Abstract
Introduction
Fast delivery of food to the terminal ileum is thought to be pathophysiologically responsible for type 2 diabetes remission after obesity surgery. Imitating this effect, ileal transposition (IT) is designed as initiating diabetes remission for non-obese patients.
Aim
To date, it is not clear which length of the transposed segment achieves the best glucose lowering results. As previous rodent data mostly rely on a 10 cm IT, the current study evaluated a long segment IT (20 cm) in the diabetic obese Zucker rat.
Material and methods
Twenty male diabetic obese Zucker rats (Crl:ZUC-Leprfa) were randomly assigned to undergo either a long segment (20 cm; ∼ 50% of ileum) IT or sham surgery. Glucose control was determined by an oral glucose tolerance test (OGTT) on day −7, 0, 14 and 20. Analysis of the incretin hormones glucagon-like peptide 1 (GLP-1), peptide YY (PYY) and insulin was included in the first and third OGTT.
Results
Ileal transposition animals showed an early improvement of glucose control after 14 days (area under the curve: IT vs. baseline 314.7 ±229.0 mmol/l × min vs. 564.6 ±268.5 mmol/l × min; p < 0.05). Compared to sham animals, glucose-stimulated GLP-1 and PYY levels were raised (5.75 ±3.73 pmol/l vs. 18.52 ±14.22 pmol/l, p < 0.05; 129.7 ±64.62 pmol/l vs. 164.0 ±62.26 pmol/l, p < 0.05). Body weight gain from postoperative day 5 was greater for sham animals (50.22 ±20.93 γ vs. 16.4 ±25.93 g; p < 0.01).
Conclusions
Long segment IT shows a rapid rise in GLP-1 and PYY levels, thus leading to early amelioration of glucose control.
Keywords: ileal transposition, ileal transposition, type 2 diabetes mellitus, obesity surgery, metabolic surgery
Introduction
Current diabetes estimates suggest a general prevalence of 6.4% with remarkably higher rates for Europe and North America [1]. About 95% of these patients suffer from type 2 diabetes mellitus (T2DM) [2]. Besides life-style changes, conventional diabetes therapy relies mainly on long-term pharmacological treatment. Due to the progressive nature of the disease, most patients eventually require insulin therapy [3]. Initially mainly performed for weight loss, bariatric surgery remarkably leads to an improvement and even remission of T2DM before significant weight loss has occurred [4]. In light of this potential, indications for classical obesity surgery are expanded and new techniques implemented, although sufficient animal research is not available at present. The Diabetes Surgery Summit Consensus Conference (New York, 2008) strongly urges more research in this area with emphasis on efforts to advance pathophysiological understanding [5].
The current hypotheses of T2DM remission after obesity surgery are based on the foregut and hindgut theories. According to the hindgut hypothesis, fast delivery of nutrients to the terminal ileum leads to the secretion of incretins [6]. Today, glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) are thought to be the pathophysiologically relevant incretins in this context [4–6]. Glucagon-like peptide 1 is known to stimulate insulin release from pancreatic β-cells and inhibit secretion of glucagon from α-cells. Glucagon-like peptide 1 further improves peripheral insulin sensitivity and promotes β-cell proliferation [7]. Peptide YY lowers gastric and small intestinal motility, resulting in a longer mouth-to-caecum transit time. Peptide YY 3-36 further suppresses appetite and promotes weight loss [8]. The foregut hypothesis, claiming that bypassing the first part of the intestine will lead to reduced secretion of anti-incretins, is pathophysiologically less well characterized to date.
Initially designed as weight loss surgery, an ileal transposition (IT) was performed in Sprague-Dawley rats by Koopmans et al. after having observed notably reduced food intake after jejunal-ileal bypass [9]. The operation physiologically evokes hormonal alterations due to stimulation of enteroendocrine GLP-1 and PYY-secreting L-cells, which are located at a higher concentration in the terminal ileum [10].
Aim
As the development of the IT operation progresses, the operative technique needs to be more clearly defined. Current rodent studies are mainly based on transposition of the distal 10 cm of the ileum [11–15]. The aim of the current study was to establish a long segment IT (distal 50% of the ileum, approx. 20 cm) in the diabetic obese Zucker rat, investigating body weight, glucose control and changes in GLP-1 and PYY secretion.
Material and methods
Diets and animals
Eleven to twelve-week old male diabetic obese Zucker rats (Crl:ZUC-Lepr fa) were purchased from Charles River Breeding Laboratories (Wilmington, MA). Rats were housed individually and maintained on a 12/12 light/dark cycle with free access to water and rat chow diet (Provimi Kliba AG, Kaiseraugst, Switzerland). Chow contained 24% protein, 4.9% fat, 7% crude ashes, 4.7% crude fibre, lysine (13.6 g/kg), calcium (12 g/kg), methionine (4.5 g/kg) and phosphorus (8.3 g/kg). Rats were fasted overnight before surgery and oral glucose tolerance tests (OGTTs). All animal experimental protocols were approved by the Ethics Committee of the University of Freiburg.
