Abstract
Purpose
Chemotherapy-induced peripheral neuropathy (CIPN) is common and leads to suboptimal treatment. Acetyl-L-carnitine (ALC) is a natural compound involved in neuronal protection. Studies have suggested ALC may be effective for the prevention and treatment of CIPN.
Patients and Methods
A 24-week randomized double-blind trial comparing ALC (3,000 mg per day) with placebo in women undergoing adjuvant taxane-based chemotherapy was conducted. The primary objective was to determine if ALC prevents CIPN as measured by the 11-item neurotoxicity (NTX) component of the Functional Assessment of Cancer Therapy (FACT) –Taxane scale at 12 weeks. Secondary objectives included changes in 24-week end points, functional status (FACT–Trial Outcome Index [TOI]), fatigue (Functional Assessment of Chronic Illness Therapy [FACIT] –Fatigue), and NTX grade.
Results
A total of 409 patients were evaluable (208 received ALC; 201, placebo). In a multivariate linear regression, week-12 scores were 0.9 points lower (more CIPN) with ALC than placebo (95% CI, −2.2 to 0.4; P = .17), whereas week-24 scores were 1.8 points lower with ALC (95% CI, −3.2 to −0.4; P = .01). Patients receiving ALC were more likely to have a > 5-point decrease in FACT-NTX scores (38% v 28%; P = .05), and FACT-TOI scores were 3.5 points lower with ALC (P = .03). Grade 3 to 4 neurotoxicity was more frequent in the ALC arm (eight v one). No differences between arms were observed for FACIT-Fatigue or other toxicities. Serum carnitine level increased with ALC but remained stable with placebo.
Conclusion
There was no evidence that ALC affected CIPN at 12 weeks; however, ALC significantly increased CIPN by 24 weeks. This is the first study to our knowledge showing that a nutritional supplement increased CIPN. Patients should be discouraged from using supplements without proven efficacy.
INTRODUCTION
Chemotherapy-induced peripheral neuropathy (CIPN) is a common and disabling adverse effect of some widely used anticancer agents, including taxanes, vinca alkaloids, platinum salts, epothilones, and thalidomide. This has important clinical significance because neurotoxicity (NTX) may be a dose-limiting adverse effect leading to early treatment discontinuation. Large clinical trials have demonstrated a survival benefit with taxanes administered in the adjuvant setting for breast cancer.1 Unfortunately, taxanes are associated with CIPN, which is predominantly a distal sensory neuropathy characterized by pain, paresthesias, and reduced functional capacity.2 Up to 80% of patients treated with taxanes report some neuropathy, and approximately 25% to 30% report severe neuropathy.3–5 The symptoms usually begin during chemotherapy, can increase after completion of therapy, and may have a waxing and waning course.
The only approach to managing the symptoms of CIPN has been limiting total dose, reducing individual doses, or discontinuing therapy. However, once significant CIPN develops, these measures may be ineffective. Symptomatic treatments for CIPN include antidepressants, anticonvulsants, and nonnarcotic and narcotic analgesics.6 However, results from drug treatment studies have generally been conflicting, with small sample sizes and limitations resulting from adverse effects. None of these studies have prospectively evaluated these agents for the prevention of the long-term effects of CIPN in the adjuvant breast cancer treatment setting.
Acetyl-L-carnitine (ALC) is a natural compound that has a role in intermediary metabolism. In mitochondria, it ensures the availability of acetyl-CoA for the elimination of toxic metabolic byproducts. ALC is involved in the acetylation of tubulin,7 which plays an important role in neuronal protection.8 In animal models of CIPN, ALC improved sensory neuropathy and reduced the severity of neuropathy development.9 ALC has also been shown to improve neuropathy in patients with diabetes10,11 and HIV.12 In a single-arm phase II study, 25 patients with grade 2 to 3 CIPN were treated with 3 g of ALC daily.13 Sensory neuropathy improved in 15 patients (60%), with six patients improving by 2 points and nine patients improving by 1 point, according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) 5-point scale. Of 14 patients with motor neuropathy, 11 patients (79%) improved. Toxicity was limited to mild nausea.13 On the basis of these preliminary data, we conducted a prospective randomized double-blind placebo-controlled clinical trial to test the hypothesis that ALC prevents symptoms of CIPN in women undergoing taxane-based adjuvant therapy for early-stage breast cancer.
PATIENTS AND METHODS
The study was activated in September 2009 and closed to accrual in February 2011. Patients were informed of the investigational nature of the study and signed informed consent. The study was conducted after appropriate approval by individual institutional review boards in compliance with the provisions of the Declaration of Helsinki and Good Clinical Practice guidelines.
Patient Characteristics
Women age > 18 years with a history of stage I to III breast cancer scheduled to undergo adjuvant taxane-based chemotherapy with one of the following standard regimens were included: paclitaxel 80 mg/m2 once per week for 12 cycles, paclitaxel 175 mg/m2 once every 2 weeks for four cycles, paclitaxel 175 mg/m2 once every 2 weeks for six cycles, docetaxel 75 mg/m2 once every 3 weeks for four cycles, or docetaxel 75 mg/m2 once every 3 weeks for six cycles. Patients were required to have a Zubrod performance status of 0 to 2. Serum creatinine ≤ 2.5× the institutional upper limit of normal was required. Patients with a history of diabetes, history of peripheral neuropathy resulting from other causes, or seizure disorder were excluded. Patients receiving any of the following medications used to treat CIPN were excluded: vitamin E, glutamine, gabapentin, nortriptyline, amitriptyline or duloxetine hydrochloride (HCL), and other nutritional supplements used to treat CIPN.
