Abstract
Purpose
Prior studies have suggested that patients with stage II/III colon cancer receive similar benefit from intravenous (IV) fluoropyrimidine adjuvant therapy regardless of age. Combination regimens and oral fluorouracil (FU) therapy are now standard. We examined the impact of age on colon cancer recurrence and mortality after adjuvant therapy with these newer options.
Patients and Methods
We analyzed 11,953 patients age < 70 and 2,575 age ≥ 70 years from seven adjuvant therapy trials comparing IV FU with oral fluoropyrimidines (capecitabine, uracil, or tegafur) or combinations of fluoropyrimidines with oxaliplatin or irinotecan in stage II/III colon cancer. End points were disease-free survival (DFS), overall survival (OS), and time to recurrence (TTR).
Results
In three studies comparing oxaliplatin-based chemotherapy with IV FU, statistically significant interactions were not observed between treatment arm and age (P interaction = .09 for DFS, .05 for OS, and .36 for TTR), although the stratified point estimates suggested limited benefit from the addition of oxaliplatin in elderly patients (DFS hazard ratio [HR], 0.94; 95% CI, 0.78 to 1.13; OS HR, 1.04; 95% CI, 0.85 to 1.27). No significant interactions by age were detected with oral fluoropyrimidine therapy compared with IV FU; noninferiority was supported in both age populations.
Conclusion
Patients age ≥ 70 years seemed to experience reduced benefit from adding oxaliplatin to fluoropyrimidines in the adjuvant setting, although statistically, there was not a significant effect modification by age, whereas oral fluoropyrimidines retained their efficacy.
INTRODUCTION
Colorectal cancer accounts for approximately 10% of all new patient cases of cancer and cancer-related deaths in the United States. In 2012, an estimated 143,460 new patient cases of colorectal cancer were diagnosed.1 Worldwide, the incidence of colorectal cancer is > 1,000,000 per year.2 The probability of developing colorectal cancer increases from 0.07% in the first four decades of life to 4.5% to 5% in the seventh decade of life.3
The Adjuvant Colon Cancer End Points (ACCENT) group (Appendix, online only) originally assembled individual patient data from 18 trials in the United States, Canada, Australia, and Europe testing fluoropyrimidine-based adjuvant therapy for patients with stage II or III colon cancer. Previous analyses of data from trials included in ACCENT comparing surgery alone with surgery followed by fluorouracil (FU) -based chemotherapy demonstrated that patients age ≥ 70 years experienced a similar benefit from chemotherapy compared with younger patients.4 Recently, data from seven newer studies comparing either intravenous (IV) combination regimens with oxaliplatin or irinotecan or oral fluoropyrimidine chemotherapy with IV FU and leucovorin (LV) in stages II and III colon cancer were added to ACCENT (Table 1).5–10 These studies included > 14,500 participants, of whom 18% were age ≥ 70 years. We sought to determine the impact of age on cancer recurrence and mortality after combination chemotherapy or oral fluoropyrimidines compared with single-agent IV FU as adjuvant colon cancer treatment.
Table 1.
Adjuvant Colon Cancer Trials Included
| Trial | Accrual Period | No. of Patients | Patients Age ≥ 70 Years (%) | Experimental Treatment Arm* | Stage III (%)† |
|---|---|---|---|---|---|
| Oxaliplatin | |||||
| MOSAIC | 1998 to 2001 | 2,246 | 14 | FOLFOX4 | 60 |
| NSABP-C07 | 2000 to 2002 | 2,434 | 16 | FLOX | 71 |
| XELOXA | 2003 to 2004 | 1,862 | 22 | XELOX | 100 |
| Irinotecan | |||||
| CALGB-89803 | 1999 to 2001 | 1,263 | 24 | IFL | 98 |
| PETACC-3 | 2000 to 2002 | 3,186 | 13 | FOLFIRI | 71 |
| Oral fluoropyrimidine | |||||
| NSABP-C06 | 1997 to 1999 | 1,557 | 23 | Uracil/tegafur | 53 |
| X-ACT | 1998 to 2001 | 1,983 | 20 | Capecitabine | 100 |
Abbreviations: CALGB, Cancer and Leukemia Group B; FLOX, bolus intravenous fluorouracil, leucovorin, and oxaliplatin; FOLFIRI, infusional fluorouracil, leucovorin, and irinotecan; FOLFOX, infusional fluorouracil, leucovorin, and oxaliplatin; IFL, bolus intravenous fluorouracil, leucovorin, and irinotecan; MOSAIC, Multicenter International Study of Oxaliplatin/Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer; NSABP, National Adjuvant Breast and Bowel Project; PETACC, Pan-European Trials in Adjuvant Colon Cancer; X-ACT, Xeloda in Adjuvant Colon Cancer Therapy; XELOX, Xeloda and oxaliplatin; XELOXA, Xeloda and Oxaliplatin in Adjuvant Colon Cancer Treatment.
Compared with control arm of intravenous flourouracil and leucovorin.
Remaining patients had stage II disease or unknown stage.