Experimental protocol
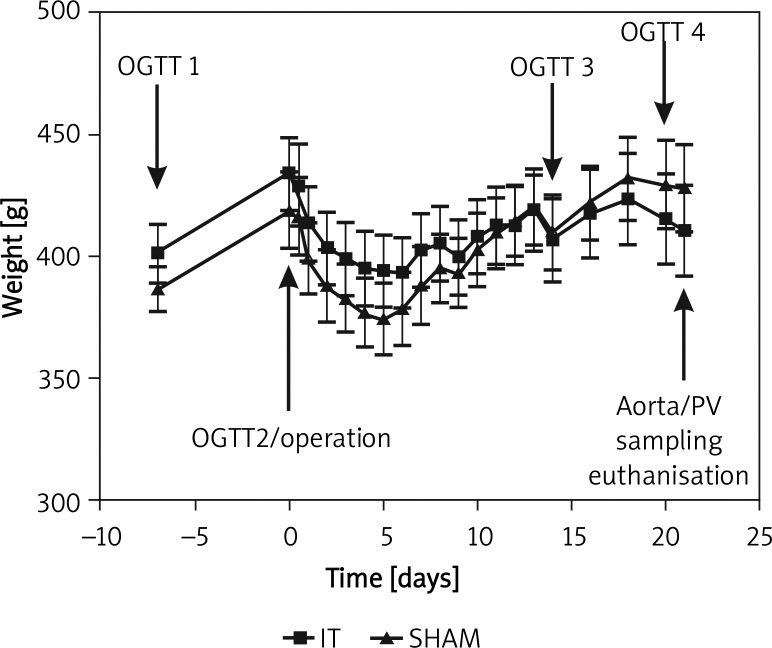
Rats were purchased and left to acclimatize with free access to food and water for 7 to 8 days. A total of 4 OGTTs were performed to assess glucose metabolism. Relative to surgery, time points were −7, 0, 14 and 20 days (Figure 1). The first and third OGTTs included blood collection from the right tail vein for hormone analysis. Before surgery, rats were randomly assigned to the IT or sham surgery group using sealed envelopes. On postoperative day 21, blood for additional hormone analysis was collected simultaneously from the aorta and portal vein. Euthanasia followed the last procedure on postoperative day 21 with a lethal intracardiac dose of potassium chloride.
Figure 1.
Body weight ± SD and time schedule. From day 5, sham animals gained significantly more weight (p < 0.05)
Body weight was recorded daily for 14 days following surgery and every second day for the remaining time period. Food and water consumption were recorded for 7 days postoperatively.
Surgery
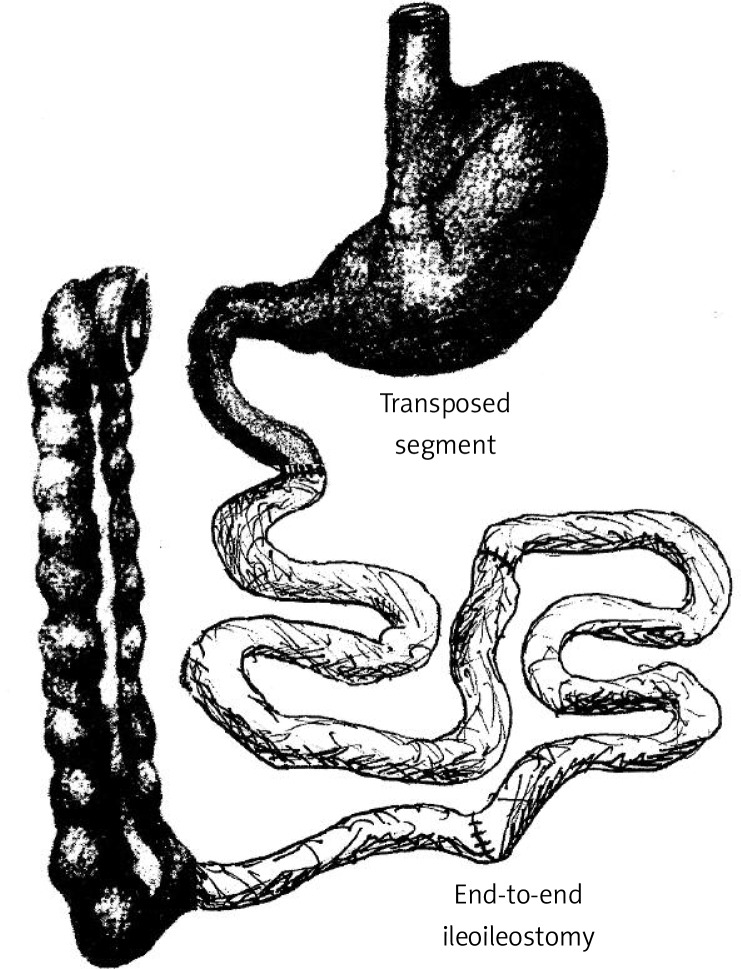
Animals were fasted for 12 h. Anaesthesia was induced and maintained using isoflurane 2% (Abbott GmbH & Co. KG, Wiesbaden, Germany) and oxygen flow at 2 l/min under spontaneous breathing. After a midline incision of 4-5 cm to gain abdominal access, the total length of the small intestine was determined (86.1 ±6.47 cm, n = 20). Thereafter, Bauhin's valve was identified and the first transection was conducted approximately 5 cm orally. To account for interindividual differences, the length of the transposed segment was defined as one-quarter of the total length of the small intestine (∼ distal 50% of ileum). Therefore, the second division of the ileum was performed 20 cm (21.5 ±1.6 cm, n = 20) orally to the first transection. All anastomoses were performed as interrupted end-to-end extramucosal anastomoses using Prolene 6/0 (Ethicon), as described earlier by our research group [16].
