Abstract
RING finger protein 11 (RNF11), a negative regulator of NF-κB signaling pathway, colocalizes with α-synuclein and is sequestered in Lewy bodies in Parkinson’s disease (PD). Since persistent NF-κB activation is reported in PD, in this report we investigated if RNF11 expression level is correlated to activated NF-κB in PD. We examined RNF11 expression levels in correlation to phospho-p65, a marker for activated NF-κB, in control and PD brain tissue from cerebral cortex. In addition we performed double immunofluorescence labeling experiments to confirm this correlation. Our investigations demonstrated that the neuronal RNF11 expression was down-regulated in PD and was usually associated with increased expression of phospho-p65. Double labeling confirmed that loss of neuronal RNF11 was linked to increased phospho-p65 expression, suggesting that persistent presence of NF-κB activation could be due to decreased levels of its negative regulator. Our data exemplifies the relevance of RNF11 and persistent NF-κB activation in PD.
Keywords: phospho-p65, immunofluorescence, cingulate cortex, substantia nigra
1. Introduction
RNF11 is one of several candidate genes within the PARK10 locus, a 9-megabase region on chromosome 1 that is associated with increased risk of Parkinson’s disease (PD, [8]). Previously we have demonstrated that RNF11 is expressed in dopaminergic neurons in the substantia nigra (SN), a region most vulnerable to cell loss and Lewy body pathology in PD [1]. More importantly, in PD brains RNF11 was found to co-localize with α-synuclein in Lewy bodies and neurites [1]. Recently we have demonstrated that RNF11, as a negative regulator of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling pathway [5, 13], can modulate neuronal response to toxicity in both cellular and rodent models of PD [14].
The NF-κB signaling pathway is evolutionarily conserved with respect to its role in immunity and inflammation [17]. In addition, NF-κB’s contribution to neurodegeneration in PD has been well documented [6, 7]. We reasoned that if RNF11-mediated NF-κB activity is pertinent to PD pathogenesis then activated NF-κB will be accompanied with altered RNF11 expression in PD affected brain regions. Understanding RNF11-mediated regulation of NF-κB will be highly relevant in PD, where (a) activated NF-κB is observed in surviving SN neurons [6, 9] and microglial cells [6], (b) increased NF-κB level is detected in striatum and SN [12] and (c) persistent NF-κB activation is associated with increased inflammatory responses and degeneration of dopaminergic neurons in animal models of PD [6, 10]. In this study, we examined RNF11 expression in conjunction with NF-κB activation in PD brains. Using bright-field microscopy and semi-quantitative analysis of RNF11 immunoreactivity, we report here that neuronal RNF11 protein expression is reduced in PD. More interestingly, we demonstrate here with certainty that reduced RNF11 is associated with activated NF-κB in cortical and nigral neurons.
2. Materials and Method
2.1 Human tissue
Human brain tissues used for immunohistochemical analysis were derived from 20 autopsy brains fixed in paraformaldehyde, which were pathologically evaluated for Parkinson’s disease (PD), from the Emory University Alzheimer’s Disease Research Center and NINDS Neurosciences Core Facilities Brain Bank (Atlanta, GA, USA). The neuropathologic diagnosis of PD was based on the presence of nigral degeneration and Lewy bodies. Control cases had no clinical history or neuropathologic diagnosis of neurologic disease. Two groups were compared: 10 subjects aged 62 to 79 years (mean = 71) with clinically and pathologically confirmed PD, and 10 control subjects aged 52 to 92 years (mean = 71).
