Abstract
Objective
To estimate the global burden of congenital toxoplasmosis (CT), which results from infection of pregnant women with Toxoplasma gondii.
Methods
The authors systematically searched 9 major databases for published and unpublished sources and established direct contact with the authors of source materials. Searches were country-specific. To be included, studies had to report on the incidence of CT, on positivity to Toxoplasma-specific IgM in infants and pregnant women (including seroconversion results) or on positivity to Toxoplasma-specific IgG in the general population. Various modelling techniques were used, depending on the country-specific data available, to estimate the CT incidence and burden in each country. These data were then synthesized into an estimate of the global incidence of CT and of the global burden of CT in disability-adjusted life years (DALYs).
Findings
The global annual incidence of congenital toxoplasmosis was estimated to be 190 100 cases (95% credible interval, CI: 179 300–206 300). This was equivalent to a burden of 1.20 million DALYs (95% CI: 0.76–1.90). High burdens were seen in South America and in some Middle Eastern and low-income countries.
Conclusion
Congenital toxoplasmosis poses a substantial burden of poor health globally. Toxoplasmosis should be included in future updates of the global burden of disease and the corresponding data should be used to support public health interventions to reduce disease burden.
Résumé
Objectif
Faire une estimation de la charge mondiale de la toxoplasmose congénitale (TC), qui résulte de l'infection des femmes enceintes par le Toxoplasma gondii.
Méthodes
Les auteurs ont recherché des sources publiées et non publiées dans neuf bases de données principales et sont entré en contact direct avec les auteurs des documents source. Les recherches ont été spécifiques par pays. Pour être incluses, les études devaient décrire l'incidence de la TC sur les résultats positifs aux IgM spécifiques de la toxoplasmose chez les nourrissons et les femmes enceintes (y compris les résultats de séroconversion) ou les résultats positifs aux IgG spécifiques de la toxoplasmose dans la population en général. Différentes techniques de modélisation ont été employées, selon les données disponibles spécifiques par pays, pour estimer l'incidence et la charge de la TC dans chaque pays. Ces données ont été synthétisées en une estimation de l'incidence globale de la TC et de la charge globale de la TC en années de vie corrigées du facteur invalidité (AVCI).
Résultats
L'incidence annuelle globale de la toxoplasmose congénitale a été estimée à 190 100 cas (intervalle de crédibilité, IC de 95%: 179 300–206 300), ce qui équivaut à une charge de 1,20 millions d'AVCI (IC de 95%: 0,76–1,90). Des taux élevés ont été observés en Amérique du Sud et dans certains pays du Moyen-Orient et à faibles revenus.
Conclusion
La toxoplasmose congénitale représente une charge de morbidité considérable au niveau mondial; elle devrait donc être incluse dans les futures mises à jour de la charge mondiale de morbidité et les données correspondantes devraient être employées pour soutenir les interventions en santé publique visant à réduire la charge de morbidité.
Resumen
Objetivo
Calcular la carga mundial de la toxoplasmosis congénita (TC) derivada de la infección de mujeres embarazadas con el parásito Toxoplasma gondii.
Métodos
Los autores buscaron sistemáticamente en nueve de las principales bases de datos de fuentes publicadas y no publicadas y establecieron contacto directo con los autores del material original. Se realizaron búsquedas específicas para cada país. Para ser incluidos, los estudios debían informar sobre la incidencia de la TC, la positividad de IgM específicos de Toxoplasma en niños y mujeres embarazadas (incluidos los resultados de la seroconversión) o la positividad de IgG específicos de Toxoplasma en la población general. Se utilizaron diferentes técnicas de modelización, dependiendo de los datos específicos disponibles de cada país, con el objetivo de calcular la incidencia y la carga de TC en cada país. A continuación se sintetizarod esos datos en una estimación de la incidencia mundial de la TC y la carga mundial de la TC en los años de vida con discapacidad.
Resultados
Se calculó que la incidencia anual de la toxoplasmosis congénita a nivel mundial es de 190 100 casos (un 95% de intervalo de confianza: 179 300—206 300). Esto equivale a una carga de 1,20 millones de años de vida con discapacidad (un 95% de intervalo de confianza: 0,76 – 1,90). En Sudamérica, algunos países de Oriente Medio y en los países de renta baja se observaron cargas elevadas.
Conclusión
La toxoplasmosis congénita plantea una carga importante de salud deficiente a nivel mundial. La toxoplasmosis debería incluirse en las actualizaciones futuras de la carga mundial de morbilidad y deberán utilizarse los correspondientes datos para apoyar las intervenciones en materia de salud pública destinadas a reducir la carga de morbilidad.