For IT, the first anastomosis was subsequently formed as an end-to-end ileoileostomy in order to restore ileal continuity, excluding the transposed segment. As the next step, the ligament of Treitz was identified and the jejunum divided 5 cm aborally. The ileal loop was then interpositioned in an isoperistaltic fashion forming two end-to-end anastomoses (Figure 2). Mesenteric openings were closed with Prolene 6/0 (Ethicon).
Figure 2.
Ileal transposition: 50% of the ileum is transposed 5 cm behind the ligament of Treitz in an isoperistaltic fashion
For sham surgery, a division of the small intestine with subsequent anastomoses was performed at the 3 corresponding positions (5 cm and 25 cm orally to Bauhin's valve and 5 cm aborally to the ligament of Treitz) without IT. Fascia and skin closure were performed as a continuous suture using Monocryl 4/0 and Vicryl 4/0. Postoperative analgesia was ensured via subcutaneous carprofen (Rimadyl, Pfizer, Switzerland) injection (4 mg/kg) at the beginning of the operation. Animals were fasted on day 1 after the operation with free access to tap water. Oral food was continuously increased to free access until day 6 (3, 6, 10, 15 g rat chow on day 2, 3, 4, 5 respectively) [16].
Oral glucose tolerance test and blood collection from tail vein
In total, four OGTTs were performed (Figure 1). The OGTT 1 and OGTT 3, conducted at −7 and 14 days relative to surgery, included a blood sample collection 30 min after glucose load for hormonal measurement. The OGTT 2 and OGTT 4, performed at 0 and 20 days, did not involve blood sampling.
For OGTTs, anaesthesia was induced and maintained using isoflurane 2% and oxygen flow at 2 l/ min under spontaneous breathing. The OGTT was initiated after placement of an orogastric tube (central venous catheter, Arrow International Inc., USA) infusing a 40% glucose solution to a dosage of 1.5 g/kg. Glucose was determined via tail snip at 0, 10, 30, 60, 90 and 120 min using a glucometer (Ascensia Elite, Bayer Corp., Germany).
Hormonal assessment
For hormonal evaluation, access to the tail vein was gained via a 2-cm incision above the tail vein. Then the vein was dissected and cannulated using a 26 gauge cannula. Blood was drawn via the cannula at 0 and 30 min after oral glucose was given, using tubes containing 10 µl EDTA (Sigma-Aldrich, St. Louis, USA) and 4 µl DPP-4 inhibitor (DRG Instruments GmbH, Marburg, Germany). After centrifugation at 4000 U for 10 min at 4°C, samples were snap-frozen in liquid nitrogen and stored at −80°C until analysis. Insulin, GLP-1 (7-36) and PYY (3-36) were measured by sandwich ELISA.
In brief, for GLP-1 (7-36), samples were added directly to a streptavidin-coated microtitre plate (DRG Instruments GmbH, Marburg, Germany). Subsequently, a mixture of a biotinylated specific GLP-1 antibody and a horseradish peroxidase-conjugated GLP-1 antibody was added to form a “Streptavidin–Biotin-Antibody–GLP-1(7-36)–HRP-conjugated-antibody” immunocomplex after incubation. For detection, each well was incubated with a substrate solution and measured in a microplate reader. For PYY (3-36), goat anti-rabbit IgG-coated microplates were used (DRG Instruments GmbH, Marburg, Germany). Determination was based on a competitive immunoreaction between sample PYY and biotinylated mouse PYY with a specific PYY (3-36) rabbit anti-mouse antibody. Detection was based on HRP substrate reaction. High-range rat insulin ELISA was a solid phase two-site enzyme immunoassay using HRP reaction for detection (DRG Instruments GmbH, Marburg, Germany).