PATIENTS AND METHODS
Study Design and Trial Selection
The ACCENT group identifies and obtains individual patient data from phase III adjuvant trials in patients with colon cancer. Our analysis used seven phase III trials recently added to ACCENT that either compared standard IV FU and LV with combination regimens or oral fluoropyrimidine therapy.5–10 The trials accrued 14,528 patients between 1997 and 2004 (Table 1). Beyond age, available data include patient sex, disease stage, treatment arm, survival status, and recurrence status at last follow-up time point. Data on toxicity and comorbidities were not consistently available across all studies and were therefore not included in this analysis.
Statistical Considerations
All patients were included in the analyses according to the intention-to-treat principle. Disease-free survival (DFS) was defined as the time from random assignment to either recurrent disease or death, whichever occurred first. Overall survival (OS) was defined as the time from random assignment to death resulting from any cause. Time to recurrence (TTR) was defined as the time to colon cancer recurrence; deaths without recurrence were censored at the time of death. Second primary colon or noncolon tumors were not counted as events in the DFS and TTR analyses. The definitions of DFS and TTR used in the primary analysis of each individual trial were not consistent across the seven trials; thus, the definitions for our analyses may differ from those used in the original trials. The primary analyses pooled individual patient data from the seven trials, stratified by the patient's original trial, and considered age as a two-level dichotomous variable: age < 70 versus ≥ 70 years. Hazard ratios (HRs) were calculated using the Cox proportional hazards regression model, adjusting for sex, treatment arm, and disease stage. Models tested for an age-by-treatment interaction using the likelihood ratio test. Additional models for age were explored using three age groups (< 70, 70 to 74, and ≥ 75 years) and using age as a continuous variable. Age as a continuous variable was modeled using a subpopulation treatment effect pattern plot (STEPP) analysis, which plots treatment effects within a clinical trial by a covariate (eg, age), allowing validation of cutoff points for treatment effects.11
RESULTS
Patient Enrollment and Characteristics
In the seven trials included in our analyses, 11,953 patients were age < 70 years, and 2,575 were age ≥ 70 years. There were no appreciable differences in sex, stage of disease, or treatment assignment by age, although 4% more patients age < 70 years had stage II disease compared with those age ≥ 70 years (Table 2).
Table 2.
Overall Baseline Patient Characteristics
| Characteristic | Age < 70 Years (%; n = 11,953) | Age ≥ 70 Years (%; n = 2,575) |
|---|---|---|
| Sex | ||
| Female | 45 | 45 |
| Male | 55 | 55 |
| Stage | ||
| II | 23 | 19 |
| III | 77 | 81 |
| Treatment arm | ||
| Control | 49 | 52 |
| Experimental | 51 | 48 |
Efficacy
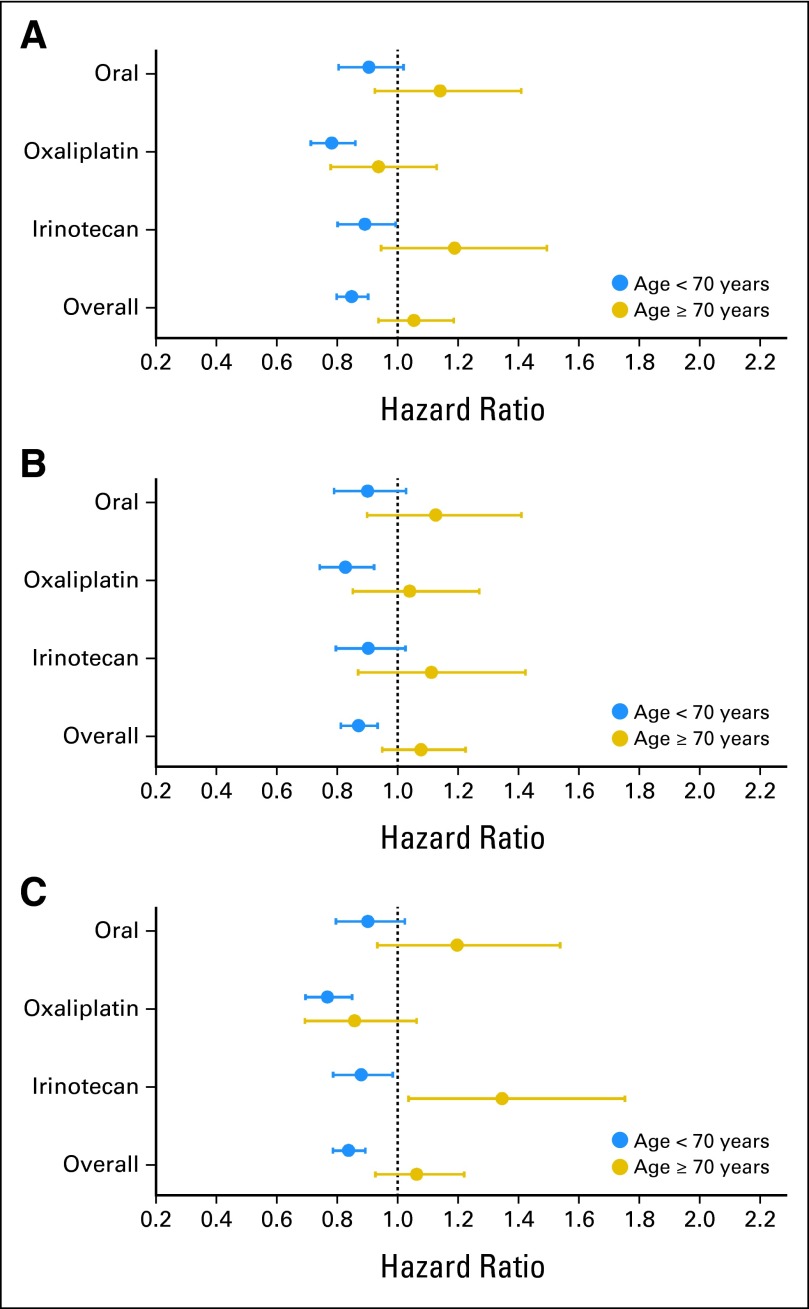