ملخص
الغرض
تقدير العبء العالمي لداء المقوسات الخلقي (CT)، الذي ينجم عن إصابة السيدات الحوامل بعدوى المقوسة الغوندية .
الطريقة
قام المؤلفون بالبحث على نحو منهجي في 9 قواعد بيانات رئيسية من أجل الحصول على المصادر المنشورة وغير المنشورة والاتصال المباشر مع مؤلفي مواد المصادر. وكانت الأبحاث خاصة بكل بلد. ولكي يتم إدراج الدراسات، يتعين عليها الإبلاغ عند وجود داء المقوسات الخلقي، بوجود إيجابية للعوز المناعي مفرط الأيج M (IgM) الخاص بالمقوسة لدى الرضع والسيدات الحوامل (بما في ذلك نتائج انقلاب تفاعلية المصل) أو وجود إيجابية لمستضد غلوبين غاما (IgG) الخاص بالمقوسة لدى عامة السكان، وهو ما تقرر إدراجه. وتم استخدام عدة أساليب نمذجة، بناءً على البيانات المتاحة الخاصة بكل بلد، لتقدير معدل الإصابة بداء المقوسات الخلقي والعبء في كل بلد. وتم بعد ذلك استخلاص هذه البيانات في تقدير معدل الإصابة العالمي بداء المقوسات الخلقي والعبء العالمي لداء المقوسات الخلقي بالنسبة لسنوات العمر المصححة باحتساب مدد العجز.
النتائج
بلغ معدل الإصابة العالمي السنوي بداء المقوسات الخلقي وفق التقديرات 190100 حالة (فاصل الثقة 95 %، فاصل الثقة: من 179300 إلى 206300). وكان هذا المعدل معادلاً لعبء 1.20 مليون سنة من سنوات العمر المصححة باحتساب مدد العجز (فاصل الثقة 95 %: من 0.76إلى 1.90). ولوحظت أعباء عالية في أمريكا الجنوبية وفي بعض بلدان الشرق الأوسط والبلدان المنخفضة الدخل.
الاستنتاج
يفرض داء المقوسات الخلقي عبئاً كبيراً على سوء الظروف الصحية على الصعيد العالمي. وينبغي إدراج داء المقوسات في التحديثات المستقبلية للعبء العالمي للمرض وينبغي استخدام البيانات المقابلة لدعم تدخلات الصحة العمومية بُغية تقليل عبء المرض.
摘要
目的
评估先天性弓形虫病(CT)的全球负担,这种病因孕妇感染刚地弓形虫而导致。
方法
作者系统地搜查公布和未公布来源的9 个主要数据库并与来源资料的作者建立直接联系。搜索按国家进行。纳入的研究必须有CT发病率、婴儿和孕妇的弓形虫特定IgM阳性(包含血清转换结果)或者一般人群的弓形虫特定IgG阳性的相关报告。根据各个国家可用的特定数据,使用各种建模技术,估计每个国家的CT发病率和负担。这些数据而后进行合成,以评估CT全球发病率和以伤残调整数字年(DALY)计量的CT全球负担。
结果
全球先天性弓形虫病每年发病率估计为190100 例(95%置信区间,CI:179300–206300)。这相当于120 万DALY(95% CI:0.76–1.90)。南美洲和一些中东国家以及低收入国家的负担高。
结论
先天性弓形虫病在全球带来不良健康状况的沉重负担。在全球疾病负担的未来更新中应包括弓形体病,并应使用相应的数据以支持公共卫生干预措施,从而减少疾病负担。
Резюме
Цель
Оценить глобальное бремя врожденного токсоплазмоза (ВТ), который возникает в результате инфицирования беременных женщин Toxoplasma gondii.
Методы
Авторы провели систематический поиск опубликованных и неопубликованных источников в 9 основных базах данных и установили прямой контакт с авторами исходных материалов. Поиск проводился с учетом особенностей каждой конкретной страны. Для включения в обзор в исследованиях должно было сообщаться о случаях ВТ, о положительных пробах на присущий Toxoplasma иммуноглобулин М у младенцев и беременных женщин (включая результаты сероконверсии) или положительных пробах на присущий Toxoplasma иммуноглобулин М среди населения в целом. Для оценки уровня заболеваемости и бремени ВТ в каждой стране использовались различные методы моделирования в зависимости от имеющихся данных по конкретной стране. Затем эти данные были обобщены в оценке глобального уровня заболеваемости и глобального бремени ВТ, выраженной в потерянных годах жизни, скорректированных на инвалидность (DALY).