2.2 Immunohistochemistry
Blocks of formalin fixed tissue at the level of cingulate cortex, frontal cortex and substantia nigra were sectioned on a freezing microtome. Free-floating sections (50 µm) were stored at −20 °C in ethylene glycol/sucrose cryopreservative. Coronal sections from control and PD cases were immunostained for RNF11 [1] or phospho-p65 (3036S; Cell Signaling Technology, Beverly, MA, USA), a marker of activated NF-κB [16, 18, 20, 21]. In brief, sections were incubated in primary antibody (Rabbit polyclonal RNF11 (1:100, [1]); mouse monoclonal phospho-p65, (1:250, Cell Signaling) for 72 h at 4°C, followed by incubation in appropriate biotinylated secondary antibody (1 h at room temp), and avidin-biotin peroxidase complex (1 h at room temp; ABC Elite Kit, Vector Laboratories, Burlingame, CA). Diaminobenzedine (DAB) was used as a chromophore to detect immunoreactivity as previously described [3]. Semiquantitation - RNF11 immunoreactivity for each case was blind-scored by 4 individuals on a scale of 1 to 4 with 1 being the lowest and 4 being the highest intensity. The average score for each case was represented as scatter plots for the two groups, control and PD cases. Statistical analysis was performed using student’s t-test to compare control versus PD cases. A P value less than 0.05 was accepted as indicating a significant difference.
2.3 Double immunofluorescence labeling
For double immunofluorescence labeling, brain sections were stained using a standard immunofluorescence protocol [2]. Briefly, sections were blocked with 10% normal horse serum and 10% Triton-X in TBS. Sections were incubated with a combination of primary antibodies (RNF11 and phospho-p65) overnight at 4°C followed by appropriate fluorescent conjugated secondary antibodies (Alexa 488 or Cy3 goat antimouse/rabbit, Molecular Probes, Eugene, OR). Sections were incubated with Hoechst 333258 to counter-stain nuclei. Autoflourescence eliminator reagent (Chemicon, CA) was used to eliminate background autoflourescence in human tissue. Primary antibodies were omitted as a negative control. Fluorescent images were captured using Olympus wide-field fluorescence microscopy or Zeiss LSM 510 laser scanning confocal microscope. For final output, images were processed using Adobe Photoshop 7.0 software.
2.4 Quantification of colocalization coefficients
