United States). This value of Kbol was determined by simulation using measured bolus data as previously described [12]. List mode data were reframed into a dynamic sequence of 33 frames (6 × 30 sec, 3 × 1 min, 2 × 2 min, 22 × 5 min). Dynamic images (207 slices; pixel size = 1.2 mm) were reconstructed using OSEM including an event-by-event motion correction algorithm (MOLAR; Carson IEEE-NSS). Motion correction was measured using an optical motion tracking tool fastened to the subjects head via a lycra swim-cap (Vicra, NDI systems, Waterloo, Canada).
Image analysis
Each participant's summed early PET image (0-10 min) was used for coregistration to the individual subject's MRI, which was then coregistered to a template MRI (Automated Anatomical Labeling template). Decay-corrected time activity curves were generated for regions-of-interest including caudate, putamen, ventral striatum, pallidum, substantia nigra, thalamus and cerebellum (left and right regions-of-interest were averaged for caudate, putamen and substantia nigra).
Outcome Measures
Regional [11C]-(+)-PHNO uptake was quantified as binding potential (BPND), which is proportional to the density of available receptors. BPND was calculated both by equilibrium ratios and by the simplified reference tissue model (SRTM) [13]. The cerebellum was used as a reference region. To assess the quality of equilibrium, the rate of change in activity between 60 and 90 min was calculated as the slope divided by the average concentration. Equilibrium ratios were based on an average of the data between 60 and 90 minutes whereas the SRTM used the full scan duration of 120 minutes. Simulations were performed where Kbol was varied, e.g., under- and over-estimated, and the error ((measured-true)/true) in estimates of BPND induced was estimated and the effect on rates of change between 60 and 90 min and bias in BPND in the putamen were determined.
Statistical Analyses
Paired t-tests (two-tailed; p<0.05 was considered statistically significant) were used to compare [11C]-(+)-PHNO injection characteristics including injected dose, specific activity and mass between test and retest. Test-retest variability (TRV) was calculated as follows: 2[BPtest − BPretest]/[BPtest + BPretest] × 100. The test-retest consistency was measured with an intraclass correlation coefficient (ICC).
Results
Estimation of Kbol
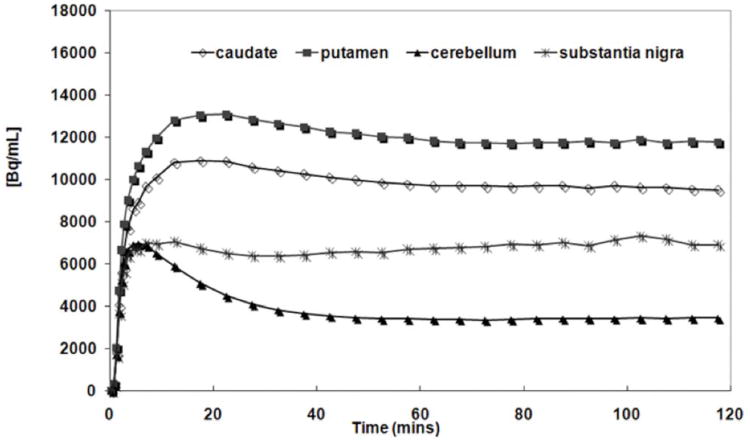
The Kbol value (the ratio of bolus amount to infusion rate) was determined using the regional time activity curves from a human [11C]-(+)-PHNO bolus study (120 min). The optimal Kbol was 80 min, which predicted that equilibrium would be achieved by 60 min in the caudate, putamen, substantia nigra, and cerebellum (Figure 1).
Figure 1.
Simulations of predicted tissue response to bolus plus constant infusion of [11C]-(+)-PHNO using Kbol = 80 min (curves are decay corrected).
[11C]-(+)-PHNO injection characteristics and pharmacological effects
No statistically significant differences were observed in specific activity or injected mass between test and retest scans. Two of eight enrolled subjects experienced side effects (nausea and emesis), which have been previously reported for [11C]-(+)-PHNO[9, 14]. In both cases the infusion and scan were stopped within 5 minutes of the start of injection. The mass dose for the bolus phase of [11C]-(+)-PHNO for these subjects (0.02 μg/kg) was within the range of the other subjects (0.01-0.04 μg/kg).