In analyses that combined data from all seven trials (Table 3; Fig 1), older patients did not experience statistically significant benefit from combination therapy or oral fluoropyrimidines in terms of DFS (HR, 1.05; 95% CI, 0.94 to 1.19), OS (HR, 1.08; 95% CI, 0.95 to 1.23), or TTR (HR, 1.06; 95% CI, 0.93 to 1.22). In contrast, patients age < 70 years experienced statistically significant improvements in all three end points for the experimental regimens compared with standard IV FU and LV. Tests for interaction between age and treatment were statistically significant for all three end points (P interaction = .001 for DFS, .004 for OS, and .002 for TTR). Although rates of death within 6 months of initiation of therapy were higher in older patients (2.80% v 0.85%), there were no significant differences in death rates at 6 months comparing age categories within experimental (age < 70 v ≥ 70 years, 3.10% v 2.52%; P = .4) and control arms (age < 70 v ≥ 70 years, 0.91% v 0.80%; P = .5). Thus, early deaths resulting from toxicity are unlikely to explain the significant interaction between treatment and age observed for DFS and OS. We then performed analyses by drug class, given that the current standard of care for adjuvant therapy is oxaliplatin-based chemotherapy, IV FU and LV, or oral fluoropyrimidines.
Table 3.
Outcomes of Experimental (combination or oral FU) Versus Control Arm (IV FU) by Treatment and Age
| Treatment Arm | DFS** |
OS* |
TTR* |
Deaths Within 6 Months, Experimental Versus Control |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | HR | 95% CI | % | P | |
| All trials | ||||||||
| Age < 70 years (n = 11,953) | 0.85 | 0.80 to 0.90 | 0.87 | 0.81 to 0.93 | 0.84 | 0.79 to 0.89 | 0.91 v 0.80 | .5 |
| Age ≥ 70 years (n = 2,575) | 1.05 | 0.94 to 1.19 | 1.08 | 0.95 to 1.23 | 1.06 | 0.93 to 1.22 | 3.10 v 2.52 | .4 |
| P interaction | .001 | .004 | .002 | |||||
| Oxaliplatin based | ||||||||
| Age < 70 years (n = 5,420) | 0.78 | 0.71 to 0.86 | 0.83 | 0.74 to 0.92 | 0.77 | 0.69 to 0.85 | 0.88 v 0.82 | .8 |
| Age ≥ 70 years (n = 1,119) | 0.94 | 0.78 to 1.13 | 1.04 | 0.85 to 1.27 | 0.86 | 0.69 to 1.06 | 3.15 v 2.25 | .4 |
| P interaction | .09 | .05 | .36 | |||||
| Irinotecan based | ||||||||
| Age < 70 years (n = 3,750) | 0.89 | 0.80 to 0.99 | 0.90 | 0.80 to 1.03 | 0.88 | 0.79 to 0.98 | 0.90 v 0.43 | .1 |
| Age ≥ 70 years (n = 699) | 1.19 | 0.95 to 1.49 | 1.11 | 0.87 to 1.42 | 1.35 | 1.04 to 1.76 | 3.96 v 2.43 | .3 |
| P interaction | .02 | .13 | .002 | |||||
| Oral fluoropyrimidine | ||||||||
| Age < 70 years (n = 2,783) | 0.91 | 0.80 to 1.02 | 0.90 | 0.79 to 1.03 | 0.90 | 0.80 to 1.02 | 0.98 v 1.25 | .5 |
| Age ≥ 70 years (n = 757) | 1.14 | 0.92 to 1.41 | 1.13 | 0.90 to 1.41 | 1.20 | 0.93 to 1.54 | 2.24 v 3.00 | .5 |
| P interaction | .13 | .16 | .09 | |||||
Abbreviations: DFS, disease-free survival; FU, fluorouracil; HR, hazard ratio; IV, intravenous; OS, overall survival; TTR, Time to recurrence.
Values < 1 favor experimental arm.
Fig 1.
Forest plots by drug class, all stages. (A) Disease-free survival; (B) overall survival; (C) time to recurrence.