The degree of colocalization was determined by calculating the overlap coefficient according to Manders in single confocal images using the “colocalization” feature of Imaris 7.6.0, 64-bit version (Bitplane AG) as described previously [14]. Automated thresholding to correct the background was used. Three sections were analyzed for each case and 3 cases were analyzed for each group. Values are reported as mean Mander’s coefficient ± standard deviation.
3. Results
Since our in vitro and in vivo studies [13, 14] demonstrated neuronal RNF11 to be a negative regulator of NF-κB, we wanted to investigate if activated NF-κB in PD is associated with reduced levels of neuronal RNF11 expression.
3.1 RNF11 expression in human brains
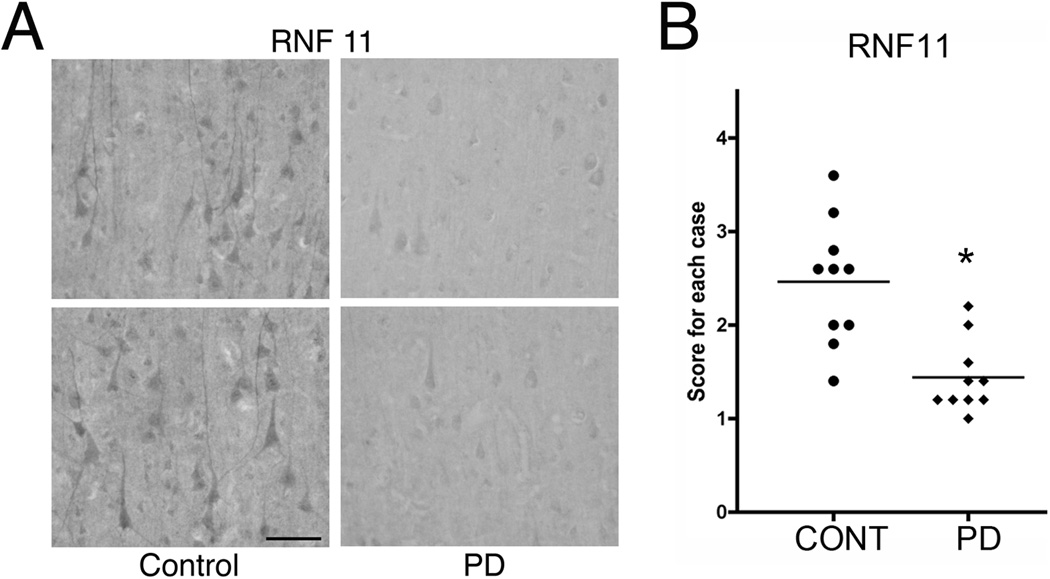
As previously described [1, 15], RNF11 has a differential distribution in the normal brain with specific regions of enrichment and low expression in white matter. Both neuronal and glial cells express RNF11 but expression level is much higher in neurons compared to glial cells. RNF11 expression can be detected in cerebral cortex (Fig 1, 3A) where it is concentrated in somatodendritic compartments of pyramidal and non-pyramidal neurons. This normal distribution of RNF11 was altered in PD brains. Qualitative preliminary studies comparing RNF11 expression in control and PD sections suggested that neuronal RNF11 levels were reduced in PD (Fig 1A). Furthermore, semi-quantitative evaluation of RNF11-immunoreactivity confirmed that RNF11 expression was indeed reduced in PD (Fig 1B).
Fig 1. RNF11 expression is reduced in PD.
A - Sections through the cingulate cortex from control (A, left panel) and PD (A, right panel) brains were immunostained for RNF11 using diaminobenzadine (DAB) as the chromophore. Representative images are shown in (A). B - The sections were blind scored by four independent individuals on a rating scale of 1 (low RNF11 immunoreactivity as in PD sections) to 4 (high RNF11 immunoreactivity as in control sections). Average score for each case was plotted with the mean for each group demonstrated by the bar in (B). * P < 0.05. Note the reduced RNF11-immunoreactivity in PD confirmed by semi-quantitative analysis. Scale bar − 100µm.
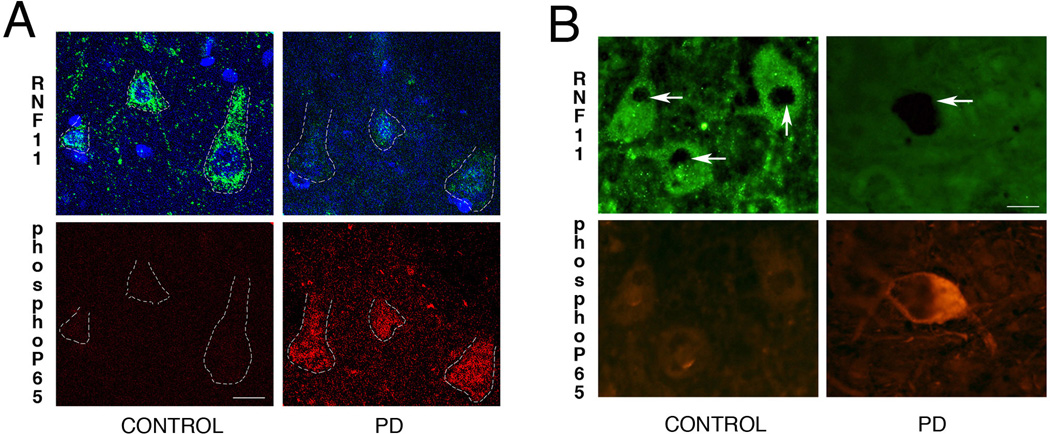
Fig 3. Reduced RNF11 is associated activated NF-κB in PD.