Final blood collection from the aorta and portal vein
Blood sampling from the aorta and portal vein (PV) was conducted at 21 days post surgery. Anaesthesia was induced and maintained as described above. After abdominal access was gained via a midline incision, the aorta and PV were dissected. Blood collection was performed using a 24 and 21 gauge cannula for the portal vein and aorta, respectively. Samples were treated as described above. Potassium chloride was injected intracardially for euthanasia (2 mmol/kg bw).
Statistical analysis
Statistical analysis was conducted using Prism 5 for Mac OS X (GraphPad Software, Inc.). Area under the curve (AUC) was applied when comparing OGTTs. Due to incomplete data at 120 min, 90 min AUC levels were used for statistical evaluation. The Mann-Whitney-U test was used for group comparison. Curves were analysed with two-way ANOVA, where applicable. Values of p < 0.05 was considered significant.
Results
Glucose metabolism
Oral glucose tolerance tests
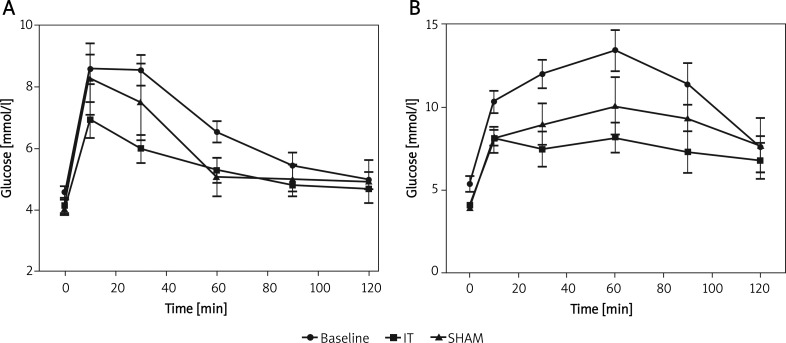
Oral glucose tolerance tests were conducted with and without simultaneous blood collection. Preoperative comparison of these two groups revealed significantly impaired glucose control when blood collection was conducted simultaneously, although OGTTs were otherwise performed under identical conditions (area under the curve (AUC) pre OP: 564.6 ±268.5 mmol/l × min vs. 230.8 ±91.77 mmol/l × min, p < 0.0001, Table I). Whereas OGTT 2 and 4 data showed a common glucose peak at 10 min, blood collection delayed the glucose peak to 60 min (Figure 3). Further statistical analysis was hence conducted comparing only OGTTs 1 and 3 (with blood collection) and OGTT 2 and 4 (without blood collection) in order to exclude blood collection as a confounder.
Table I.
AUC (mmol/l × min, 90 min) levels ± SD before and 2-3 weeks after IT/sham operation
| Variable | Baseline | IT | SHAM | |||
|---|---|---|---|---|---|---|
| OGTT 1 | OGTT 2 | OGTT 3 | OGTT 4 | OGTT 3 | OGTT 4 | |
| AUC | 564.6 ±268.5 | 230.8 ±91.77 | 314.7 ±229.0 | 135.5 ±59.27 | 451.4 ±327.6 | 207.5 ±106.1 |
| Value of p * | < 0.0001** | < 0.05 | < 0.01 | 0.154 | 0.460 | |
vs. baseline
OGTT 1 vs. 2
Figure 3.
Serial OGTTs performed at day –7, 0, 14, 20 (1.5 g/kg glucose orally). OGTT 2 and 4 were performed as single procedures (A), OGTT 1 and 3 with simultaneous blood collection (B). Ileal transposition led to a significant reduction in AUC compared to baseline levels at 2 weeks (p < 0.05, n = 9, Figure B) and 3 weeks (p < 0.01, n = 7, Figure A)
Evaluating OGTT 1 and 3, IT animals presented with a significantly reduced AUC compared to preoperative data, therefore showing an amelioration of glucose control as early as 2 weeks postoperatively (AUC: 314.7 ±229.0 mmol/l × min vs. 564.6 ±268.5 mmol/l × min, p < 0.05, Table I, Figure 3B). This effect could be reconfirmed 3 weeks postoperatively, again showing a significant reduction of AUC compared to preoperative data (AUC: 230.8 ±91.77 mmol/l × min vs. 135.5 ±59.27 mmol/l × min, p < 0.01, Table I, Figure 3A). Reduction rates in AUC for IT animals were 41% and 44% at 2 and 3 weeks, respectively.
In sham animals, no improvement in glucose metabolism was noted at 2 and 3 weeks postoperatively (AUC OGTT 1 vs. 3: 564.6 ±268.5 mmol/l × min vs. 451.4 ±327.6 mmol/l × min, p = 0.154 and OGTT 2 vs. 4: 230.8 ±91.77 mmol/l × min vs. 207.5 ±106.1 mmol/l × min, p = 0.460, Table I).
