Abstract
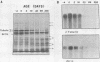
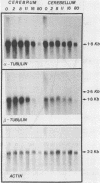
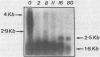
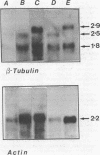
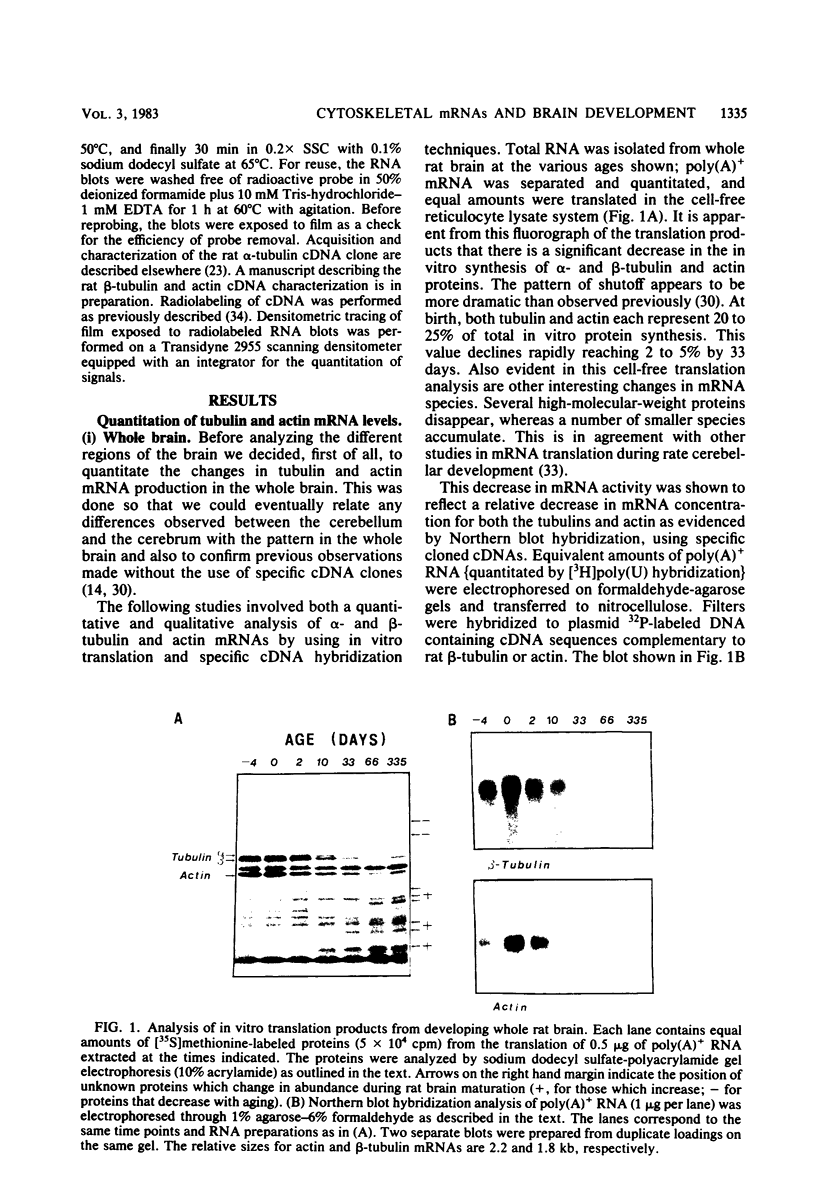
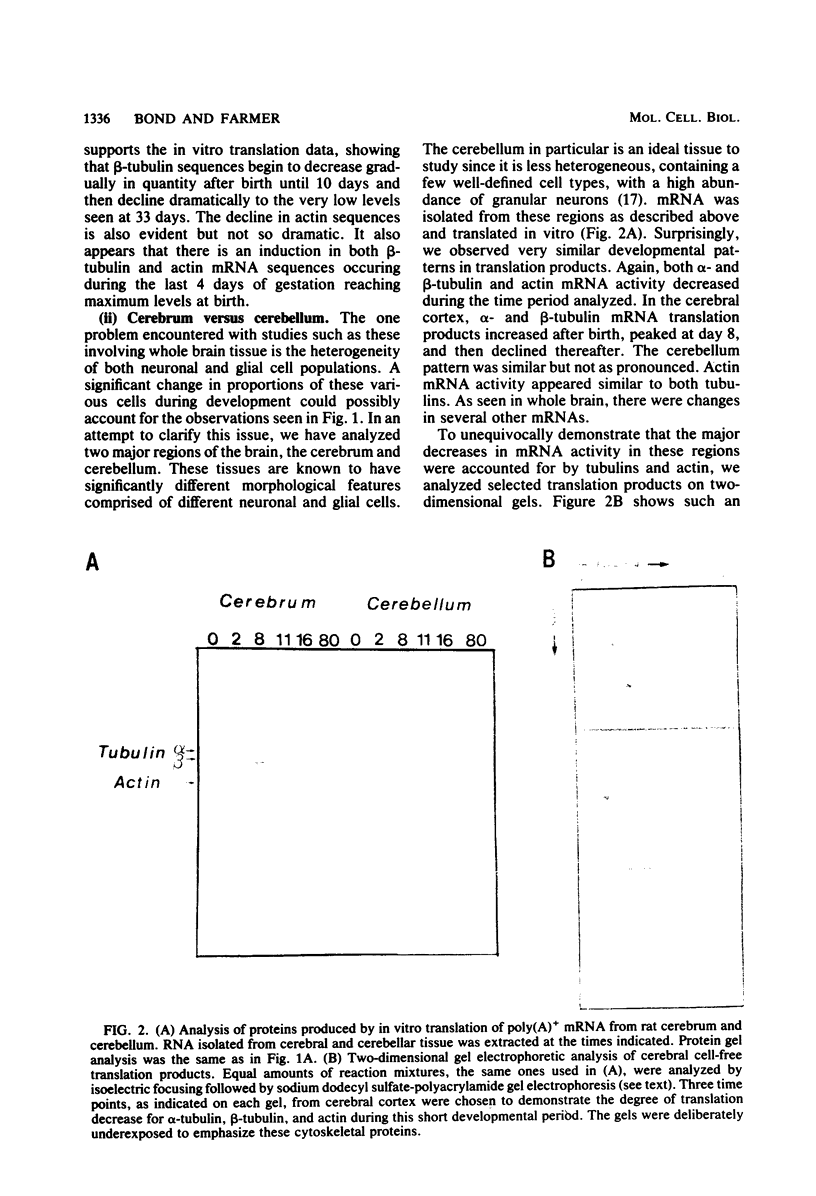
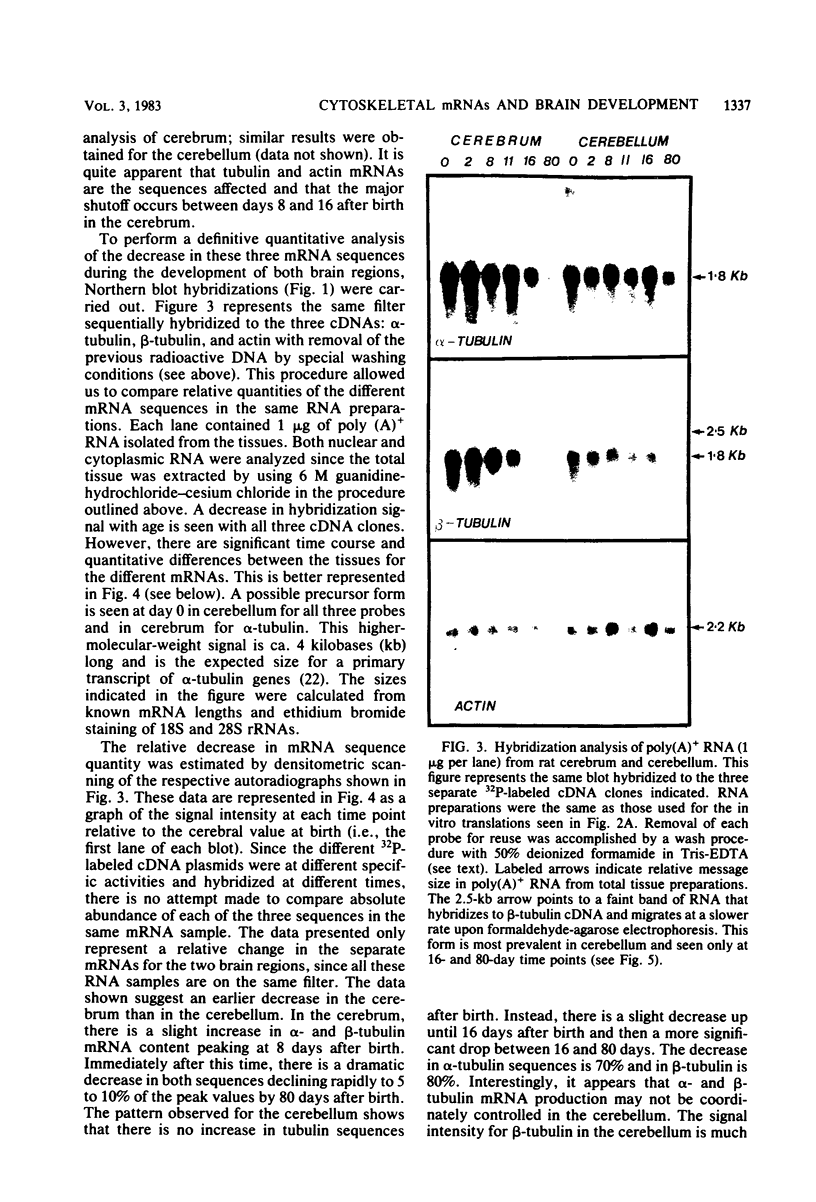
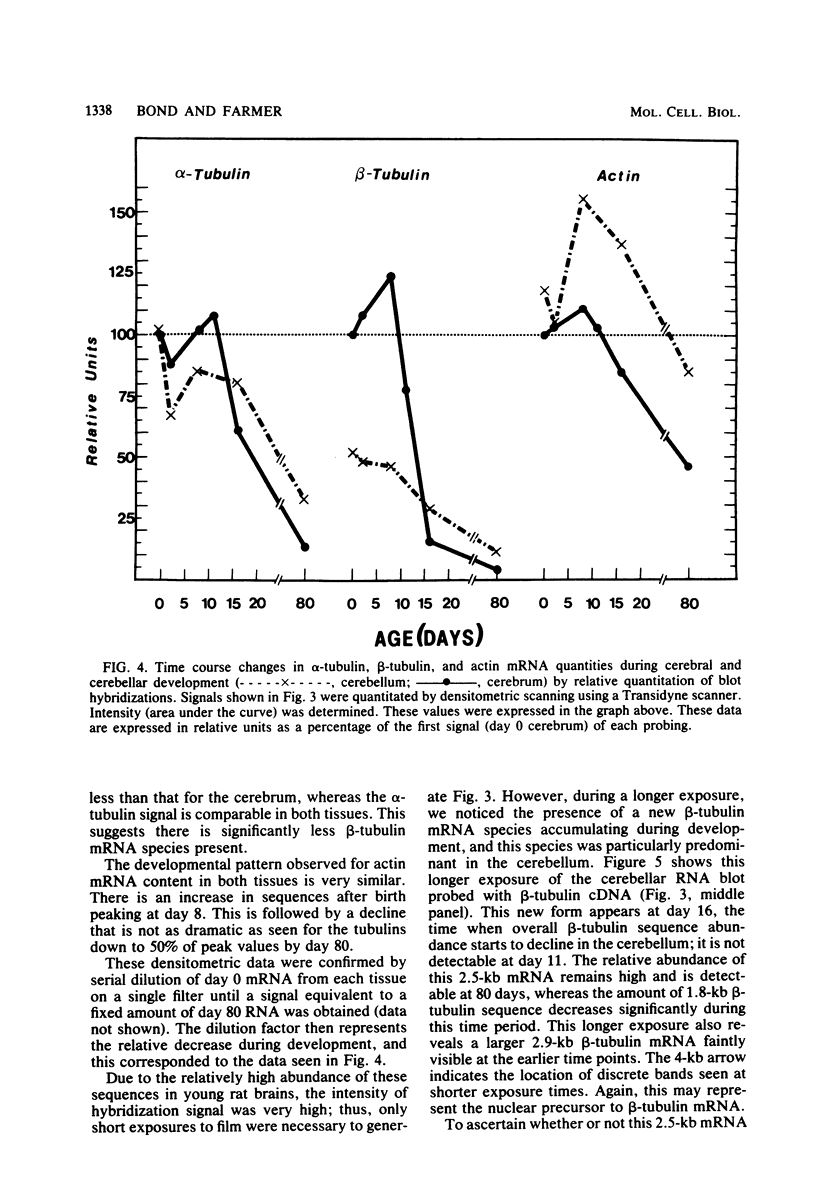
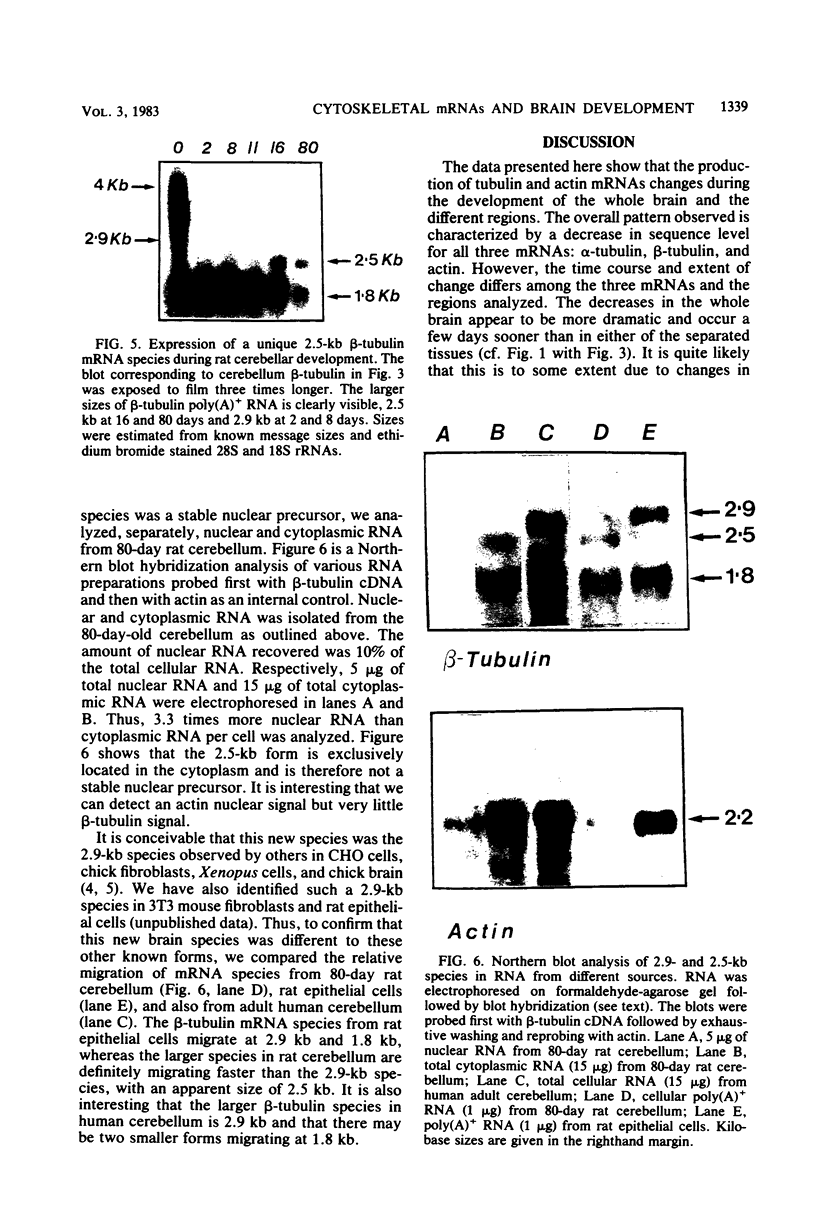
The expression of alpha-tubulin, beta-tubulin, and actin mRNA during rat brain development has been examined by using specific cDNA clones and in vitro translation techniques. During brain maturation (0 to 80 days postnatal), these mRNA species undergo a significant decrease in abundance. The kinetics of this decrease varies between the cerebrum and the cerebellum. These mRNAs are most abundant in both tissues during week 1 postnatal, each representing 10 to 15% of total mRNA activity. Both alpha- and beta-tubulin mRNA content decreases by 90 to 95% in the cerebrum after day 11 postnatal, and 70 to 80% decreases in the cerebellum after day 16. Actin sequences also decrease but to a lesser extent in both tissues (i.e., 50%). These decreases coincide with the major developmental morphological changes (i.e., neurite extension) occurring during this postnatal period. These studies