Cortical (A) and nigral (B) sections from brains of control (left panel) and PD (right panel) cases were double labeled for RNF11 and phospho-p65, a marker for activated NF-κB pathway. (A) Confocal images in the top panel demonstrate RNF11 immunoreactivity (green) and bottom panel demonstrates phospho-p65 immunoreactivity (red) in cingulate cortex. Nuclei were stained with Hoechst 333258 (blue). (B) Photomicrographs of nigral sections from brains of control (left panel) and PD (right panel) patients demonstrating immunoreactivity for RNF11 (green, top panel) and phosph-p65 (red, bottom panel). Nigral dopaminergic neurons are identified by dense neuromelanin pigmentation (arrow). These representative images demonstrate that in PD brain reduced RNF11 immunoreactivity is associated with increased phospho-p65 immunoreactivity. The ‘gain’ was adjusted to avoid over-saturation while taking fluorescent images. Scale bar of (A) − 10µm and (B) − 20µm.
3.2 NF-κB in human brains
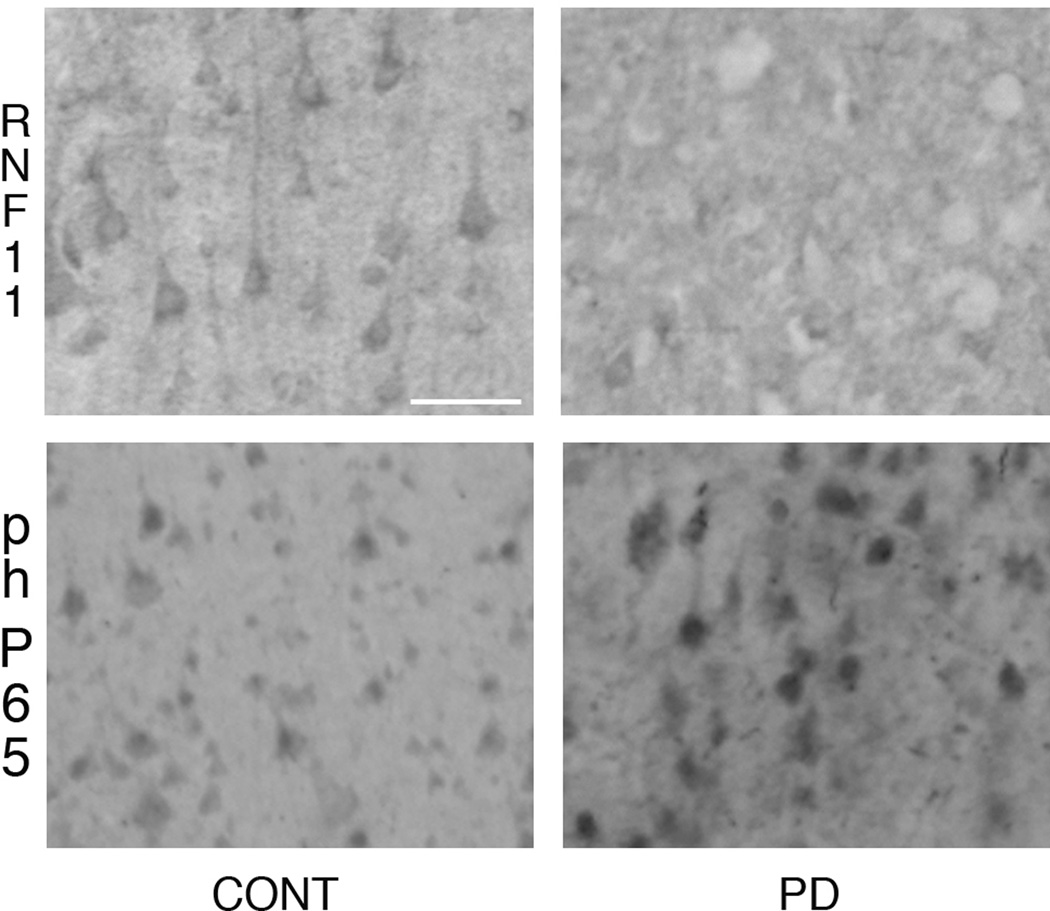
Since phospho-p65 is routinely used as a marker for activated NF-κB [16, 18, 20, 21] we used a mouse monoclonal antibody against phospho-p65 to examine NF-κB activation in human control and diseased brains. Phospho-p65 expression levels were detected in control, and PD cases, however expression levels of phospho-p65 were elevated in PD cases as compared to controls (Fig 2). No phospho-p65 immunoreactivity was observed following primary antibody omission or preabsorption with excess peptide (data not shown).