Fasting glucose
Fasting glucose levels were not significantly different between sham and IT animals (3.96 ±0.41 mmol/l vs. 4.12 ±0.42 mmol/l, p = 0.479; 4.03 ±0.50 mmol/l vs. 4.11 ±0.58 mmol/l; p = 0.943 at 2 and 3 weeks). Compared to preoperative data, 3-week fasting glucose levels did not differ in sham or IT animals (sham: 4.57 ±0.77 mmol/l vs. 4.03 ±0.50 mmol/l; p = 0.110; IT: 4.57 ±0.77 mmol/l vs. 4.11 ±0.58 mmol/l; p = 0.121).
Body weight
Body weight development was largely parallel in both groups (Figure 2). Weight was lowest at postoperative day 5 corresponding to the slow feeding in the first postoperative days. IT animals, however, had limited ability to regain body weight. Until the end of the experiment, sham animals gained significantly more weight (50.22 ±20.93 γ vs. 16.4 ±25.93 g; p < 0.01).
Hormonal assessment
Glucagon-like peptide 1
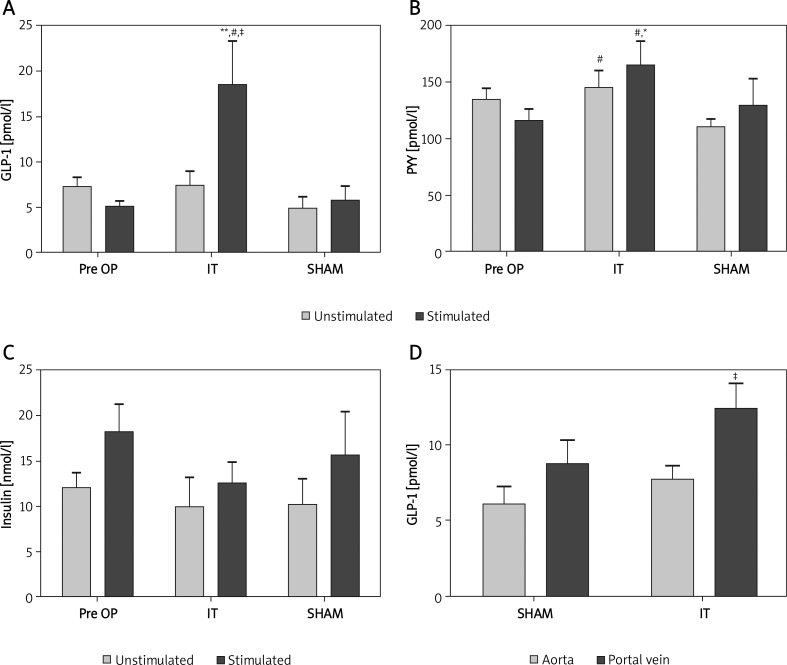
Ileal transposition surgery had a significant impact on GLP-1 secretion. Glucose-stimulated GLP-1 levels were significantly higher in the IT group compared to preoperative data and to sham animals (18.52 ±14.22 pmol/l vs. 5.07 ±2.44 pmol/l and 5.75 ±3.73 pmol/l, p < 0.01 and p < 0.05, Table II, Figure 4A). Sham animals displayed no change in stimulated GLP-1 levels (7.20 ±4.55 pmol/l vs. 5.75 ±3.73 pmol/l, p = 0.606). Unstimulated GLP-1 levels remained unchanged in both groups with no apparent trend. Glucose significantly stimulated GLP-1 secretion only in IT animals (7.50 ±4.37 pmol/l vs. 18.52 ±14.22 pmol/l, p < 0.05, Table II).
Table II.
Serum levels ± SD of incretins before and 2 weeks after IT/sham operation
| Variable | Baseline | IT | SHAM | |||
|---|---|---|---|---|---|---|
| Pre glucose | Post glucose | Pre glucose | Post glucose | Pre glucose | Post glucose | |
| GLP-1 | 7.20 ±4.55 | 5.07 ±2.44 | 7.50 ±4.37 | 18.52 ±14.22** | 4.92 ±2.82 | 5.75 ±3.73 |
| PYY | 134.6 ±36.83 | 115.9 ±41.64 | 145.0 ±46.76# | 164.0 ±62.26* | 110.0 ±19.38 | 129.7 ±64.62 |
| Insulin | 12.07 ±7.19 | 18.22 ±12.99 | 9.95 ±9.73 | 12.66 ±6.70 | 10.27 ±7.95 | 15.65 ±13.37 |
p < 0.05 vs. baseline
p < 0.01 vs. baseline
p < 0.5 vs. SHAM
Figure 4.