Study Design
We conducted a randomized double-blind multicenter trial comparing 3,000 mg per day (six capsules) of ALC (Thorne Research, Sandpoint, ID) versus matching placebo daily for 24 weeks. Each active capsule contained ALC HCL 590 mg and cellulose 10 mg. The 590 mg of ALC HCL provided 500 mg of ALC. Each placebo capsule contained 600 mg of cellulose. Patient randomization was stratified by planned taxane-based chemotherapy treatment and age (< 60 v ≥ 60 years). Study drug was initiated at the start of taxane chemotherapy. After completion of the study, the capsules were retested for stability and composition. Serum was collected at baseline and week 12, stored at −80°C, and tested centrally for carnitine levels (Appendix, online only).
Outcome Measures
Patients were assessed at baseline (before starting taxane therapy and ALC or placebo) for peripheral neuropathy using the 11-item NTX component of the Functional Assessment of Cancer Therapy (FACT) –Taxane (FACT-NTX) symptom module as a continuous measure. A lowering in the FACT-NTX score (worse CIPN) > 10% or 5 points is considered clinically significant.14 For secondary objectives, functional status was measured with the FACT-Taxane Trial Outcome Index (TOI; FACT-TOI) score.14 Fatigue was measured with the 13-item Functional Assessment of Chronic Illness Therapy (FACIT) –Fatigue symptom module score; lower scores reflect more fatigue.14,15 Patients were assessed at baseline and weeks 12, 24, 36, 52, and 104. Collection of long-term follow-up data is ongoing.
National Cancer Institute CTCAE Version 3.0 Neuropathy
Clinician grading of adverse events on a scale of 0 to 5 was performed for motor neuropathy (asymptomatic weakness, symptomatic weakness, and weakness interfering with activities of daily living) and sensory neuropathy (asymptomatic, loss of deep tendon reflex, sensory alteration or paresthesias, and sensory alteration or paresthesias interfering with activities of daily living). Other adverse events were measured similarly.
Statistical Considerations
The primary end point was the absolute difference between arms in the change in FACT-NTX score from baseline to 12 weeks. Power estimates were based on a two-arm normal design. Assuming a standard deviation of 9.4, a sample size of 380 patients (190 per arm) was sufficient for 80% power to detect a 3-point difference in the change in FACT-NTX subscale between groups, using a two-sided test with α = .05 and accounting for up to a 10% dropout rate and 5% nonadherence. Secondary objectives were to determine differences in FACT-TOI, FACIT-Fatigue, and neurotoxicity grade at 12 and 24 weeks.
The primary analysis adjusted for baseline FACT-NTX score and stratification factors. Linear and logistic regression (10%; 5-point change) analyses were used in the multivariate analysis.
RESULTS
Accrual, Eligibility, and Evaluability
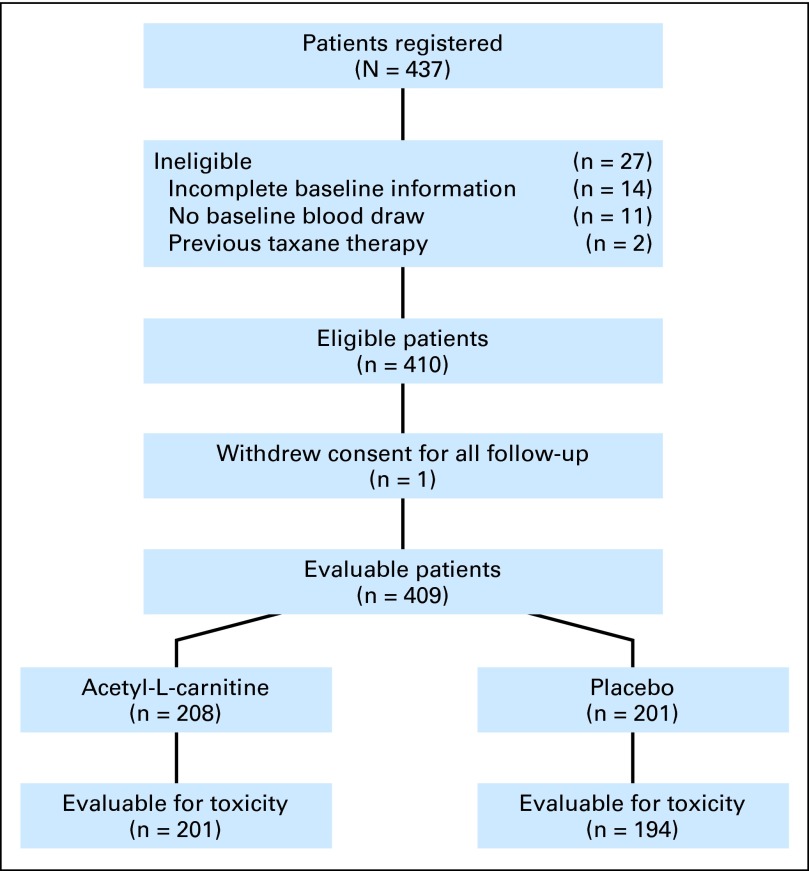
From September 2009 to February 2011, 437 patients were accrued. Twenty-seven patients were ineligible for the following reasons: prior taxane therapy (n = 2), no baseline blood draw (n = 11), and incomplete baseline information (n = 14). One eligible patient who withdrew consent for all study follow-up was not analyzable for any end point, leaving 409 evaluable patients, of whom 208 received active agent and 201 received placebo (Fig 1). There was no differential dropout or difference in drug compliance between arms as assessed by pill counts.
Fig 1.
CONSORT diagram.
Patient Characteristics
Patient characteristics by intervention assignment are listed in Table 1. No notable imbalances by arm were observed by age, ethnicity, race, planned taxane-based chemotherapy treatment, performance status, stage, or serum carnitine level.
Table 1.