Результаты
Глобальная ежегодная заболеваемость врожденным токсоплазмозом оценивается на уровне 190 100 случаев (95%-ный доверительный интервал, ДИ: 179 300–206 300). Это эквивалентно бремени 1,2 млн потерянных лет жизни, скорректированных на инвалидность (95%-ный ДИ: 0,76-1,90). Высокие уровни бремени были отмечены в Южной Америке, некоторых ближневосточных странах и странах с низким уровнем доходов.
Вывод
Врожденный токсоплазмоз создает значительное бремя плохого здоровья во всем мире. Токсоплазмоз должен быть включен в будущие уточненные варианты глобального бремени болезней, и соответствующие данные должны быть использованы для поддержки мероприятий в области общественного здравоохранения для снижения бремени данной болезни.
Introduction
Toxoplasmosis is present in every country and seropositivity rates range from less than 10% to over 90%.1 The causative agent, Toxoplasma gondii, has a complex life cycle and is an important foodborne pathogen. Human infection can result from the ingestion or handling of undercooked or raw meat containing tissue cysts. Alternatively, it can result from direct contact with cats or from the consumption of water or food contaminated by oocysts excreted in the faeces of infected cats.2
Congenital toxoplasmosis (CT) occurs in infants following maternal transmission. It can result in fetal death and abortion and in syndromes that include neurologic and neurocognitive deficits and chorioretinitis.3 We aimed to estimate the global incidence and burden of CT as part of a larger study on the global burden of foodborne toxoplasmosis arising from an initiative coordinated by the Food Borne Disease Burden Epidemiology Reference Group of the World Health Organization (WHO).4,5
In our systematic review we searched specifically for data on CT incidence in infants (seropositivity to Toxoplasma-specific IgM or confirmed case series) or on the rate of maternal transmission, from which to estimate the incidence of CT. We also searched for country-specific data on seropositivity to Toxoplasma-specific IgM and IgG among women of reproductive age and in the general population, with and without age stratification. We used various models to estimate country-specific CT incidences from the data we obtained.
Methods
Literature and data searches
We applied a “best available evidence” approach by conducting a systematic review of the literature on the incidence of CT and on the prevalence of seropositivity to Toxoplasma-specific IgM. Box 1 shows the databases that we accessed and the search terms that we used. A PRISMA statement is provided in Appendix A (available at: www.vetepi.uzh.ch/research/Diseaseburden/Burden_CT-Appendices.pdf). We sent the authors of retrieved publications an Excel (Microsoft, Redmond, United States of America) spreadsheet with data for them to check and amend as appropriate. We cross-checked any amendments suggested and incorporated them into our database where appropriate.
Box 1. Databases accessed and search terms used.
The following databases were accessed during the literature review:
Web of knowledge: http://apps.webofknowledge.com
Embase: http://www.embase.com/
Google Scholar: http://scholar.google.com/
Scopus : http://www.scopus.com/home.url
SciELO : http://www.scielo.br/
MyAIS : http://myais.fsktm.um.edu.my
Science Links Japan: http://sciencelinks.jp/
Index Medicus for South-East Asian Region: http://imsear.hellis.org/
Free Medical Journals: http://www.freemedicaljournals.com/
Asia Journals on Line: http://www.asiajol.info/
African Journals on Line: http://ajol.info/
African Medical Journals (WHO Regional Office for Africa): http://indexmedicus.afro.who.int/Journals/Indexj.htm
Eastern Mediterranean Region Journal Information Directory (WHO Regional Office for the Eastern Mediterranean): http://www.emro.who.int/emrjorlist/Online.aspx
China National Knowledge Infrastructure: http://en.cnki.com.cn/
Eastview (Russian, eastern European and other Eurasian Countries): http://www.eastview.com
We employed the following search terms/keywords: (toxoplasmosis or toxoplasma or TORCH) AND (immunity OR susceptibility OR seroprevalence OR sero-prevalence OR seroepidemiology OR sero-epidemiology OR serology OR seroprofile OR antibod* OR incidence) AND (pregnan* OR reproductive age OR mother* OR childbearing OR women OR antenatal). These search terms were also used in Russian, Spanish and Chinese in appropriate databases. Initially only publication dates between 1 January 2000 and 31 December 2010 with available abstract were used. Publications relating only to animal studies were excluded. If no data were available for a country, the search was repeated to include publications as early as 1980. The search was also widened in such cases by removing references to women of child-bearing age from the search terms. Finally, during the preparation of the article, searches were repeated for countries with missing data for 2011–2012.