GLP-1, PYY and insulin levels, determined by multiplex ELISA 2 weeks postoperative. A – Glucosestimulated GLP-1 levels in the IT group (n = 9) were significantly raised, compared to preop. levels (**p < 0.01), SHAM (# p < 0.05) and fasted (unstimulated) IT animals (‡ p < 0.05). B – IT animals displayed raised PYY levels compared to SHAM animals (#Unstim./stim. p < 0.05 for both) and compared to preoperative levels (*stimulated p < 0.05). C – Insulin levels remained unchanged when compared to preoperative levels and sham animals. D – Simultaneously determined, portal GLP-1 levels were higher compared to aortic levels in IT animals (‡ p < 0.05)
Table III.
Serum levels ± SD of incretins in portal vein and aorta at postoperative day 21
| Variable | IT | Sham | ||
|---|---|---|---|---|
| Aorta | Portal vein | Aorta | Portal vein | |
| GLP-1 | 7.78 ±2.30 | 12.41 ±5.3‡ | 6.07 ±3.25 | 8.74 ±4.45 |
| PYY | 172.2 ±66.19 | 168.6 ±72.68 | 182.5 ±58.16 | 155.4 ±77.01 |
| Insulin | 12.6 ±20.2 | 13.65 ±18.09 | 8.53 ±3.77 | 7.11 ± 3.90 |
p < 0.05
Peptide YY
Peptide YY levels showed a similar pattern, but revealing distinct differences. Glucose stimulated levels also proved to be higher for IT animals compared to preoperative data and to sham animals (164.0 ±62.26 pmol/l vs. 115.9 ±41.64 and 129.7 ±64.62 pmol/l respectively; p < 0.05 for all, Table II, Figure 4B). Furthermore, unstimulated PYY levels were significantly elevated in IT compared to sham animals (145.0 ±46.76 pmol/l vs. 110.0 ±19.38 pmol/l; p < 0.05). In contrast to GLP-1, glucose could not significantly raise PYY levels in the IT group (p = 0.863, Table II). SHAM animals displayed no change in respect to preoperative data (unstimulated 134.6 ±36.83 pmol/l vs. 110.0 ±19.38 pmol/l, p = 0.071; stimulated 115.9 ±41.64 pmol/l vs. 129.7 ±64.62 pmol/l, p = 0.821, Table II).
Insulin
Insulin secretion did not differ between IT and sham animals for unstimulated and stimulated data (Figure 4C). Glucose failed to stimulate insulin pre- and postoperatively (preop. 12.07 ±7.19 nmol/l vs. 18.22 ±12.99 nmol/l, p = 0.070; IT: 9.95 ±9.73 nmol/l vs. 12.66 ±6.70 nmol/l, p = 0.136; sham: 10.27 ±7.94 nmol/l vs. 15.65 ±13.37 nmol/l, p = 0.382, Table II).
Hormonal assessment in aorta versus portal vein
Simultaneous determination of hormone levels in the aorta and PV revealed significantly higher levels of GLP-1 in the portal vein of IT animals (7.78 ±2.30 pmol/l vs. 12.41 ±5.3 pmol/l, p < 0.05, Figure 4D). In sham rats, GLP-1 levels were equal in aorta and PV (6.07 ±3.25 pmol/l vs. 8.74 ±4.45 pmol/l, p = 0.235). Although a significant difference in arterial and PV levels was detected only in IT animals, direct comparison of arterial and PV GLP-1 levels of sham and IT animals revealed no significance (portal: 8.74 ±4.45 pmol/l vs. 12.41 ±5.3 pmol/l, p = 0.237; arterial: 6.07 ±3.25 pmol/l vs. 7.78 ±2.30 pmol/l, p = 0.161).
Discussion
Ileal transposition surgery is an upcoming new procedure targeting the operative remission of T2DM. Widely accepted, IT overall leads to an amelioration of glucose control based on enteroendocrine hormone stimulation [11, 12, 14, 15].
Inter-study comparison of single effects of IT is, however, limited since the study design varies considerably and the postoperative time-point of data sampling is particularly relevant due to rapid incretin secretion changes within the first postoperative months. In this respect, Wang et al. noted a continuous increase of fasting GLP-1 levels after IT over a time period of 6 months [15]. In the current study, a long segment transposition was performed with analysis concentrating on immediate postoperative changes.