Patient Demographic and Clinical Characteristics
| Characteristic | ALC (n = 208) |
Placebo (n = 201) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 52 | 50 | ||
| Range | 27-80 | 26-77 | ||
| < 60 | 149 | 72 | 143 | 71 |
| ≥ 60 | 59 | 28 | 58 | 29 |
| Hispanic | ||||
| Yes | 21 | 10 | 15 | 7 |
| No | 181 | 87 | 177 | 88 |
| Unknown | 6 | 3 | 9 | 4 |
| Race | ||||
| White | 160 | 77 | 165 | 82 |
| Black | 17 | 8 | 20 | 10 |
| Asian | 12 | 6 | 8 | 4 |
| Pacific Islander | 4 | 2 | 2 | 1 |
| Native American | 1 | 0 | 0 | 0 |
| Multiracial | 2 | 1 | 1 | 0 |
| Unknown | 12 | 6 | 5 | 2 |
| Treatment | ||||
| Paclitaxel once per week, 12 cycles | 73 | 35 | 68 | 34 |
| Paclitaxel every 2 weeks for four cycles | 48 | 23 | 51 | 25 |
| Paclitaxel every 2 weeks for six cycles | 3 | 1 | 5 | 2 |
| Docetaxel every 3 weeks for four cycles | 51 | 25 | 48 | 24 |
| Docetaxel every 3 weeks for six cycles | 33 | 16 | 29 | 14 |
| Performance status | ||||
| 0 | 156 | 75 | 146 | 73 |
| 1 | 51 | 25 | 54 | 27 |
| 2 | 1 | < 1 | 1 | < 1 |
| Breast cancer stage | ||||
| I | 57 | 27 | 50 | 25 |
| II | 110 | 53 | 107 | 54 |
| III | 41 | 20 | 43 | 21 |
| Serum carnitine level, ng/mL* | ||||
| Baseline† | ||||
| Mean | 5,562 | 5,447 | ||
| SD | 2,525 | 2,005 | ||
| 12 weeks‡ | ||||
| Mean | 7,176 | 5,493 | ||
| SD | 2,104 | 2,224 | ||
Abbreviations: ALC, acetyl-L-carnitine; SD, standard deviation.
Sample size (n = 206) with both baseline and week-12 carnitine scores (ALC, n = 108; placebo, n = 98).
Difference between baseline and 12-week carnitine level in ALC group (P < .001).
Difference between 12-week carnitine level in ALC and placebo groups (P < .001).
FACT-NTX Subscale Score
A total of 191 of the 208 evaluable patients in the ALC arm (92%) and 181 of the 201 eligible patients in the placebo arm (90%) underwent week-12 FACT-NTX assessments. The mean observed FACT-NTX subscale score was 5.2 points lower (worse CIPN) at 12 weeks compared with baseline in the intervention arm and 4.5 points lower in the placebo arm (Table 2). In a linear regression assessing week-12 subscale scores by treatment and adjusting for baseline score, planned taxane-based treatment type, and age, week-12 scores were 0.9 points lower in the ALC arm than in the placebo arm (95% CI, −2.2 to 0.4; P = .17; Table 3).
Table 2.
Mean FACT-NTX, Functional Status, and Fatigue Scores at 12 and 24 Weeks
| Analysis | Observed Baseline |
Observed |
Fitted |
P* | |||
|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | ||
| FACT-NTX† | |||||||
| Week-12 analysis (n = 372)‡ | .17 | ||||||
| ALC (n = 191) | 40.6 | 40.1-41.1 | 35.4 | 34.3-36.4 | 35.4 | 34.1-36.7 | |
| Placebo (n = 181) | 40.8 | 40.2-41.5 | 36.4 | 35.4-37.4 | 36.3 | 35.0-37.6 | |
| Week-24 analysis (n = 347)§ | .01 | ||||||
| ALC (n = 174) | 40.6 | 40.0-41.1 | 35.3 | 34.1-36.5 | 35.5 | 34.2-36.9 | |
| Placebo (n = 173) | 41.1 | 40.5-41.6 | 37.5 | 36.6-38.5 | 37.3 | 36.0-38.7 | |
| Functional status† | |||||||
| Week-12 analysis (n = 372)‡ | .92 | ||||||
| ALC (n = 191) | 99.3 | 97.4-101.3 | 91.9 | 89.5-94.2 | 92.1 | 89.3-94.9 | |
| Placebo (n = 181) | 99.9 | 97.7-102.1 | 92.5 | 90.1-95.0 | 92.3 | 89.4-95.1 | |
| Week-24 analysis (n = 347)§ | .03 | ||||||
| ALC (n = 174) | 100.0 | 98.0-102.0 | 95.1 | 92.5-97.7 | 95.2 | 92.2-98.3 | |
| Placebo (n = 173) | 100.1 | 98.0-102.3 | 98.9 | 96.5-101.2 | 98.7 | 95.7-101.8 | |
| Fatigue† | |||||||
| Week-12 analysis (n = 371)‡ | .20 | ||||||
| ALC (n = 191) | 37.3 | 35.7-38.9 | 36.4 | 34.9-37.9 | 36.6 | 34.7-38.6 | |
| Placebo (n = 180) | 37.9 | 36.2-39.5 | 35.5 | 33.9-37.1 | 35.3 | 33.4-37.3 | |
| Week-24 analysis (n = 348)§ | .51 | ||||||
| ALC (n = 174) | 37.6 | 35.9-39.3 | 39.3 | 37.9-40.7 | 39.5 | 37.6-41.3 | |
| Placebo (n = 174) | 38.1 | 36.4-39.8 | 40.3 | 38.7-41.8 | 40.1 | 38.2-42.0 | |
Abbreviations: ALC, acetyl-L-carnitine; FACT-NTX, neurotoxicity component of Functional Assessment of Cancer Therapy–Taxane scale.