have also identified the appearance of a new 2.5-kilobase beta-tubulin mRNA species, which is more predominant in the cerebellar cytoplasm. The appearance of this form occurs at a time when the major 1.8-kilobase beta-tubulin mRNA levels are declining. The possibility that the tubulin multigene family is phenotypically expressed and then this expression responds to the morphological state of the nerve cells is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ze'ev A., Farmer S. R., Penman S. Mechanisms of regulating tubulin synthesis in cultured mammalian cells. Cell. 1979 Jun;17(2):319–325. doi: 10.1016/0092-8674(79)90157-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Rosbash M. Polynucleotide sequences in eukaryotic DNA and RNA that form ribonuclease-resistant complexes with polyuridylic acid. J Mol Biol. 1974 May 5;85(1):75–86. doi: 10.1016/0022-2836(74)90130-2. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Carney D. H. Evidence that microtubule depolymerization early in the cell cycle is sufficient to initiate DNA synthesis. Cell. 1981 Jan;23(1):61–71. doi: 10.1016/0092-8674(81)90270-1. [DOI] [PubMed] [Google Scholar]
- Crossin K. L., Carney D. H. Microtubule stabilization by taxol inhibits initiation of DNA synthesis by thrombin and by epidermal growth factor. Cell. 1981 Dec;27(2 Pt 1):341–350. doi: 10.1016/0092-8674(81)90417-7. [DOI] [PubMed] [Google Scholar]
- Cumming R., Burgoyne R. D., Lytton N. A. Differential immunocytochemical localisation of alpha-tubulin and beta-tubulin in cerebellum using monoclonal antibodies. Cell Biol Int Rep. 1982 Nov;6(11):1047–1053. doi: 10.1016/0309-1651(82)90021-2. [DOI] [PubMed] [Google Scholar]
- Farmer S. R., Wan K. M., Ben-Ze'ev A., Penman S. Regulation of actin mRNA levels and translation responds to changes in cell configuration. Mol Cell Biol. 1983 Feb;3(2):182–189. doi: 10.1128/mcb.3.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman J., Greenspan H. P. Influence of geometry on control of cell growth. Biochim Biophys Acta. 1975 Dec 31;417(3-4):211–236. doi: 10.1016/0304-419x(75)90011-6. [DOI] [PubMed] [Google Scholar]
- Folkman J., Moscona A. Role of cell shape in growth control. Nature. 1978 Jun 1;273(5661):345–349. doi: 10.1038/273345a0. [DOI] [PubMed] [Google Scholar]
- Friedkin M., Legg A., Rozengurt E. Antitubulin agents enhance the stimulation of DNA synthesis by polypeptide growth factors in 3T3 mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3909–3912. doi: 10.1073/pnas.76.8.