Oxaliplatin-Based Trials
Three trials tested the addition of oxaliplatin to fluoropyrimidines—two with IV FU and LV and one with oral capecitabine (Table 3). Patients age < 70 years experienced significant improvements in DFS, OS, and TTR, whereas those age ≥ 70 years did not experience significant improvements for any of these end points. Older patients also had an appreciably greater risk of dying in the first 6 months from start of therapy, with or without oxaliplatin. There was, however, not a significant interaction by age for OS (P = .05), DFS (P = .09), or TTR (P = .36). Thus, although the HRs suggest reduced benefit from oxaliplatin in older patients, the P values for interactions ≥ .05 indicate lack of significant effect modification by age for all three end points.
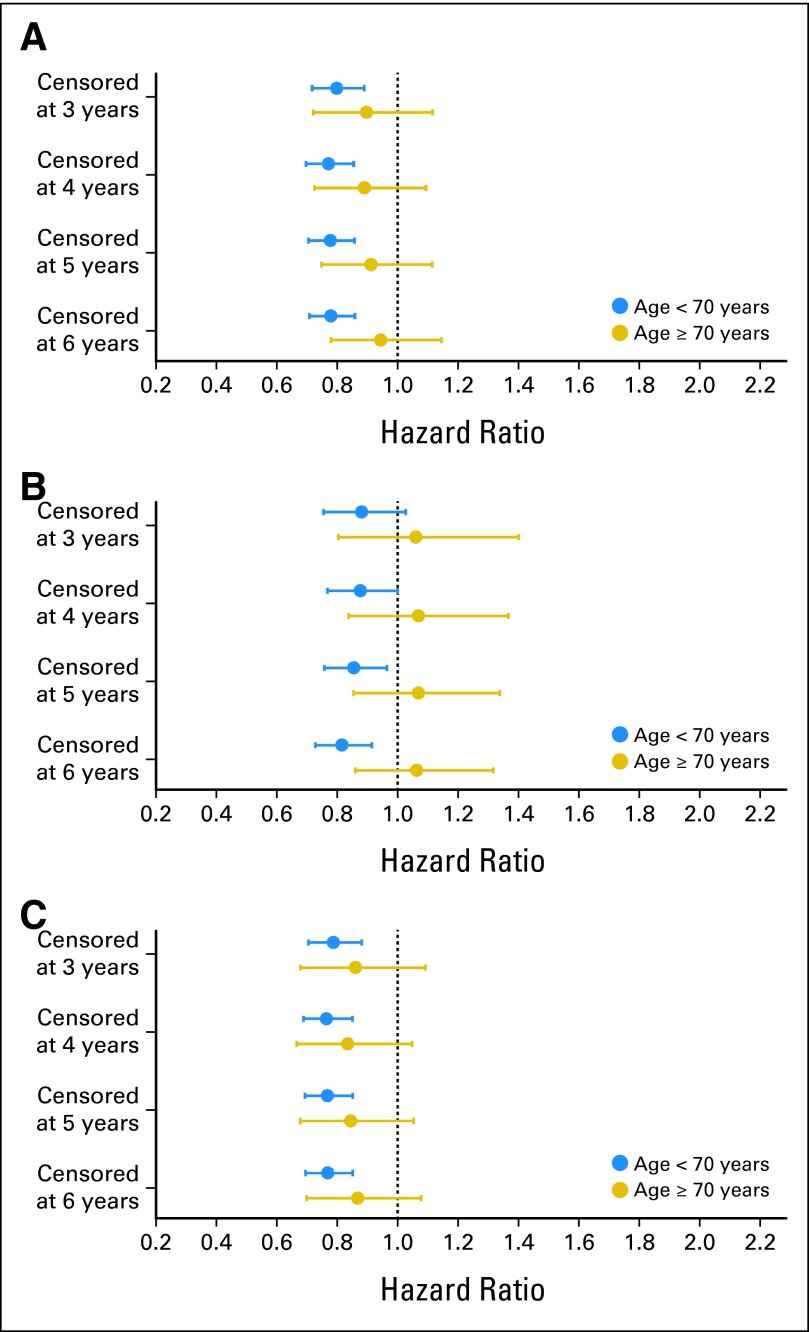
To further explore the efficacy of oxaliplatin in older patients and the smaller apparent gain in DFS and OS based on the subgroup HR point estimates, we censored the analyses at 3, 4, 5, and 6 years after study entry, presuming that the analyses with longer follow-up would be affected more by non–cancer-related deaths (Fig 2). As shown in Figure 2, the HRs for DFS remained consistent at each censoring time point for younger patients but become increasingly closer to 1 (null hypothesis) for elderly patients. For OS, the HRs for younger patients demonstrated an increasingly stronger point estimate as time evolved, but for older patients, they were > 1 at each censoring year. These analyses suggest that oxaliplatin may provide a short-term reduction in the risk of recurrence in elderly patients, but by 3 years after surgery, a sufficient proportion of elderly patients die as a result of other causes so that no long-term DFS or OS benefit is present.
Fig 2.
Oxaliplatin trials with censoring analyses of different time points. (A) Disease-free survival; (B) overall survival; (C) time to recurrence.