From multivariate analysis adjusting for age, baseline FACT-NTX, and planned taxane treatment.
Higher scores reflect less neurotoxicity, better functional status, and less fatigue, respectively.
Among patients with week-12 scores only.
Among patients with week-24 scores only.
Table 3.
Multivariate Analyses Comparing FACT-NTX Scores Between ALC and Placebo Arms at 12 and 24 Weeks
| Linear Regression Analyses*† | Week-12 Analysis (n = 372) |
Week-24 Analysis (n = 347) |
||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | |
| Intercept | 7.26 | 0.38 to 14.14 | .04 | 4.35 | −3.61 to 12.31 | .28 |
| Treatment‡ | −0.91 | −2.21 to 0.39 | .17 | −1.80 | −3.16 to −0.43 | .01 |
| Baseline NTX score | 0.69 | 0.52 to 0.86 | < .001 | 0.82 | 0.62 to 1.01 | < .001 |
| Age (< 60 v ≥ 60 years)§ | −0.78 | −2.25 to 0.69 | .30 | −1.66 | −3.22 to −0.11 | .04 |
| Planned taxane treatment∥ | ||||||
| Paclitaxel once per week for 12 cycles | 0.00 | Referent | 0.00 | Referent | ||
| Paclitaxel every 2 weeks for four cycles | 0.20 | −1.50 to 1.90 | .82 | −0.10 | −1.90 to 1.69 | .91 |
| Paclitaxel every 2 weeks for six cycles | 1.62 | −3.61 to 6.85 | .54 | −0.06 | −5.37 to 5.25 | .98 |
| Docetaxel every 3 weeks for four cycles | 2.17 | 0.43 to 3.91 | .02 | 0.14 | −1.70 to 1.98 | .89 |
| Docetaxel every 3 weeks for six cycles | 2.60 | 0.52 to 4.68 | .02 | 0.41 | −1.79 to 2.61 | .72 |
| Logistic Regression Analyses¶ | Week-12 Analysis (n = 372) |
Week-24 Analysis (n = 347) |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |
| Treatment‡ | ||||||
| Placebo | 1.00 | Referent | 1.00 | Referent | ||
| ALC | 1.48 | 0.96 to 2.29 | .08 | 1.57 | 0.99 to 2.49 | .05 |
| Age, years§ | ||||||
| < 60 | 1.00 | Referent | 1.00 | Referent | ||
| ≥ 60 | 1.15 | 0.71 to 1.87 | .57 | 2.05 | 1.24 to 3.37 | .005 |
| Planned taxane treatment¶ | ||||||
| Paclitaxel once per week for 12 cycles | 1.00 | Referent | 1.00 | Referent | ||
| Paclitaxel every 2 weeks for four cycles | 0.87 | 0.50 to 1.50 | .61 | 1.07 | 0.59 to 1.93 | .84 |
| Paclitaxel every 2 weeks for six cycles | 0.75 | 0.13 to 4.29 | .75 | 0.41 | 0.05 to 3.63 | .42 |
| Docetaxel every 3 weeks for four cycles | 0.57 | 0.32 to 1.02 | .06 | 0.72 | 0.38 to 1.32 | .27 |
| Docetaxel every 3 weeks for six cycles | 0.49 | 0.24 to 0.99 | .05 | 1.02 | 0.50 to 2.06 | .96 |
Abbreviations: ALC, acetyl-L-carnitine; FACT-NTX, neurotoxicity component of Functional Assessment of Cancer Therapy–Taxane scale; NTX, neurotoxicity; OR, odds ratio.
Week-12 linear regression analysis represents planned primary end point analysis.
Separate linear regression models that evaluated interaction of treatment and baseline NTX score resulted in regression coefficients for interaction term of 0.18 (95% CI, −0.14 to 0.51; P = .27) for week-12 analysis and −0.05 (95% CI, −0.42 to 0.32; P = .80) for week-24 analysis.
Coded as placebo = 0; ALC = 1.
Coded as < 60 = 0; ≥ 60 = 1.
Coded as indicator variables, with weekly paclitaxel for 12 weeks as the reference group.
Dependent variable for logistic regression analyses was > 5-point decrease in FACT-NTX score at follow-up assessment time.
Multivariate analyses based on the change in score from baseline to week 12 yielded similar results (P = .23). No interactions were observed by age (P = .39) or baseline FACT-NTX score (P = .27). Also at 12 weeks, 38% of those receiving ALC had a > 5-point decrease in score compared with 30% receiving placebo (odds ratio [OR], 1.48; P = .08; Table 3).
A total of 174 of the 208 eligible patients in the intervention arm (84%) and 173 of the 201 eligible patients in the placebo arm (86%) underwent week-24 FACT-NTX assessments. The mean observed FACT-NTX subscale score was 5.3 points lower at 24 weeks in the intervention arm and 3.6 points lower in the placebo arm (Table 3). In the multivariate linear regression model, week-24 scores were 1.8 points lower in the ALC arm than in the placebo arm (95% CI, −0.4 to −3.2; P = .01), representing a statistically significant trend toward more self-reported neuropathy symptoms in the ALC arm (Table 3).
Multivariate analyses based on the change in score from baseline to week 24 yielded similar results (P = .02). No differential effects of intervention assignment on outcome were observed by age (P = .74) or baseline FACT-NTX level (P = .80). At 24 weeks, 38% of those receiving ALC had a > 5-point decrease in score compared with 28% receiving placebo (OR, 1.57; P = .05; Table 3).