Feasibility of the bolus plus infusion paradigm
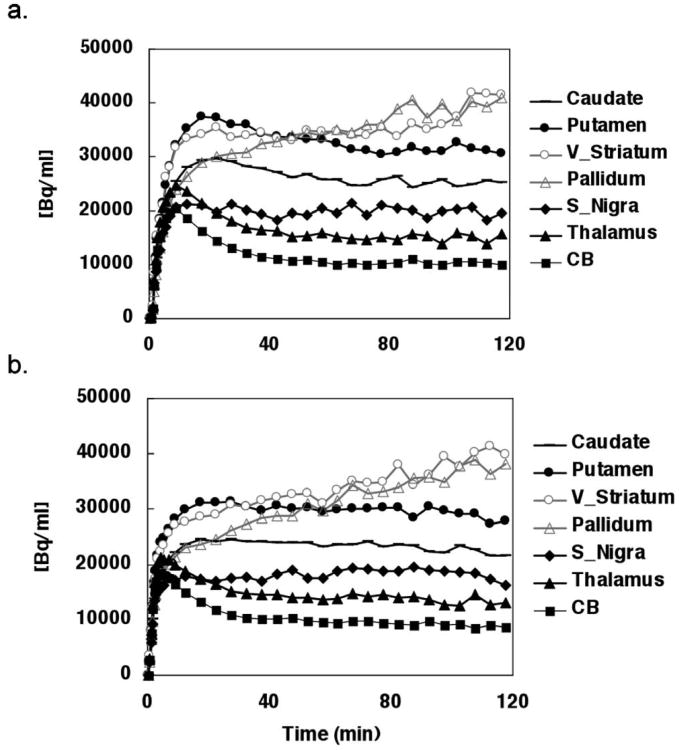
Time-activity curves (Figure 2) demonstrate low rates of change between 60 and 90 min in the caudate (−4 ± 13%/hr), putamen (−8 ± 8 %/hr), substantia nigra (−6 ± 19 %/hr), thalamus (− 6 ± 14 %/hr) and cerebellum (−2 ± 12 %/hr). However, high rates of change were observed in the ventral striatum (10 ± 17 %/hr) and pallidum (20 ± 13 %/hr).
Figure 2.
Time-activity curves of bolus plus constant infusion (Kbol=80min) of [11C]-(+)-PHNO for test (a) and retest (b) of a representative subject (curves are decay corrected).
Variability and Reliability of the Test and Retest Scans
The test-retest BPND estimates using the equilibrium method yielded high inter-scan correlation coefficients (r2=0.98) in all regions. Similarly, the inter-scan correlation coefficients of BPND using SRTM across all regions were high (r2=0.97).
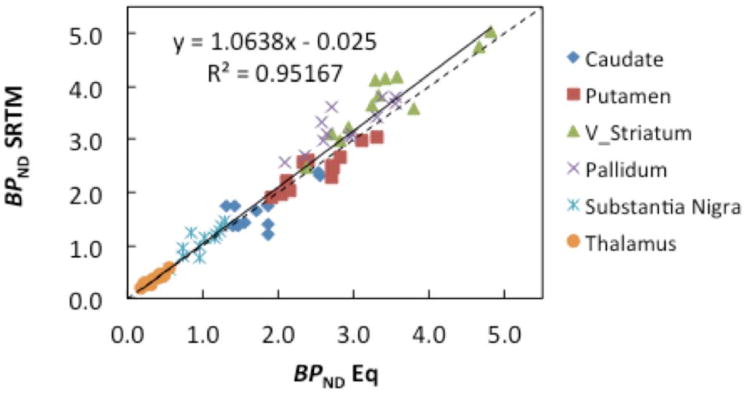
The rank order of BPND (ventral striatum > pallidum > putamen > caudate > substantia nigra > thalamus) was consistent for equilibrium and SRTM methods. The mean BPND estimates were similar on both scan days using the equilibrium approach (range: 0.36 ± 0.12 to 3.41 ± 0.74 and using SRTM (range: 0.37 ± 0.11 to 3.75 ± 0.75) (Table 1). A comparison of BPND estimates from equilibrium and SRTM methods for all regions and all scans indicates they were highly correlated (r2=0.95) across methods for all regions (Figure 3).