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., Sweadner K. J. Multiple tubulin forms are expressed by a single neurone. Nature. 1981 Dec 3;294(5840):477–480. doi: 10.1038/294477a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., de Baetselier A., Littauer U. Z. Translation in vitro of rat brain mRNA coding for a variety of tubulin forms. Eur J Biochem. 1980 Jan;103(1):13–20. doi: 10.1111/j.1432-1033.1980.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Maness P. F., Walsh R. C., Jr Dihydrocytochalasin B disorganizes actin cytoarchitecture and inhibits initiation of DNA synthesis in 3T3 cells. Cell. 1982 Aug;30(1):253–262. doi: 10.1016/0092-8674(82)90031-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Partlow L. M., Larrabee M. G. Effects of a nerve-growth factor, embryo age and metabolic inhibitors on growth of fibres and on synthesis of ribonucleic acid and protein in embryonic sympathetic ganglia. J Neurochem. 1971 Nov;18(11):2101–2118. doi: 10.1111/j.1471-4159.1971.tb05069.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Schmitt H., Gozes I., Littauer U. Z. Decrease in levels and rates of synthesis of tubulin and actin in developing rat brain. Brain Res. 1977 Feb;121(2):327–342. doi: 10.1016/0006-8993(77)90155-x. [DOI] [PubMed] [Google Scholar]