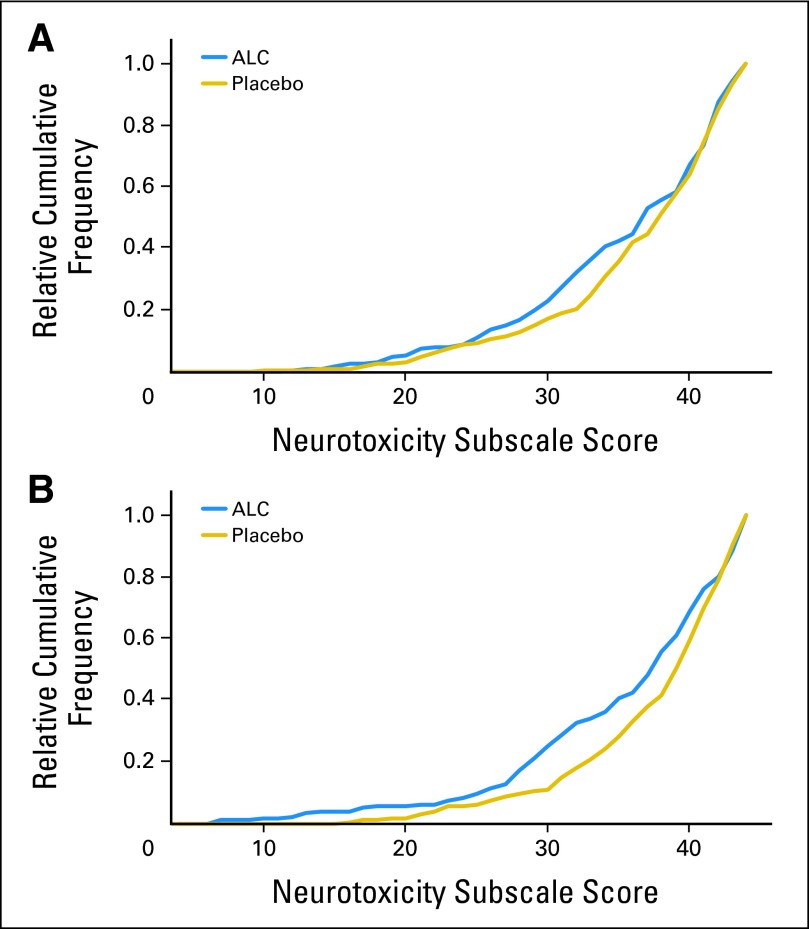
Cumulative Frequency of Week-12 and Week-24 FACT-NTX Scores
The relative cumulative frequencies of week-12 and week-24 FACT-NTX scores by arm are shown in Figures 2A and 2B, respectively. The separation between the curves indicates the shift in distribution between arms with respect to NTX score. There is a higher frequency of lower FACT-NTX scores for the ALC arm (worse CIPN), a difference that is maintained throughout the spectrum of scores. Thus, the shift in the distribution of scores by arm at week 24 is consistent across a wide range of scores and is not just because of a few extreme values influencing the results.
Fig 2.
Cumulative probability of neurotoxicity subscale for acetyl-L-carnitine (ALC) and placebo at (A) 12 and (B) 24 weeks.
Carnitine Levels
For the purposes of this analysis, patients in the lowest quartile carnitine level were defined as carnitine deficient. There was no difference by arm in the proportion of patients who were carnitine deficient at baseline (P = .87). The addition of baseline carnitine deficiency status to the multivariate model had minimal impact on results by treatment. Week-12 scores were 0.9 points lower in the ALC arm than in the placebo arm (95% CI, −2.5 to 0.6; P = .23). Week-24 scores were −2.3 points lower in the ALC arm than in the placebo arm (95% CI, −4.0 to −0.6; P = .007).
Functional Status
Functional status was assessed using the FACT-TOI score. In the ALC arm, the mean baseline FACT-TOI score was 99.3, and the mean week-12 score was 91.9—a drop of 7.4 points (decreased functional status). In the placebo arm, there was a similar drop of 7.4 points. In adjusted linear regression, week-12 scores were 0.2 points lower in the ALC arm than in the placebo arm (95% CI, −3.0 to 2.7; P = .92; Table 2). Week-24 scores were 3.5 points lower in the ALC arm than in the placebo arm (95% CI, −6.5 to −0.4; P = .03), representing a statistically significant decrease in functional status in the ALC arm.
Fatigue
Fatigue was assessed using the FACT-Fatigue score. In the ALC arm, the mean FACT-Fatigue score dropped 0.9 points (worsening fatigue). In the placebo arm, the mean FACT-Fatigue score dropped 2.4 points. In adjusted linear regression, there was no difference by arm in week-12 scores (ALC minus placebo, 1.3 points; 95% CI, −0.7 to 3.3; P = .20; Table 2).
At 24 weeks, in the ALC arm, the mean FACT-Fatigue score had increased 1.7 points; in the placebo arm, the score had increased 2.2 points. In adjusted linear regression, there was no difference by arm in week-24 scores (ALC minus placebo, −0.6 points; 95% CI, −2.5 to 1.3; P = .51).
Adverse Events
Two patients experienced grade 3 toxicities resulting from study treatment; one patient in the ALC arm experienced grade 3 vomiting, and one patient in the placebo arm experienced grade 3 insomnia. There were eight cases of grade 3 to 4 neuropathy reported in the ALC arm and one in the placebo arm resulting from taxane (P = .46; Table 4). There was no difference in treatment delays or dose reductions of taxane-based chemotherapy between the arms.
Table 4.