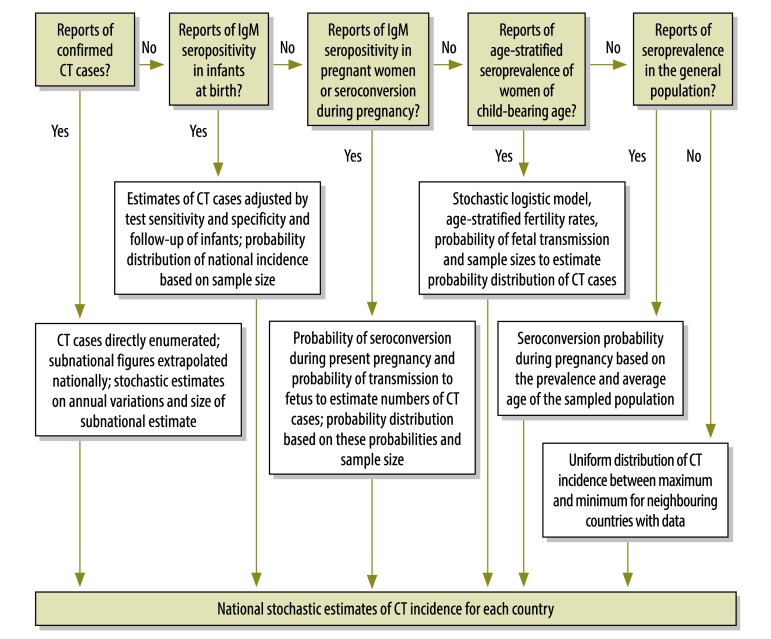
We used an algorithm to select the best available evidence for each country (Fig. 1). From each source we extracted the following when available: incidence of CT, positivity to Toxoplasma-specific IgM in infants and in pregnant women, rate of seroconversion in pregnant women, and prevalence of CT in the general population, with and without age stratification; study period; study subjects; and sampling method (where and how the subjects were selected). No study was excluded on the basis of language. In countries where only one study was available, we used that study. When two or more publications with data of similar quality were available, we used the mean value given in the most recent publication and in the more valid studies and summed up the results of these, or we used the data in one publication to verify the estimates generated by the other(s). Where no data could be found, we extrapolated estimates from neighbouring countries from the same WHO region and in the same WHO mortality stratum. We entered all data extracted onto an Excel spreadsheet for further analysis.
Fig. 1.
Flowchart illustrating the algorithm for the best evidence approach
CT, congenital toxoplasmosis.
Incidence of congenital toxoplasmosis
Because a variety of methods are used in toxoplasmosis surveillance globally, we used different methods depending on the type and quality of the data available. In a few instances we were able to directly calculate the number of incident CT cases from reports of confirmed CT cases in a given country. When the data pertained only to a sample of all possible cases, we used stochastic uncertainty analysis based on sample size as a proportion of the estimated number of births in the country.
In the absence of recorded confirmed CT cases, it is possible to estimate the incidence of CT from serological surveys of infants at birth. In the studies we analysed, blood was obtained by heel pricks or from the umbilical cord to measure Toxoplasma-specific IgM titres. Positive samples were usually confirmed with a second test because a single IgM test has poor positive predictive value. Data from such studies also needed to be adjusted for poor diagnostic sensitivity. We identified four studies in which a cohort of children were tested for IgM at birth and then monitored for at least 1 year (Appendix B, available at: www.vetepi.uzh.ch/research/Diseaseburden/Burden_CT-Appendices.pdf). Of these children, 385 were diagnosed with CT during infancy but only 205 were seropositive at birth, which shows that IgM testing at birth has a sensitivity of 53.3%.
The presence of Toxoplasma-specific IgM in pregnant women indicates recent infection because IgM antibodies are relatively short-lived. They can, however, remain detectable in blood for long after the pregnancy has ended. To estimate the proportion of women who were seropositive as a result of seroconversion during their current pregnancy, we constructed a simple mathematical model, using Monte-Carlo simulations, based on the kinetics of loss of Toxoplasma–specific IgM and on the length of time that the woman had been seropositive (Appendix C, available at: www.vetepi.uzh.ch/research/Diseaseburden/Burden_CT-Appendices.pdf).6 Since most studies failed to report the stage of pregnancy when women were tested, we assumed that each woman was midway through her pregnancy when her blood was sampled. Using this approach, we estimated the probability that a pregnant woman who was IgM-positive had seroconverted during the pregnancy as being between 15.4 and 24.3% (mean: 19.9%).