Fig 2. RNF11 and activated NF-κB in PD.
Cortical sections from brains of control (left panel) and PD (right panel) cases were immunolabeled with RNF11 (top) and phospho-p65 (bottom, a marker of activated NF-κB). Representative photomicrographs demonstrate that reduced RNF11-immunoreactivity (ir) is associated with increased phospho-p65-ir in PD in comparison to control cases. Scale bar − 50 µm.
3.3 Activated NF-κB is associated with reduced RNF11 expression in PD
To confirm the above-mentioned association of RNF11 and NF-κB activation in brain tissue, co-localization experiments were performed using double immunolabeling with phospho-p65, a phosphorylated protein that up-regulates upon activation of the NF-κB pathway. For this set of experiments we used brain tissue from three control and PD cases each. The fluorescence intensities were manipulated such that difference in immunostaining was evident and fluorescence signals were not saturated for high expression levels of our proteins of interest. As demonstrated in Fig 3, reduced RNF11 expression level in cortical (Fig 3A, Mander’s coefficient − 0.07± 0.5) and nigral (Fig3B, Mander’s coefficient − 0.10± 0.7) neurons was associated with increased phospho-p65 expression levels, indicating a reverse association of RNF11 expression with activated NF-κB pathway.
4. Discussion
4.1 Phosho-p65 as an indicator of NF-κB activation
Phospho-p65 has been extensively used as a marker to detect activated NF-κB [16, 18, 20, 21]. Moreover, presence of phospho-p65 has been consistent with NF-κB p65 translocation to the nuclei [16], the more traditional marker of activated NF-κB. Antibody specificity for phospho-p65 has been documented and established previously [18]. Moreover, our studies demonstrated that staining for phosphop65 on human brain tissue was specific since omission of primary antibody or preabsorption with excess peptide resulted in absence of immunoreactivity.
4.2 NF-κB in PD
Several lines of investigation have demonstrated that activation of NF-κB is associated with PD pathology and pathogenesis [6, 9]. Activation of NF-κB has been observed in SN neurons of PD patients. Our investigations confirmed these previous findings that NF-κB is activated in nigral neurons. Furthermore we observed activated NF-κB in cortical neurons in PD brains. This information is especially important given that cortical regions are one of the last areas to be afflicted with PD pathology [4].
4.3 RNF11 and NF-κB in PD
We have previously shown that RNF11 mRNA expression levels are reduced in PD [14]. Here we demonstrate that RNF11 protein levels are also reduced in PD. Given that RNF11 is a negative regulator of NF-κB activity [13, 19] such that loss of RNF11 augments NF-κB activity, this is not surprising. Indeed, in this report we illustrate, for the first time, that reduced RNF11 expression in cortical and nigral neurons of PD brains is associated with increased NF-κB activation.
4.4 Relevance to PD pathogenesis
Role of NF-κB activity in neuronal death has been ambiguous. Based on cell culture studies it has been inferred that activated NF-κB in nigral neurons is an early sign of degeneration [9]. However numerous studies have also suggested that activated NF-κB can confer neuroprotection [11, 14]. Given that activated NF-κB and apoptotic features are not detected in the same nigral neurons [9] reported previously, we could infer that activation of NF-κB in neurons is protective. This inference is strongly supported by our in vitro and in vivo studies in models of PD [14] where we clearly demonstrate that activation of NF-κB is neuroprotective. Moreover we go on to demonstrate that loss of RNF11 is neuroprotective while RNF11-mediated inhibition of NF-κB activity in dopaminergic cells exaggerates 6-OHDA toxicity [14].