Irinotecan-Based Trials
Two adjuvant irinotecan trials were incorporated into the ACCENT database, although the administration of FU and LV differed between the two trials (bolus regimen in CALGB-89803 [Cancer and Leukemia Group B] and infusional regimen in PETACC-3 [Pan-European Trials in Adjuvant Colon Cancer]). Older patients did not benefit from the addition of irinotecan to FU and LV in DFS, OS, or TTR (Table 3). However, patients age < 70 years seemed to experience significant improvement in DFS (HR, 0.89; 95% CI, 0.80 to 0.99) and TTR (HR, 0.88; 95% CI, 0.79 to 0.98). There was a significant interaction by age for DFS (P = .02) and TTR (P = .002) but not OS (P = .13). It should be noted that the definition of DFS used in these analyses included time to disease recurrence, new primary colon cancer, or death resulting from any cause (which was the definition of recurrence-free survival in PETACC-3).
Oral Fluoropyrimidines
For oral fluoropyrimidines, interaction testing between treatment and age for DFS (P = .13), OS (P = .16), and TTR (P = .09) were not significant. Both oral fluoropyrimidine trials were designed to test noninferiority compared with IV FU and LV. These data suggest that the benefit of oral fluoropyrimidines is similar to that of IV FU regardless of age.
Stage III Disease
We tested whether older patients with stage III disease benefited from newer therapies, excluding stage II patients. The point estimates of the HRs for DFS for older patients, comparing experimental with control arms, were all > 1 (overall stage III population: HR, 1.06; 95% CI, 0.94 to 1.21; oral fluoropyrimidines: HR, 1.19; 95% CI, 0.95 to 1.50; irinotecan-based therapy: HR, 1.21; 95% CI, 0.95 to 1.55); for oxaliplatin-based therapy, it was slightly < 1 (HR, 0.91; 95% CI, 0.74 to 1.11). The P value for interaction for DFS between age and treatment arm was .15 when restricted to the three oxaliplatin-based therapy trials; it was .002 for all seven trials. Although this analysis suggests that there is a lack of DFS benefit among older patients with stage III disease for combination regimens, a significant P value for a treatment interaction was not seen.
Alternative Models of Age
The lack of recurrence-free or OS benefit is not altered when alternative modeling of age is used. In secondary analyses, we divided age into three categories: < 70 (n = 11,953), 70 to 74 (n = 1,989), and ≥ 75 years (n = 586); two of the trials (MOSAIC [Multicenter International Study of Oxaliplatin/Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer] and PETACC-3) limited eligibility to those age ≤ 75 years and thus contributed minimally to the oldest category. For the overall population, the HRs for DFS for experimental versus control treatment were 0.85 (95% CI, 0.80 to 0.90) for patients age < 70, 1.09 (95% CI, 0.95 to 1.25) for patients age 70 to 74, and 0.97 (95% CI, 0.77 to 1.23) for patients age ≥ 75 years. In analyses by chemotherapy regimen, for oxaliplatin-based chemotherapy versus FU and LV, HRs for DFS were 0.78 (95% CI, 0.71 to 0.86) for patients age < 70, 0.91 (95% CI, 0.73 to 1.13) for patients age 70 to 74, and 1.01 (95% CI, 0.72 to 1.42) for patients age ≥ 75 years. For oral versus IV FU chemotherapy, HRs for DFS were 0.91 (95% CI, 0.80 to 1.02) for patients age < 70, 1.23 (95% CI, 0.96 to 1.57) for patients age 70 to 74, and 0.93 (95% CI, 0.61 to 1.41) for patients age ≥ 75 years.
Furthermore, we conducted analyses using the STEPP method.12 This method estimates the treatment benefit for a sequence of patient subgroups defined by a characteristic of interest (such as age) without set cut points. For the oxaliplatin cohort, improved DFS was estimated by the STEPP method for patients age 50 to 65 years, with no benefit estimated for the experimental versus control treatment for patients age approximately 65 years (Fig 3).
Fig 3.
Subpopulation treatment effect pattern plot of disease-free survival (DFS) by age for oxaliplatin-based therapy.
DISCUSSION
In the ACCENT database, older patients (age ≥ 70 years) experienced mixed benefit from newer adjuvant cytotoxic chemotherapy regimens. When initially reported in 2009,9 only two of the oxaliplatin-based trials were available for analyses (MOSAIC and NSABP-C07 [National Surgical Adjuvant Breast and Bowel Project]), and there were statistically significant P values for interaction in DFS and OS comparing older and younger patients with the addition of oxaliplatin. With the addition of a third oxaliplatin-based treatment study, XELOXA [Xeloda and Oxaliplatin in Adjuvant Colon Cancer Treatment], the P value for interaction for age and oxaliplatin treatment was no longer statistically significant for DFS, suggesting that older patients may experience a DFS benefit from oxaliplatin in the adjuvant setting. However, the lack of an OS benefit among older patients receiving oxaliplatin in the adjuvant setting remains consistent with prior analysis.9
Recent studies have focused on combination regimens for patients with metastatic disease.12,13 In a retrospective pooled analysis of 3,742 participants (16% age ≥ 70 years) enrolled onto four clinical trials of FOLFOX (infusional FU, LV, and oxaliplatin; three metastatic trials and one adjuvant trial), individuals age ≥ 70 years had similar recurrence or relapse-free and OS as well as overall toxicity rates compared with those age < 70 years with oxaliplatin-based therapy.12 Folprecht et al13 published a combined analysis of four phase III studies enrolling 2,691 patients (22% age ≥ 70 years) receiving first-line irinotecan-based therapy in the metastatic setting. Combination regimens with irinotecan were associated with a significant improvement in progression-free survival and a trend toward improvement in OS for older patients, compared with FU and LV alone. Our analysis sought to test the impact of age in the adjuvant setting because benefits of treatment in the metastatic setting do not always translate to the adjuvant setting in colorectal cancer.