No. of Patients With Given Type and Grade of Adverse Event*
| Adverse Event | ALC (n = 202) |
Placebo (n = 194) |
P† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade |
Grade |
||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 0 | 1 | 2 | 3 | 4 | 5 | ||
| Insomnia | 180 | 16 | 6 | 0 | 0 | 0 | 170 | 18 | 5 | 1 | 0 | 0 | .76 |
| Involuntary movement | 194 | 7 | 1 | 0 | 0 | 0 | 191 | 3 | 0 | 0 | 0 | 0 | .25 |
| Nausea | 181 | 16 | 5 | 0 | 0 | 0 | 172 | 16 | 6 | 0 | 0 | 0 | .89 |
| Vomiting | 195 | 3 | 3 | 1 | 0 | 0 | 190 | 1 | 3 | 0 | 0 | 0 | .59 |
| Neurotoxicity | |||||||||||||
| Motor neuropathy | 193 | 3 | 4 | 2 | 0 | 0 | 184 | 5 | 5 | 0 | 0 | 0 | .93 |
| Sensory neuropathy | 125 | 57 | 14 | 5 | 1 | 0 | 112 | 68 | 13 | 1 | 0 | 0 | .46 |
Abbreviation: ALC, acetyl-L-carnitine.
Investigators were instructed to document only these adverse events related to blinded protocol treatment (ALC or placebo) and neuropathy resulting from taxane treatment. Other toxicities related to taxane regimen were not documented.
Comparing grade ≥ 1.
DISCUSSION
Despite the supportive evidence from preclinical and phase II studies that ALC may be effective for both treatment and prevention of taxane-induced symptoms of CIPN, in this large placebo-controlled trial, we found no evidence that ALC had a positive impact on the prevention of CIPN at 12 weeks in women undergoing adjuvant breast cancer therapy. Unexpectedly, we did find that ALC actually increased CIPN and decreased functional status at 24 weeks, as measured by both patient-reported outcomes (FACT-NTX, FACT-TOI) and the CTCAE motor and sensory NTX scales. Supplementation resulted in an increase in serum carnitine in the ALC arm but no change in the placebo arm over time. No differences were observed between groups with regard to fatigue or other adverse events.
The need for well-tolerated effective therapy for CIPN is a high priority in oncology because an increasing number of effective cancer agents result in dose-limiting neurologic toxicity. Despite efforts by many investigators testing a number of promising agents, results from prior studies evaluating tricyclic antidepressants, anticonvulsants, amifostine, and a variety of natural products such as glutamine, vitamin E, and glutathione have been disappointing.16–19 A recent trial evaluating a topical combination of baclofen, amitriptyline, and ketamine for treating CIPN demonstrated a trend toward improvement in sensory neuropathy and a statistically significant improvement in motor neuropathy, although the effect size was small.20 Also of interest, an unblinded pilot trial recently reported that a patient-specific electrostimulation device (MC5-A Calmare; Competetive Technologies, Wakefield, RI) reduced neuropathic pain by 59%.21 Additional studies are ongoing to better define the benefit of this new device. Limitations from prior controlled trials include enrollment of patients with advanced-stage cancer, heterogeneous chemotherapy exposure, and nonspecific outcome measures.
Unlike prior studies that failed to show benefit from the intervention, we found that at 24 weeks, there was a significant detrimental effect of the ALC intervention, with worse scores on the FACT-NTX and FACT-TOI and more grade 3 to 4 NTX. With regard to clinically meaningful results, 38% of those receiving ALC had a > 5-point decrease in FACT-NTX score compared with 28% of those receiving placebo (OR, 1.57; P = .05). Furthermore, baseline carnitine deficiency status was not associated with the development of CIPN in either arm of the study. We questioned why this occurred. In addition to their role in the acetylation of tubulin, carnitines exert a substantial antioxidant action, thereby providing a protective effect against oxidative stress.22 Reactive oxygen species cause damage to DNA and proteins and trigger apoptosis, which can result in tumor- and normal-cell death. Antioxidants may also block treatment-generated reactive oxygen species, leading to lower levels of cell damage.22,23 Several other clinical trials of nutritional supplements that showed promise in preclinical and observational studies were found to be detrimental in clinical trials. Examples include increases in lung cancer among men receiving beta carotene,24 second primary cancers with alpha tocopherol,25 and prostate cancer risk with vitamin E.26 We will be conducting a variety of correlative studies to better understand the mechanism of CIPN.
Use of complementary medicine, particularly antioxidant supplements, is widespread among patients with cancer.27 Among 663 participants in the Long Island Breast Cancer Study who completed follow-up interviews, 401 (60.5%) reported using antioxidants during adjuvant treatment, and of these, 278 women (69.3%) used high doses.28 Americans spend > $12 billion per year on dietary supplements.29 This is of particular concern, given the paucity of evidence of benefit and the increasing evidence of harm.
Use of ALC was based on numerous animal studies using an established CIPN rat model showing direct neuronal benefits of ALC for preventing both taxane and oxaliplatin NTX.30–33 These studies showed that the incidence of swollen and vacuolated C-fiber mitochondria in peripheral nerve axons was decreased with the administration of ALC and that ALC prevented slowing of sensory neurons as measured by nerve conduction studies.30,31 It is possible that we would have seen similar results if nerve biopsies or nerve conduction studies had been performed. We relied on patient-reported outcomes and believe that these are clinically meaningful end points known to correlate to objective measures.
Given the strength of preclinical evidence, one may question if methodologic choices in the study design contributed to these unexpected findings. First, we chose a dose of 1,000 mg three times per day. This was based on a dose-escalation study that found the supplement L-carnitine to be safe in doses up to 3,000 mg per day, with improvements seen in measures of fatigue and depression and no adverse effects observed.34 It was also based on the unblinded phase II treatment trial that tested oral ALC (1,000 mg three time per day) for 8 weeks in 25 patients with CIPN and found the total neuropathy score improved in 23 patients (92%).13 However, there was no placebo arm in this study. It is not known if differences between the metabolites of L-carnitine and ALC may have different neuroprotectant effects. One may also question the patient-reported outcome measure we used. Cella et al15 documented the validity of the FACT-NTX questionnaire in 230 patients with stage IIIB to IV lung cancer receiving carboplatin and paclitaxel and in patients with colon cancer treated with oxaliplatin in the NSABP (National Surgical Adjuvant Breast and Bowel Project) C-07 trial.35 It is unclear whether other measures, like the EORTC (European Organisation for Research and Treatment of Cancer) CIPN-20,36 may have been more sensitive. Lastly, the negative finding in the FACT-NTX scores at week 24 did not represent the primary end point; rather, it was the main secondary end point, and therefore, this may have been the result of chance. However, in retrospect, the 24-week end point may have been a more appropriate primary end point because a majority of patients had not completed taxane treatment 12 weeks from registration.