5. Conclusions
Our investigations, while confirming previous reports that NF-κB is activated in PD, demonstrate that RNF11, a negative regulator of NF-κB, is down-regulated in cerebral cortex and substantia nigra regions in PD cases. More importantly, using double labeling techniques we have illustrated, for the first time, that NF-κB activation is accompanied with reduced RNF11 expression levels. Our data suggests the presence of RNF11- NF-κB association in human brain tissue with significance to PD pathology and pathogenesis.
Highlights.
Neuronal RNF11-immunoreactivity is down-regulated in PD
Activated NF-κB is observed in neurons of the cerebral cortex in PD
RNF11 is inversely associated with activated NF-κB in cortical and nigral neurons in PD.
Acknowledgements
We thank members of the Lah/Levey lab for constructive discussion regarding this manuscript and the National Institutes of Health through the Alzheimer’s Disease Research Center grant (AG025688), NIEHS ES015777 (RSB), NIEHS ES012870 (ELP), and NINDS NS007480 (ELP). This research project was supported in part by the microscopy cores of the Emory Neuroscience NINDS Core Facilities grant, P30NS055077.
Abbreviations
- RNF11
RING finger protein 11
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PD
Parkinson’s disease
- SN
substantia nigra
- DAB
diaminobenzedine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elaine Pranski, Email: epransk@emory.edu.
Carson .D. Van Sanford, Email: cvansan2@gmail.com.
Nirjari Dalal, Email: nvdalal@emory.edu.
Adam L. Orr, Email: aorr25@gmail.com.
Dipan Karmali, Email: karmali.dipan@gmail.com.
Deborah S Cooper, Email: dscoope@emory.edu.
Marla Gearing, Email: mgearin@emory.edu.
James J. Lah, Email: jlah@emory.edu.
Allan I. Levey, Email: alevey@emory.edu.
Ranjita Betarbet, Email: rbetarb@emory.edu.
References
- 1.Anderson LR, Betarbet R, Gearing M, Gulcher J, Hicks AA, Stefansson K, Lah JJ, Levey AI. PARK10 candidate RNF11 is expressed by vulnerable neurons and localizes to Lewy bodies in Parkinson disease brain. J Neuropathol Exp Neurol. 2007;66:955–964. doi: 10.1097/nen.0b013e3181567f17. [DOI] [PubMed] [Google Scholar]
- 2.Betarbet R, Anderson LR, Gearing M, Hodges TR, Fritz JJ, Lah JJ, Levey AI. Fas-associated factor 1 and Parkinson's disease. Neurobiol Dis. 2008;31:309–315. doi: 10.1016/j.nbd.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease [In Process Citation] Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 5.Dalal NV, Pranski EL, Tansey MG, Lah JJ, Levey AI, Betarbet RS. RNF11 modulates microglia activation through NF-kappaB signalling cascade. Neurosci Lett. 2012;528:174–179. doi: 10.1016/j.neulet.2012.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson's disease. Proc Natl Acad Sci U S A. 2007;104:18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hicks AA, Petursson H, Jonsson T, Stefansson H, Johannsdottir HS, Sainz J, Frigge ML, Kong A, Gulcher JR, Stefansson K, Sveinbjornsdottir S. A susceptibility gene for late-onset idiopathic Parkinson's disease. Ann Neurol. 2002;52:549–555. doi: 10.1002/ana.10324. [DOI] [PubMed] [Google Scholar]