Table 1. Regional [11C]PHNO BPND values and their test-retest characteristics.
| Scan 1 | Scan 2 | Both Scans | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| M ± s.d. | cov (%) | M ± s.d. | COV (%) | M ± s.d. | COV (%) | p value | % TRV | ICC | ||
| BPND Eq | Caudate | 1.69 ±0.47 | 28 | 1.81 ±0.40 | 22 | 1.75 ±0.42 | 24 | 0.64 | 8.4 ±9.5 | 0.94 |
| Putamen | 2.49 ± 0.51 | 21 | 2.55 ± 0.40 | 16 | 2.52± 0.44 | 17 | 0.84 | 4.9 ±3.1 | 0.99 | |
| Ventral Striatum | 3.38 ± 0.80 | 24 | 3.45 ± 0.75 | 22 | 3.41 ± 0.74 | 22 | 0.88 | −2.6 ±6.7 | 0.97 | |
| Pallidum | 2.85 ± 0.42 | 15 | 2.94 ±0.57 | 19 | 2.89 ± 0.48 | 17 | 0.76 | −2.4 ±8.9 | 0.92 | |
| Substantia Nigra | 0.94 ±0.21 | 23 | 1.03 ±0.26 | 26 | 0.99 ± 0.23 | 24 | 0.52 | −8.1 ±21.8 | 0.72 | |
| Thalamus | 0.33 ±0.08 | 25 | 0.39 ±0.14 | 37 | 0.36±0.12 | 32 | 0.40 | −12.2 ±31.9 | 0.71 | |
| BPSRIM | Caudate | 1.57 ±0.41 | 26 | 1.72 ±0.36 | 21 | 1.65 ±0.37 | 23 | 0.5 | 9.4 ± 8.9 | 0.95 |
| Putamen | 2.38 ± 0.43 | 18 | 2.49 ± 0.33 | 13 | 2.44 ± 0.37 | 15 | 0.5 | 6.3 ±5.7 | 0.96 | |
| Ventral Striatum | 3.62 ± 0.82 | 23 | 3.88 ± 0.72 | 19 | 3.75± 0.75 | 20 | 0.6 | −7.9 ±10.3 | 0.92 | |
| Pallidum | 3.12 ±0.36 | 12 | 3.39 ± 0.44 | 13 | 3.26 ±0.41 | 13 | 0.3 | −8.0 ±10.3 | 0.66 | |
| Substantia Nigra | 1.07 ±0.19 | 18 | 1.08 ±0.35 | 32 | 1.08 ±0.27 | 25 | 1.0 | 3.2 ± 28.5 | 0.60 | |
| Thalamus | 0.31 ± 0.07 | 20 | 0.40 ±0.14 | 35 | 0.37 ±0.11 | 30 | 0.3 | −2.1 ± 25.7 | 0.80 | |
Figure 3.
Correlation of BPND estimates using the equilibrium (60-90 min) and SRTM. The results of the linear regression analysis are given (solid line). The dotted line is the line of identity.
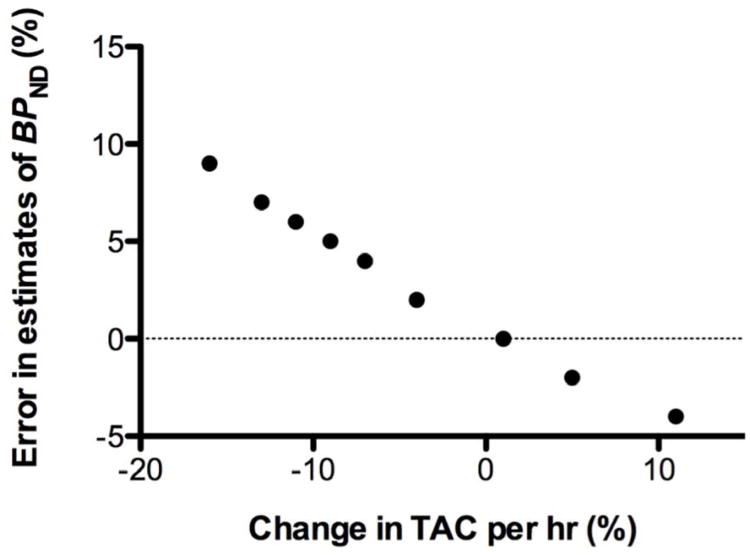
In order to assess the effects of lack of equilibrium on bias in equilibrium BPND values, simulations were performed where Kbol was varied, e.g., under- and over-estimated, and the effect on rates of change between 60 and 90 min and bias in BPND in the putamen was determined. Results indicate that rates of change up to 10%/hr results in a bias in BPND of <5% (Figure 4).
Figure 4.
Simulated error in estimated BPND using the equilibrium method (%) as a function of change in time activity curve (TAC) per hour (%) determined by simulating over- and underestimates of the infusion parameter Kbol.
Lastly, no age or gender differences in outcome measures were observed.