Initial studies in the adjuvant setting of fluoropyrimidine therapy showed similar survival benefit regardless of age. One prior analysis of the efficacy of adjuvant IV FU and either levamisole or LV therapy compared with surgery alone in elderly patients using pooled data from seven randomized trials found no significant interaction between age and efficacy of treatment.4 Our current data do not contradict those findings, because all studies in the current analysis involved chemotherapy in both arms.
The seven trials included in this analysis were those with mature data testing regimens beyond variations in dosing schedule of IV FU and LV therapy. Five trials (MOSAIC, XELOXA, NSABP-C07, CALGB-89803, and PETACC-3) were designed to demonstrate superiority of the experimental regimen to IV FU and LV to significantly improve DFS or OS. Two trials (X-ACT [Xeloda in Adjuvant Colon Cancer Therapy] and NSABP-C06) were designed to demonstrate noninferiority of oral fluoropyrimidine therapy compared with IV FU and LV; the analyses presented here are supportive of the conclusion that the effect of oral versus IV therapy is similar regardless of age.
In contrast to our initial report reflecting two adjuvant studies of oxaliplatin, expansion of the data to include a third study, the XELOXA trial, changed the P value for age/oxaliplatin from significant (P = .016)9 to nonsignificant (P = .09). It is important to note that the XELOXA trial differed from MOSAIC because it used bolus instead of infusional FU/LV for the control arm and oral fluoropyrimidine (capecitabine) with oxaliplatin (instead of infusional FU/LV) for the experimental arm. The age-by-treatment benefit interaction became P = .05 for OS, whereas that for TTR remained nonsignificant, suggesting that oxaliplatin provides a reduction in the risk of recurrence in a subset of elderly patients. However, after a period of time, a sufficient number of elderly patients died as a result of other causes, diminishing the OS benefit. The nonsignificant P interaction for age with oxaliplatin treatment suggests that a subset of elderly patients derives a DFS benefit from oxaliplatin. This hypothesis is supported by exploratory analyses in which the data from the three oxaliplatin trials were censored at different time points (Fig 2). If this is truly the explanation, it raises the clinical question of whether increased toxicity from more intensive therapy to delay recurrence is a clinically meaningful benefit, given the competing risks of toxicity and death in older adults.
Our study did not include evaluation of biologic agents (bevacizumab and cetuximab) among older patients in the adjuvant setting.14–19 However, given the lack of survival benefit noted in the general adjuvant treatment setting, subgroup analyses by age may be less relevant.
The ACCENT database provides the advantage of pooling large, mature clinical trials to test hypotheses that are difficult or impossible to test within individual trials. Given the fact that none of the included trials had > one quarter of the patients age ≥ 70 years, subset analyses by age in the individual studies have limited power. Pooling of the data from these seven trials resulted in > 2,000 patients age ≥ 70 years to be studied, larger than any subgroup analysis from individual studies. Nonetheless, there are limitations to this analysis. First, the ACCENT analysis lacks toxicity or comorbidity data. Given the potential benefit of oxaliplatin DFS but not OS, consideration of the toxicity from oxaliplatin should be weighed in decision making regarding the use of oxaliplatin in elderly patients. Second, we do not have data on dose-intensity or proportion of doses delivered compared with the total dose planned. Thus, we were unable to comment on the extent to which the amount of actual dose received may have accounted for this lack of benefit among older patients receiving adjuvant therapy. However, in previously published analyses from the MOSAIC and XELOXA trials, dose-intensity did not alter efficacy of treatment.11,20 Also, only a minority of the elderly receive adjuvant therapy in practice, and an even smaller proportion of highly selected patients enter clinical trials. Therefore, in an unselected population of patients who may have been ineligible for such trials for many reasons, the degree of benefit may be less than the results reported here. Our study suggests that the competitive risk of dying as a result of other causes may be a mechanism by which older patients do not experience a DFS or OS benefit with combination therapy in the adjuvant setting, in contrast to that noted in the metastatic setting. The age cutoff of 70 years was selected to be consistent with past studies evaluating the impact of chemotherapy regimens in older patients with colorectal cancer and was also supported using a STEPP analysis. However, other factors not collected or evaluated in our study may explain the observed lack of benefit for older patients. These factors may include measures of comorbidity or functional decline collected in a comprehensive geriatric assessment that are not captured by Eastern Cooperative Oncology Group performance status.21–25 Such factors may directly or indirectly lead to lower survival via competing mortality or modification of dose-intensity or dose schedule.23,26–30
In conclusion, our findings suggest that the benefit of oxaliplatin compared with IV FU and LV is restricted to patients age < 70 years for OS. For patients age ≥ 70 years, oxaliplatin may provide a DFS benefit for a subset of older adults; however, we cannot establish which subsets of older adults experience benefit. For this reason, the data also support fluoropyrimidine monotherapy as an appropriate treatment option. Fluoropyrimidine monotherapy with either FU/LV or capecitabine is an appropriate adjuvant treatment option for patients age ≥ 70 years. Clinical studies incorporating measures of factors beyond physiologic age predicting response to treatment (eg, comorbid conditions, patient functional status, treatment duration, or dose-intensity, as collected by the Cancer-Specific Geriatric Assessment31) may guide clinicians in optimal treatment selection for older patients, limiting the potential lack of benefit or harm to vulnerable or frail older patients. Further study is needed to identify which subsets of older patients derive potential benefit from oxaliplatin-based chemotherapy.