- 9.Hunot S, Brugg B, Ricard D, Michel PP, Muriel MP, Ruberg M, Faucheux BA, Agid Y, Hirsch EC. Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc Natl Acad Sci U S A. 1997;94:7531–7536. doi: 10.1073/pnas.94.14.7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee JK, Chung J, McAlpine FE, Tansey MG. Regulator of G-protein signaling- 10 negatively regulates NF-kappaB in microglia and neuroprotects dopaminergic neurons in hemiparkinsonian rats. J Neurosci. 2011;31:11879–11888. doi: 10.1523/JNEUROSCI.1002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13:852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 12.Mogi M, Harada M, Kondo T, Mizuno Y, Narabayashi H, Riederer P, Nagatsu T. The soluble form of Fas molecule is elevated in parkinsonian brain tissues. Neurosci Lett. 1996;220:195–198. doi: 10.1016/s0304-3940(96)13257-2. [DOI] [PubMed] [Google Scholar]
- 13.Pranski EL, Dalal NV, Herskowitz JH, Orr AL, Roesch LA, Fritz JJ, Heilman C, Lah JJ, Levey AI, Betarbet RS. Neuronal RING finger protein 11 (RNF11) regulates canonical NF-kappaB signaling. Journal of neuroinflammation. 2012;9:67. doi: 10.1186/1742-2094-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pranski EL, Dalal NV, Sanford CV, Herskowitz JH, Gearing M, Lazo C, Miller GW, Lah JJ, Levey AI, Betarbet RS. RING finger protein 11 (RNF11) modulates susceptibility to 6-OHDA-induced nigral degeneration and behavioral deficits through NF-kappaB signaling in dopaminergic cells. Neurobiol Dis. 2013;54:264–279. doi: 10.1016/j.nbd.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pranski EL, Van Sanford CD, Dalal NV, Orr AL, Karmali D, Cooper DS, Costa N, Heilman CJ, Gearing M, Lah JJ, Levey AI, Betarbet RS. Comparative distribution of protein components of the A20 ubiquitin-editing complex in normal human brain. Neurosci Lett. 2012;520:104–109. doi: 10.1016/j.neulet.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Punsawad C, Krudsood S, Maneerat Y, Chaisri U, Tangpukdee N, Pongponratn E, Nantavisai K, Udomsangpetch R, Viriyavejakul P. Activation of nuclear factor kappa B in peripheral blood mononuclear cells from malaria patients. Malar J. 2012;11:191. doi: 10.1186/1475-2875-11-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruland J. Return to homeostasis: downregulation of NF-kappaB responses. Nat Immunol. 2011;12:709–714. doi: 10.1038/ni.2055. [DOI] [PubMed] [Google Scholar]
- 18.Sakurai H, Suzuki S, Kawasaki N, Nakano H, Okazaki T, Chino A, Doi T, Saiki I. Tumor necrosis factor-alpha-induced IKK phosphorylation of NF-kappaB p65 on serine 536 is mediated through the TRAF2, TRAF5, and TAK1 signaling pathway. J Biol Chem. 2003;278:36916–36923. doi: 10.1074/jbc.M301598200. [DOI] [PubMed] [Google Scholar]
- 19.Shembade N, Parvatiyar K, Harhaj NS, Harhaj EW. The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-kappaB signalling. The EMBO journal. 2009;28:513–522. doi: 10.1038/emboj.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spano D, Marshall JC, Marino N, De Martino D, Romano A, Scoppettuolo MN, Bello AM, Di Dato V, Navas L, De Vita G, Medaglia C, Steeg PS, Zollo M. Dipyridamole prevents triple-negative breast-cancer progression. Clin Exp Metastasis. 2013;30:47–68. doi: 10.1007/s10585-012-9506-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xuan A, Long D, Li J, Ji W, Zhang M, Hong L, Liu J. Hydrogen sulfide attenuates spatial memory impairment and hippocampal neuroinflammation in beta-amyloid rat model of Alzheimer's disease. Journal of neuroinflammation. 2012;9:202. doi: 10.1186/1742-2094-9-202. [DOI] [PMC free article] [PubMed] [Google Scholar]