Discussion
The purpose of this study was to evaluate the feasibility and test-retest reproducibility of striatal and extrastriatal D2/3 binding of [11C]-(+)-PHNO using a constant infusion paradigm. The reproducibility of [11C]-(+)-PHNO has been previous explored[2], but not with a constant infusion paradigm, which has several potential advantages over model-based methods[10]. In our hands, reproducibility of D2/3BPND using [11C]-(+)-PHNO with both the equilibrium method and SRTM was excellent, with less than 10% TRV in striatal regions. The two methods were highly correlated (r2=0.95). Our simulations suggest that minor lack of equilibrium, i.e., rates of change up to 10%/hr, produce no more than a 5% bias in BPND.
The reproducibility of within-subject [11C]-(+)-PHNO binding (bolus injection) has been previously examined with SRTM[2]. Earlier results reported TRV of [11C]-(+)-PHNO BPND in humans to be 8.7 ± 8 % (caudate), 9.9 ± 8 % (putamen), 18.6 ± 19 % (ventral striatum), and 21.3 ± 16 % (pallidum). The TRV in the substantia nigra and thalamus were not given. In the current study, TRV of BPND using SRTM was consistent in the caudate (9.4 ± 9 %) and putamen (6.3 ± 6 %), and better than previously reported in the ventral striatum (−7.9 ±10 %) and pallidum (−8 ± 10 %). Additionally, in a preliminary study in our laboratory, the TRV in the putamen with the bolus method using SRTM was 9 ± 6 %[15]. Here we report a modest decrease in TRV in the putamen (6 ± 7 %) using the bolus plus infusion and SRTM.
There are potential advantages to the equilibrium paradigm compared to the more commonly used kinetic modeling techniques in order to estimate a given outcome measure[10]. One potential advantage is that, in the case of a displacement study, a single scan, single radiotracer synthesis and single administration may be sufficient to measure both baseline and stimulus-dependent changes in receptor availability. In the current study, we examined the test-retest reproducibility of [11C]-(+)-PHNO given as a constant infusion to lay the groundwork for potential infusion-based displacement studies. In fact, the equilibrium ratio between 45-60 min was virtually identical in the caudate, putamen, substantia nigra, and thalamus to BPND values given in Table 1 for the 60-90 min time points, which allows the possibility to perform a displacement at an earlier time point.
A proper bolus-to-infusion ratio is crucial to obtain accurate outcome measures. Based on regional time activity curves of previous PHNO bolus injection studies, the optimal bolus to infusion ratio was determined to be Kbol=80 min. In simulations, this produced equilibrium in the caudate and putamen by 60 minutes of scan start. Using this bolus plus infusion schedule, rates of change <8%/hr were achieved between 60 and 90 minutes in the caudate, putamen, substantia nigra and thalamus. Our simulations suggest that variations in time-activity curves up to 10%/hr result in small <5% error in BPND estimates in the caudate and putamen. This is less than the error associated with TRV. The same infusion ratio (Kbol=80min) produced greater change per time (>10%/hr) in the ventral striatum and pallidum and resulted in underestimates of BPND with the equilibrium method compared to SRTM (Table 1). This was predicted by the simulation (Figure 1), and the error in BPND estimates in these regions is larger. Thus, for these higher binding regions that require longer time to reach equilibrium, the equilibrium model may result in less accurate estimates of BPND.
Unfortunately, two out of the eight enrolled subjects ended study participation due to nausea and/or emesis. These side effects have been observed by others[9, 14] and occurred within 5 minutes of injection. The mass dose (μg/kg) of [11C]-(+)-PHNO was not different from what was administered to other subjects. Although there was a higher total mass (0.05 + 0.02 μg/kg) injected in the current study than in previous bolus studies (0.03 μg/kg) suggested to avoid occurrences of side-effects[14], this design was a consequence of the bolus/infusion schedule and is unlikely to account for the side effects. In these two subjects, less than 50% of the total projected mass of radiotracer (0.02 μg/kg) was delivered in the bolus phase, which is in line with the recommended dose (0.03 μg/kg) of [11C]-(+)-PHNO at which there is no correlation between dose and side effects in humans[14].
In conclusion, [11C]-(+)-PHNO PET shows suitable characteristics for D2/3 receptor quantification by equilibrium modeling. A bolus plus constant infusion schedule to optimize equilibrium in the caudate nucleus, putamen, and substantia nigra and the requisite equilibrium analysis performs well in comparison with a standard model-based approach. Additionally, equilibrium estimates of BPND using a constant infusion of [11C]-(+)-PHNO were highly reproducible, illustrating the robustness of this paradigm.