In University of California at Davis type 2 diabetes mellitus (UCD-T2DM) rats Cummings et al. found a 3-fold increase in stimulated GLP-1 and PYY secretion 1 and 3.5 months after surgery. Furthermore, fasting PYY levels were elevated 8-fold in IT animals [12]. Although a quantitative rise in GLP-1 secretion is well established for most animal models, Patriti et al. could not record a such a change in glucose-stimulated GLP-1 secretion following IT in Goto-Kakizaki (GK) rats, demonstrating a more prolonged GLP-1 response to oral glucose than in sham-operated animals [13]. Culnan et al. conducted a similar experiment in Zucker rats comparing a short (10 cm) IT group with sham-operated animals over an 8-week observation period. Analysing incretins in postoperative week 7, GLP-1 plasma levels were elevated 3-4 fold in this model. After long segment IT, GLP-1 secretion was already elevated 3-fold 2 weeks after transposition. Therefore, the changes in GLP-1 serum levels reported by Culnan et al. were reached 5 weeks earlier, indicating a benefit for long segment IT [11]. Simultaneous analysis of GLP-1 in aorta and portal vein indicate a partly elevated enteric GLP-1 secretion for fasted animals also. This effect, however, is counterbalanced due to the large first-pass effect known for GLP-1, leading to equal peripheral GLP-1 levels in the fasting state [17, 18]. Accordingly, fasting glucose levels remained unchanged in IT and sham animals.
In contrast to GLP-1, fasting PYY levels were also elevated after long segment IT, and glucose could not further increase PYY secretion. Taking into account that GLP-1 and PYY are co-localized in over 80% of L-cell granules, it seems plausible that the mechanism of secretion is similar [19]. Possibly a change in granule composition occurs after IT, indicating alternate regulations of PYY and GLP-1. Cummings et al. noted significantly elevated PYY mRNA expression in the transposed segment, whereas proglucagon mRNA, a precursor of GLP-1, remained unchanged, indicating PYY regulation at an earlier level [12].
Studies show consistent amelioration of glucose control following IT as a result of increased incretin production, yet the quantity of changes reported again varies considerably. Cummings et al. note only a moderate reduction in AUC of 17%, performing an OGTT 1 month after IT [12, 14]. For GK rats, Patriti et al. demonstrated a significant decrease of the AUC compared to sham animals 45 days postoperatively. The fact that glucose control was still equal for IT and sham animals 30 days postoperatively in the same study again indicates that the effect of IT increases in the first postoperative months [13]. In the parallel trial examining Zucker rats, Culnan et al. revealed a 60% reduction of the AUC after OGTT in IT animals 7 weeks postoperatively [11]. Long segment IT led to amelioration of glucose control as early as 2 weeks postoperatively. Restrictively, improvement of glucose control was noted only compared to preoperative measurements. Blood collection from the tail vein led to largely altered, yet reproducible glucose curves. Although blood sampling was performed under general anaesthesia, this effect was most likely due to operative stress resulting from the venesection. Certainly, blood collection and OGTTs should be conducted separately in future trials in order to exclude this confounder.
Insulin levels were determined in IT and sham animals as a direct mediator of glucose control. Interestingly, previous analysis of insulin and insulin action in diabetic animals revealed that the GLP-1 effect after IT mostly relies on improved peripheral insulin sensitivity [14]. Although GLP-1 is known to directly stimulate insulin secretion, insulin levels remained unchanged or even decreased after IT, as previously demonstrated for Zucker rats [11, 14]. In our model, transposed animals presented with a 20-30% reduction of insulin secretion compared only to preoperative controls. Apparently, operative trauma and postoperative food restriction still exert a large effect on glucose control during this short observation period. Sham animals hence also presented with partly improved glucose tolerance, therefore diminishing statistical significance when comparing insulin and glucose parameters between sham and IT animals in this early postoperative time frame.
Weight change after IT remains controversial. Initially, IT was considered to achieve similar effects to Roux-en-Y gastric bypass [9, 15]. More recent IT studies in different rodent models, however, consistently reveal no impact of IT on body weight [11–13]. Our data suggest an impaired weight regain for IT animals in the immediate postoperative period. However, weight curves overall proceed virtually in parallel, thus calling a major impact of IT on body weight into question. The IT group's impaired capacity to regain body weight after surgical trauma seems plausible, since fat resorption is limited after IT due to a truncated enterohepatic cycle, and PYY is known to suppress appetite [8, 20].
In the current study, long segment IT could be established in Zucker rats. Quantitatively large increases in GLP-1 and PYY secretion were detected early after the operation, consequently leading to rapid glucose control. Changes in GLP-1 secretion and glucose control were similar to Culnan's analysis in Zucker rats, but were detected considerably earlier. It remains to be determined whether adaptive alterations, as suggested by Wang et al. [15], lead to an additional increase in incretin production in our model, thus clearly demonstrating a superiority of the long segment transposition. Interesting questions regarding the adaptive capacities of the transposed segment as well as pathophysiological changes in peripheral insulin sensitivity remain. Certainly, considerable research is essential before we will be able to safely perform an isolated ileal transposition in humans, since it proves to be a complex metabolic operation.