Appendix
The ACCENT (Adjuvant Colon Cancer End Points) Collaborative Group includes: D.J. Sargent, E. Green, A. Grothey, S.R. Alberts, Q. Shi, L. Renfro (Mayo Clinic, Rochester, MN); G. Yothers, M.J. O'Connell, N. Wolmark (National Surgical Adjuvant Breast and Bowel Project Biostatistical and Operations Centers, Pittsburgh, PA); A. de Gramont (Hôpital Saint Antoine, Paris, France); R. Gray, D. Kerr (Quasar Collaborative Group, Birmingham and Oxford, United Kingdom); D.G. Haller (Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA); J. Benedetti (Southwest Oncology Group Statistical Center, Seattle, WA); M. Buyse (International Drug Development Institute, Louvain-la-Neuve, Belgium); R. Labianca (Ospedali Riuniti, Bergamo, Italy); J.F. Seitz (University of the Mediterranean, Marseilles, France); C.J. O'Callaghan (National Cancer Institute of Canada Clinical Trials Group, Queens University, Kingston, Ontario, Canada); G. Francini (University of Siena, Siena, Italy); P.J. Catalano (Eastern Cooperative Oncology Group Statistical Center, Boston, MA); C.D. Blanke (British Columbia Cancer Agency, Vancouver, British Columbia, Canada); T. Andre (Groupe Hospitalier Piti e-Salpetriere, Paris, France); R.M. Goldberg (University of North Carolina, Chapel Hill, NC); A. Benson (Northwestern University, Chicago, IL); C. Twelves (University of Leeds, Leeds, United Kingdom); J. Cassidy (Genentech/Roche, Glasgow, United Kingdom); F. Sirzen (Roche, Basel, Switzerland); L. Cisar (Pfizer, New York, NY); E. Van Cutsem (University Hospital Gasthuisberg, Leuven, Belgium); L. Saltz (Memorial Sloan-Kettering Cancer Center, New York, NY); J. Meyerhardt, N.J. McCleary (Dana-Farber Cancer Center, Boston, MA).
Footnotes
Written on behalf of the ACCENT (Adjuvant Colon Cancer End Points) Collaborative Group.
Supported in part by the American Society of Clinical Oncology Young Investigator Award cosponsored by the Hartford Foundation, North Central Cancer Treatment Group (National Cancer Institute [NCI] Grant No. CA25224), and Dana-Farber Cancer Institute/Harvard Cancer Center SPORE (Specialized Programs of Research Excellence; NCI Grant No. P50 CA127003).