In summary, we found no evidence that ALC had a positive impact on the prevention of taxane-induced CIPN at 12 weeks. Unexpectedly, we found ALC had actually increased CIPN at 24 weeks. To our knowledge, this is the first study to show a detrimental effect of a nutritional supplement on the development of taxane-related CIPN. We do not know if similar results would be seen with other neurotoxic drugs or in the setting of CIPN treatment as opposed to prevention. Currently, there are no proven therapies for the treatment or prevention of CIPN; however, determining factors that exacerbate CIPN may provide clues to its mechanism and ways of preventing it. Patients should be discouraged from using ALC and other nutritional supplements that do not have proven efficacy, given the potential for harm.
Appendix
Methods for Carnitine Analysis
Plasma-free carnitine (C0 acylcarnitine) levels were measured using ultra performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) at both baseline and week 12 using the Agilent 6430 MS/MS and 1290 UPLC (Agilent, Santa Clara, CA). Plasma 50 μL was spiked with an internal standard mixture containing D9-carnitine. After precipitation of the proteins with acetonitrile/methanol (2:1), the supernatant was evaporated to dryness and resuspended in 20% methanol. Carnitine was separated using a water/methanol gradient (both containing 10 mmol/L heptaluorobutyric acid and 10 mmol/L ammonium acetate) with a Poroshell 120 EC-C18 2.7um 2.1 × 50 mm column (Agilent). Carnitine was quantified by positive electrospray ionization in the multiple-reaction monitoring mode relative to an external standard curve.
Footnotes
Supported by an Advanced Clinical Research Award from the American Society of Clinical Oncology Conquer Cancer Foundation, by the Avon Foundation, by Grant No. CA037429 from the National Cancer Institute Division of Cancer Prevention, and by Clinical and Translational Science Awards Grant No. ULR000040.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information: NCT00775645.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Dawn L. Hershman, Joseph M. Unger, Katherine D. Crew, Lori M. Minasian, Danika L. Lew, Heather Greenlee, Siu-Fun Wong, Kathy S. Albain
Financial support: Dawn L. Hershman
Administrative support: Dawn L. Hershman, Gabriel N. Hortobagyi
Provision of study materials or patients: Dawn L. Hershman, Heather Greenlee, Louis Fehrenbacher
Collection and assembly of data: Dawn L. Hershman, Joseph M. Unger, Danielle Awad, Danika L. Lew, Louis Fehrenbacher
Data analysis and interpretation: Dawn L. Hershman, Joseph M. Unger, Katherine D. Crew, Lori M. Minasian, Carol M. Moinpour, Lisa Hansen, Louis Fehrenbacher, James L. Wade III, Gabriel N. Hortobagyi, Frank L. Meyskens, Kathy S. Albain
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Henderson IC, Berry DA, Demetri GD, et al. Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol. 2003;21:976–983. doi: 10.1200/JCO.2003.02.063. [DOI] [PubMed] [Google Scholar]
- 2.Pazdur R, Kudelka AP, Kavanagh JJ, et al. The taxoids: Paclitaxel (Taxol) and docetaxel (Taxotere) Cancer Treat Rev. 1993;19:351–386. doi: 10.1016/0305-7372(93)90010-o. [DOI] [PubMed] [Google Scholar]
- 3.Hershman DL, Weimer LH, Wang A, et al. Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Res Treat. 2011;125:767–774. doi: 10.1007/s10549-010-1278-0. [DOI] [PubMed] [Google Scholar]
- 4.Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387. doi: 10.1200/JCO.2006.06.5391. [DOI] [PubMed] [Google Scholar]
- 6.Hegarty A, Portenoy RK. Pharmacotherapy of neuropathic pain. Semin Neurol. 1994;14:213–224. doi: 10.1055/s-2008-1041080. [DOI] [PubMed] [Google Scholar]
- 7.Bieber LL. Carnitine. Annu Rev Biochem. 1988;57:261–283. doi: 10.1146/annurev.bi.57.070188.001401. [DOI] [PubMed] [Google Scholar]
- 8.Kelly GS. L-carnitine: Therapeutic applications of a conditionally-essential amino acid. Altern Med Rev. 1998;3:345–360. [PubMed] [Google Scholar]
- 9.Pisano C, Pratesi G, Laccabue D, et al. Paclitaxel and cisplatin-induced neurotoxicity: A protective role of acetyl-L-carnitine. Clin Cancer Res. 2003;9:5756–5767. [PubMed] [Google Scholar]
- 10.De Grandis D, Minardi C. Acetyl-L-carnitine (levacecarnine) in the treatment of diabetic neuropathy: A long-term, randomised, double-blind, placebo-controlled study. Drugs R D. 2002;3:223–231. doi: 10.2165/00126839-200203040-00001. [DOI] [PubMed] [Google Scholar]
- 11.Di Giulio AM, Gorio A, Bertelli A, et al. Acetyl-L-carnitine prevents substance P loss in the sciatic nerve and lumbar spinal cord of diabetic animals. Int J Clin Pharmacol Res. 1992;12:243–246. [PubMed] [Google Scholar]
- 12.Famularo G, Moretti S, Marcellini S, et al. Acetyl-carnitine deficiency in AIDS patients with neurotoxicity on treatment with antiretroviral nucleoside analogues. AIDS. 1997;11:185–190. doi: 10.1097/00002030-199702000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Bianchi G, Vitali G, Caraceni A, et al. Symptomatic and neurophysiological responses of paclitaxel- or cisplatin-induced neuropathy to oral acetyl-L-carnitine. Eur J Cancer. 2005;41:1746–1750. doi: 10.1016/j.ejca.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 14.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: Development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 15.Cella D, Peterman A, Hudgens S, et al. Measuring the side effects of taxane therapy in oncology: The Functional Assessment of Cancer Therapy-Taxane (FACT-taxane) Cancer. 2003;98:822–831. doi: 10.1002/cncr.11578. [DOI] [PubMed] [Google Scholar]