Acknowledgments
This publication was made possible by NIH grant K01 DA20651 and by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Abbreviations
- PET
Positron emission tomography
- SRTM
simplified reference tissue model
- BPND
binding potential
- MRI
magnetic resonance imaging
- TRV
test-retest variability
References
- 1.Ginovart N, Galineau L, Willeit M, Mizrahi R, Bloomfield PM, Seeman P, et al. Binding characteristics and sensitivity to endogenous dopamine of [11C]-(+)-PHNO, a new agonist radiotracer for imaging the high-affinity state of D2 receptors in vivo using positron emission tomography. J Neurochem. 2006;97:1089–103. doi: 10.1111/j.1471-4159.2006.03840.x. doi:JNC3840 [pii] 10.1111/j.1471-4159.2006.03840.x. [DOI] [PubMed] [Google Scholar]
- 2.Willeit M, Ginovart N, Graff A, Rusjan P, Vitcu I, Houle S, et al. First human evidence of d-amphetamine induced displacement of a D2/3 agonist radioligand: A [11C]-(+)-PHNO positron emission tomography study. Neuropsychopharmacology. 2008;33:279–89. doi: 10.1038/sj.npp.1301400. doi:1301400 [pii] 10.1038/sj.npp.1301400. [DOI] [PubMed] [Google Scholar]
- 3.Narendran R, Slifstein M, Guillin O, Hwang Y, Hwang DR, Scher E, et al. Dopamine (D2/3) receptor agonist positron emission tomography radiotracer [11C]-(+)-PHNO is a D3 receptor preferring agonist in vivo. Synapse. 2006;60:485–95. doi: 10.1002/syn.20325. [DOI] [PubMed] [Google Scholar]
- 4.Searle G, Beaver JD, Comley RA, Bani M, Tziortzi A, Slifstein M, et al. Imaging dopamine D3 receptors in the human brain with positron emission tomography, [11C]PHNO, and a selective D3 receptor antagonist. Biol Psychiatry. 2010;68:392–9. doi: 10.1016/j.biopsych.2010.04.038. doi: S0006-3223(10)00467-1 [pii] 10.1016/j.biopsych.2010.04.038. [DOI] [PubMed] [Google Scholar]
- 5.Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, et al. Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther. 1994;268:417–26. [PubMed] [Google Scholar]
- 6.Seeman P, Ulpian C, Larsen RD, Anderson PS. Dopamine receptors labelled by PHNO. Synapse. 1993;14:254–62. doi: 10.1002/syn.890140403. [DOI] [PubMed] [Google Scholar]
- 7.Gallezot JD, Beaver JD, Gunn RN, Nabulsi N, Weinzimmer D, Singhal T, et al. Affinity and selectivity of [(11) C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse. 2012 doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- 8.Seeman P, McCormick PN, Kapur S. Increased dopamine D2(High) receptors in amphetamine-sensitized rats, measured by the agonist [(3)H](+)PHNO. Synapse. 2007;61:263–7. doi: 10.1002/syn.20367. [DOI] [PubMed] [Google Scholar]
- 9.Willeit M, Ginovart N, Kapur S, Houle S, Hussey D, Seeman P, et al. High-affinity states of human brain dopamine D2/3 receptors imaged by the agonist [11C]-(+)-PHNO. Biol Psychiatry. 2006;59:389–94. doi: 10.1016/j.biopsych.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 10.Carson RE. PET physiological measurements using constant infusion. Nucl Med Biol. 2000;27:657–60. doi: 10.1016/s0969-8051(00)00138-4. [DOI] [PubMed] [Google Scholar]
- 11.Wilson AA, McCormick P, Kapur S, Willeit M, Garcia A, Hussey D, et al. Radiosynthesis and evaluation of [11C]-(+)-4-propyl-3,4,4a,5,6,10b-hexahydro-2H-naphtho[1,2-b][1,4]oxazin-9 -ol as a potential radiotracer for in vivo imaging of the dopamine D2 high-affinity state with positron emission tomography. J Med Chem. 2005;48:4153–60. doi: 10.1021/jm050155n. [DOI] [PubMed] [Google Scholar]
- 12.Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, et al. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13:24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- 13.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–8. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 14.Mizrahi R, Houle S, Vitcu I, Ng A, Wilson AA. Side effects profile in humans of (11)C-(+)-PHNO, a dopamine D(2/3) agonist ligand for PET. J Nucl Med. 2010;51:496–7. doi: 10.2967/jnumed.109.072314. [DOI] [PubMed] [Google Scholar]
- 15.Gallezot JD, Matuskey D, Lim K, Zheng MQ, Lin SF, Ding YS, et al. [11C]PHNO parametric imaging and test-retest study in humans using the HRRT scanner. Abstract to BrainPET. 2011 [Google Scholar]