Acknowledgments
The authors thank Olivia Sick and Silke Hempel for assistance in statistics and ELISA measurements.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.National diabetes information clearing house (ndic) national diabetes statistics. 2007. http://diabetesniddknihgov/DM/PUBS/statistics/
- 3.Campos C. Treating the whole patient for optimal management of type 2 diabetes: considerations for insulin therapy. South Med J. 2007;100:804–11. doi: 10.1097/SMJ.0b013e3180485a9d. [DOI] [PubMed] [Google Scholar]
- 4.Thaler JP, Cummings DE. Minireview: hormonal and metabolic mechanisms of diabetes remission after gastrointestinal surgery. Endocrinology. 2009;150:2518–25. doi: 10.1210/en.2009-0367. [DOI] [PubMed] [Google Scholar]
- 5.Rubino F, Kaplan LM, Schauer PR, Cummings DE. The diabetes surgery summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251:399–405. doi: 10.1097/SLA.0b013e3181be34e7. [DOI] [PubMed] [Google Scholar]
- 6.Rubino F, Gagner M. Potential of surgery for curing type 2 diabetes mellitus. Ann Surg. 2002;236:554–9. doi: 10.1097/00000658-200211000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nauck MA. Unraveling the science of incretin biology. Eur J Intern Med. 2009;20(Suppl 2):S303–8. doi: 10.1016/j.ejim.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 8.Ballantyne GH. Peptide yy(1-36) and peptide yy(3-36): Part I. Distribution, release and actions. Obes Surg. 2006;16:651–8. doi: 10.1381/096089206776944959. [DOI] [PubMed] [Google Scholar]
- 9.Koopmans HS, Sclafani A, Fichtner C, Aravich PF. The effects of ileal transposition on food intake and body weight loss in vmh-obese rats. Am J Clin Nutr. 1982;35:284–93. doi: 10.1093/ajcn/35.2.284. [DOI] [PubMed] [Google Scholar]
- 10.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 11.Culnan DM, Albaugh V, Sun M, et al. Ileal interposition improves glucose tolerance and insulin sensitivity in the obese Zucker rat. Am J Physiol Gastrointest Liver Physiol. 2010;299:G751–60. doi: 10.1152/ajpgi.00525.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings BP, Strader AD, Stanhope KL, et al. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the ucd-t2dm rat. Gastroenterology. 2010;138:2437–46. doi: 10.1053/j.gastro.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patriti A, Aisa MC, Annetti C, et al. How the hindgut can cure type 2 diabetes. Ileal transposition improves glucose metabolism and beta-cell function in goto-kakizaki rats through an enhanced proglucagon gene expression and l-cell number. Surgery. 2007;142:74–85. doi: 10.1016/j.surg.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Strader AD, Clausen TR, Goodin SZ, Wendt D. Ileal interposition improves glucose tolerance in low dose streptozotocin-treated diabetic and euglycemic rats. Obes Surg. 2009;19:96–104. doi: 10.1007/s11695-008-9754-x. [DOI] [PubMed] [Google Scholar]
- 15.Wang TT, Hu SY, Gao HD, et al. Ileal transposition controls diabetes as well as modified duodenal jejunal bypass with better lipid lowering in a nonobese rat model of type 2 diabetes by increasing glp-1. Ann Surg. 2008;247:968–75. doi: 10.1097/SLA.0b013e318172504d. [DOI] [PubMed] [Google Scholar]
- 16.Marjanovic G, Holzner P, Kulemann B, et al. Pitfalls and technical aspects during the research of intestinal anastomotic healing in rats. Eur Surg Res. 2010;45:314–20. doi: 10.1159/000320768. [DOI] [PubMed] [Google Scholar]
- 17.Hansen L, Deacon CF, Orskov C, Holst JJ. Glucagon-like peptide-1-(7-36)amide is transformed to glucagon-like peptide-1-(9-36)amide by dipeptidyl peptidase iv in the capillaries supplying the l cells of the porcine intestine. Endocrinology. 1999;140:5356–63. doi: 10.1210/endo.140.11.7143. [DOI] [PubMed] [Google Scholar]
- 18.Deacon CF, Wamberg S, Bie P, et al. Preservation of active incretin hormones by inhibition of dipeptidyl peptidase iv suppresses meal-induced incretin secretion in dogs. J Endocrinol. 2002;172:355–62. doi: 10.1677/joe.0.1720355. [DOI] [PubMed] [Google Scholar]
- 19.Nilsson O, Bilchik AJ, Goldenring JR, et al. Distribution and immunocytochemical colocalization of peptide yy and enteroglucagon in endocrine cells of the rabbit colon. Endocrinology. 1991;129:139–48. doi: 10.1210/endo-129-1-139. [DOI] [PubMed] [Google Scholar]
- 20.Tsuchiya T, Kalogeris TJ, Tso P. Ileal transposition into the upper jejunum affects lipid and bile salt absorption in rats. Am J Physiol. 1996;271:G681–91. doi: 10.1152/ajpgi.1996.271.4.G681. [DOI] [PubMed] [Google Scholar]