- Schubert D. Induced differentiation of clonal rat nerve and glial cells. Neurobiology. 1974;4(6):376–387. [PubMed] [Google Scholar]
- Setzer D. R., McGrogan M., Schimke R. T. Nucleotide sequence surrounding multiple polyadenylation sites in the mouse dihydrofolate reductase gene. J Biol Chem. 1982 May 10;257(9):5143–5147. [PubMed] [Google Scholar]
- Soreq H., Safran A., Zisling R. Variations in gene expression during development of the rat cerebellum. Brain Res. 1982 Jan;255(1):65–79. doi: 10.1016/0165-3806(82)90076-1. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Strocchi P., Marotta C. A., Bonventre J., Gilbert J. M. The subunit composition of cerebellar tubulin: evidence for multiple beta tubulin messenger RNAs. Brain Res. 1981 Apr 27;211(1):206–210. doi: 10.1016/0006-8993(81)90085-8. [DOI] [PubMed] [Google Scholar]
- Tannenbaum J., Godman G. C. Cytochalasin D induces increased actin synthesis in HEp-2 cells. Mol Cell Biol. 1983 Jan;3(1):132–142. doi: 10.1128/mcb.3.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehner Z. E., Paterson B. M. Characterization of the chicken vimentin gene: single copy gene producing multiple mRNAs. Proc Natl Acad Sci U S A. 1983 Feb;80(4):911–915. doi: 10.1073/pnas.80.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]