- 16.Kottschade LA, Sloan JA, Mazurczak MA, et al. The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: Results of a randomized phase III clinical trial. Support Care Cancer. 2011;19:1769–1777. doi: 10.1007/s00520-010-1018-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rao RD, Michalak JC, Sloan JA, et al. Efficacy of gabapentin in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled, crossover trial (N00C3) Cancer. 2007;110:2110–2118. doi: 10.1002/cncr.23008. [DOI] [PubMed] [Google Scholar]
- 18.Rao RD, Flynn PJ, Sloan JA, et al. Efficacy of lamotrigine in the management of chemotherapy-induced peripheral neuropathy: A phase 3 randomized, double-blind, placebo-controlled trial, N01C3. Cancer. 2008;112:2802–2808. doi: 10.1002/cncr.23482. [DOI] [PubMed] [Google Scholar]
- 19.Moore DH, Donnelly J, McGuire WP, et al. Limited access trial using amifostine for protection against cisplatin- and three-hour paclitaxel-induced neurotoxicity: A phase II study of the Gynecologic Oncology Group. J Clin Oncol. 2003;21:4207–4213. doi: 10.1200/JCO.2003.02.086. [DOI] [PubMed] [Google Scholar]
- 20.Barton DL, Wos EJ, Qin R, et al. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Cancer. 2011;19:833–841. doi: 10.1007/s00520-010-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith TJ, Coyne PJ, Parker GL, et al. Pilot trial of a patient-specific cutaneous electrostimulation device (MC5-A Calmare®) for chemotherapy- induced peripheral neuropathy. J Pain Symptom Manage. 2010;40:883–891. doi: 10.1016/j.jpainsymman.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seifried HE, Anderson DE, Sorkin BC, et al. Free radicals: The pros and cons of antioxidants—Executive summary report. J Nutr. 2004;134:3143S–3163S. doi: 10.1093/jn/134.11.3143S. [DOI] [PubMed] [Google Scholar]
- 23.Norman HA, Butrum RR, Feldman E, et al. The role of dietary supplements during cancer therapy. J Nutr. 2003;133(suppl 1):3794S–3799S. doi: 10.1093/jn/133.11.3794S. [DOI] [PubMed] [Google Scholar]
- 24.The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers: The Alpha-Tocopherol, Beta Carotene Cancer Prevention Study Group. N Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 25.Bairati I, Meyer F, Gélinas M, et al. A randomized trial of antioxidant vitamins to prevent second primary cancers in head and neck cancer patients. J Natl Cancer Inst. 2005;97:481–488. doi: 10.1093/jnci/dji095. [DOI] [PubMed] [Google Scholar]
- 26.Klein EA, Thompson IM, Jr, Tangen CM, et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2011;306:1549–1556. doi: 10.1001/jama.2011.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassileth BR, Vickers AJ. High prevalence of complementary and alternative medicine use among cancer patients: Implications for research and clinical care. J Clin Oncol. 2005;23:2590–2592. doi: 10.1200/JCO.2005.11.922. [DOI] [PubMed] [Google Scholar]
- 28.Greenlee H, Gammon MD, Abrahamson PE, et al. Prevalence and predictors of antioxidant supplement use during breast cancer treatment: The Long Island Breast Cancer Study Project. Cancer. 2009;115:3271–3282. doi: 10.1002/cncr.24378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nesheim MC. What is the research base for the use of dietary supplements? Public Health Nutr. 1999;2:35–38. doi: 10.1017/s136898009900004x. [DOI] [PubMed] [Google Scholar]
- 30.Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng H, Xiao WH, Bennett GJ. Functional deficits in peripheral nerve mitochondria in rats with paclitaxel- and oxaliplatin-evoked painful peripheral neuropathy. Exp Neurol. 2011;232:154–161. doi: 10.1016/j.expneurol.2011.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao W, Naso L, Bennett GJ. Experimental studies of potential analgesics for the treatment of chemotherapy-evoked painful peripheral neuropathies. Pain Med. 2008;9:505–517. doi: 10.1111/j.1526-4637.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 33.Jin HW, Flatters SJ, Xiao WH, et al. Prevention of paclitaxel-evoked painful peripheral neuropathy by acetyl-L-carnitine: Effects on axonal mitochondria, sensory nerve fiber terminal arbors, and cutaneous Langerhans cells. Exp Neurol. 2008;210:229–237. doi: 10.1016/j.expneurol.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cruciani R, Dvorkin E, Homel P, et al. L-Carnatine supplementation improves fatigue, mood and sleep in cancer patients with fatigue and carnatine deficiency. J Clin Oncol. 2006;24(suppl):490s. abstr 8588. [Google Scholar]
- 35.Kopec J, Land S, Cecchini R. Validation of self-reported neurotoxicity scale in patients with operable colon cancer receiving oxaliplatin. J Supp Oncol. 2006;4:W1–W8. [Google Scholar]
- 36.Postma TJ, Aaronson NK, Heimans JJ, et al. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: The QLQ-CIPN20. Eur J Cancer. 2005;41:1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]