Presented at the 45th Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 29-June 2, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Aimery de Gramont, Roche (C), sanofi-aventis (C); Christopher J. Twelves, Roche (C); Leonard B. Saltz, Pfizer (C), Roche (C), sanofi-aventis (U); Daniel G. Haller, Roche (C), sanofi-aventis (C) Stock Ownership: None Honoraria: Aimery de Gramont, Roche, sanofi-aventis; Christopher J. Twelves, Roche; Daniel G. Haller, sanofi-aventis Research Funding: Eric Van Cutsem, Pfizer, sanofi-aventis; Leonard B. Saltz, Roche Expert Testimony: None Patents: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Nadine J. McCleary, Jeffrey A. Meyerhardt, Daniel G. Haller, Daniel J. Sargent
Financial support: Daniel J. Sargent
Administrative support: Greg Yothers, Daniel J. Sargent
Provision of study materials or patients: Eric Van Cutsem, Daniel G. Haller
Collection and assembly of data: Erin Green, Greg Yothers, Aimery de Gramont, Christopher J. Twelves, Leonard B. Saltz, Daniel G. Haller, Daniel J. Sargent
Data analysis and interpretation: Nadine J. McCleary, Erin Green, Aimery de Gramont, Eric Van Cutsem, Michael O'Connell, Daniel J. Sargent
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 3.Pasetto LM, Monfardini S. Colorectal cancer screening in elderly patients: When should be more useful? Cancer Treat Rev. 2007;33:528–532. doi: 10.1016/j.ctrv.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 5.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 6.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: Results from NSABP C-07. J Clin Oncol. 2007;25:2198–2204. doi: 10.1200/JCO.2006.08.2974. [DOI] [PubMed] [Google Scholar]
- 7.Lembersky BC, Wieand HS, Petrelli NJ, et al. Oral uracil and tegafur plus leucovorin compared with intravenous fluorouracil and leucovorin in stage II and III carcinoma of the colon: Results from National Surgical Adjuvant Breast and Bowel Project Protocol C-06. J Clin Oncol. 2006;24:2059–2064. doi: 10.1200/JCO.2005.04.7498. [DOI] [PubMed] [Google Scholar]
- 8.Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- 9.McCleary NJ, Meyerhardt J, Green E, et al. Impact of older age on the efficacy of newer adjuvant therapies in > 12,500 patients (pts) with stage II/III colon cancer: Findings from the ACCENT database. J Clin Oncol. 2009;27(suppl; abstr 4010):170s. doi: 10.1200/JCO.2013.49.6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan fluorouracil plus leucovorin is not superior to fluorouracil plus leucovorin alone as adjuvant treatment for stage III colon cancer: Results of CALGB 89803. J Clin Oncol. 2007;25:3456–3461. doi: 10.1200/JCO.2007.11.2144. [DOI] [PubMed] [Google Scholar]
- 11.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol. 2006;24:4085–4091. doi: 10.1200/JCO.2006.06.9039. [DOI] [PubMed] [Google Scholar]
- 13.Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: Combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008;26:1443–1451. doi: 10.1200/JCO.2007.14.0509. [DOI] [PubMed] [Google Scholar]
- 14.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taieb J, Puig PL, Bedenne L. Cetuximab plus FOLFOX-4 for fully resected stage III colon carcinoma: Scientific background and the ongoing PETACC-8 trial. Expert Rev Anticancer Ther. 2008;8:183–189. doi: 10.1586/14737140.8.2.183. [DOI] [PubMed] [Google Scholar]
- 16.de Gramont A, Tournigand C, André T, et al. Targeted agents for adjuvant therapy of colon cancer. Semin Oncol. 2006;33(suppl 11):S42–S45. doi: 10.1053/j.seminoncol.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Alberts SR, Sinicrope FA, Grothey A. N0147: a randomized phase III trial of oxaliplatin plus 5-fluorouracil/leucovorin with or without cetuximab after curative resection of stage III colon cancer. Clin Colorectal Cancer. 2005;5:211–213. doi: 10.3816/ccc.2005.n.033. [DOI] [PubMed] [Google Scholar]
- 18.Goldberg R, Sargent D, Thibodeau S, et al. Adjuvant mFOLFOX6 plus or minus cetuximab (Cmab) in patients (pts) with KRAS mutant (m) resected stage III colon cancer (CC): NCCTG intergroup phase III trial N0147. J Clin Oncol. 2010;28(suppl; abstr 3508):262s. [Google Scholar]
- 19.De Gramont A, Van Cutsem D, Tabernero J, et al. AVANT: Results from a randomized, three-arm multinational phase III study to investigate bevacizumab with either XELOX or FOLFOX4 versus FOLFOX4 alone as adjuvant treatment for colon cancer. J Clin Oncol. 2011;(suppl; abstr 362):29. [Google Scholar]
- 20.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29:1465–1471. doi: 10.1200/JCO.2010.33.6297. [DOI] [PubMed] [Google Scholar]
- 21.Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: An Italian Group for Geriatric Oncology study. J Clin Oncol. 2002;20:494–502. doi: 10.1200/JCO.2002.20.2.494. [DOI] [PubMed] [Google Scholar]
- 22.Wedding U, Röhrig B, Klippstein A, et al. Co-morbidity and functional deficits independently contribute to quality of life before chemotherapy in elderly cancer patients. Support Care Cancer. 2007;15:1097–1104. doi: 10.1007/s00520-007-0228-9. [DOI] [PubMed] [Google Scholar]
- 23.Wedding U, Röhrig B, Klippstein A, et al. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007;133:945–950. doi: 10.1007/s00432-007-0233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. J Clin Oncol. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 25.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective 500 patient multicenter study. J Clin Oncol. 2010;28(suppl; abstr 9001):636s. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients: Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 27.Eagles JM, Beattie JA, Restall DB, et al. Relation between cognitive impairment and early death in the elderly. BMJ. 1990;300:239–240. doi: 10.1136/bmj.300.6719.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landi F, Zuccalà G, Gambassi G, et al. Body mass index and mortality among older people living in the community. J Am Geriatr Soc. 1999;47:1072–1076. doi: 10.1111/j.1532-5415.1999.tb05229.x. [DOI] [PubMed] [Google Scholar]
- 29.Lee Y. The predictive value of self assessed general, physical, and mental health on functional decline and mortality in older adults. J Epidemiol Community Health. 2000;54:123–129. doi: 10.1136/jech.54.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]