Toxoplasma-specific IgG seroprevalence among women increases with age. Thus, the age-specific incidence of seroconversion in women can be estimated by using a logistic modelling approach across the group aged 15 to 50 years. Once we knew the parameter relating age to change in prevalence, we were able to calculate the change in prevalence between any age t and t + 9 months and obtain in this manner the approximate risk of seroconversion in a pregnant woman at age t. Demographic and age-stratified fertility data for each country and territory are available from the United States Census Bureau.7 We used such data to estimate the numbers of children born to women who seroconverted during pregnancy. The base year for the data used was 2008.
For some countries only non-age-stratified seroprevalence reports were available. The incidence of seroconversion (and from this the incidence of seroconversion in pregnant women) was estimated by using reports of the age of the population surveyed (or, if not available, the median age of the countries’ population from the United States Census Bureau) and the model of Larsen and Lebech.8
To estimate the proportion of seroconverting pregnant women who transmit toxoplasmosis to their offspring in utero, we identified manuscripts from the systematic review that contained data on cohorts of women who seroconverted during pregnancy. We identified 10 studies, including two systematic reviews containing collections of other studies (Appendix D, available at: www.vetepi.uzh.ch/research/Diseaseburden/Burden_CT-Appendices.pdf) that had suitable data from 6198 pregnancies during which seroconversion had occurred. This resulted in 1740 congenitally-infected infants, or a mean transmission rate of 28.1%. We used this mean transmission rate for both the IgM-positive pregnant women and for those who seroconverted during pregnancy, estimated from the formula relating increasing IgG seropositivity with increasing age.
In one study from the United Kingdom of Great Britain and Northern Ireland,9 the authors employed a systematic review of the published literature from the United Kingdom to estimate the national incidence of toxoplasmosis and used various data sources. From this toxoplasmosis incidence we estimated the number of incident CT cases using the transmission rate described above.
Some countries had no reported data for CT or any seroprevalence data. We had to model their data using data from neighbouring countries. Other countries had sparse data that may not have been nationally representative. This was assessed by examining the target population reported, to check for non-random or biased sampling that included only certain populations. In cases in which the data were sparse or of poor quality and in which alternative data were not available, we assumed a much greater uncertainty surrounding CT incidence and prevalence estimates. The quality of the data provided by each study is indicated in Appendix E (available at: www.vetepi.uzh.ch/research/Diseaseburden/Burden_CT-Appendices.pdf).
Diagnostic uncertainty
Wherever possible, we adjusted observed prevalences in accordance with the reported performance of the diagnostic test employed. We obtained the sensitivity and specificity of the diagnostic tests from either the original study, from the test manufacturer or from other reports in which the same test had been used. In many studies in which the diagnostic test had poor positive predictive value, false positives were largely eliminated by using a secondary confirmatory test and follow-up. Thus, when we used data from such studies we only had to adjust for the sensitivity of the test.
Disease model and disability weights
The disability-adjusted life year (DALY) metric was used to estimate the total disease burden comprised by CT. This followed established methods,10–12 with DALY = YLL + YLD. YLL is the number of years lost on account of death, and YLD is the number of years lived with a disability, weighted by a factor between 0 and 1 for the severity of the disability. In the main text we report the burden of CT without age weighting or discounting for either YLL or YLD calculations, as was done in the most recent global burden of disease (GBD) study.13 However, in Appendix F (available at: www.vetepi.uzh.ch/research/Diseaseburden/Burden_CT-Appendices.pdf) we included a table showing mean DALYs per case of CT calculated after age weighting and discounting. The duration of all sequelae of CT was considered to be lifelong, with the exception of a fraction of the cases of chorioretinitis, in which the onset of clinical signs can occur at a mean age of approximately 10 years, according to the evidence.10 The life table reported in the most recent GBD study with males and females having a life expectancy at birth of 86 years was used to calculate DALYs.13 The frequency of sequelae varies with the genotype of T. gondii. The incidence of sequelae for all regions except the Region of the Americas was calculated as suggested in a previous study.14 This is consistent with the CT sequelae reported when predominantly type 2 genotypes are involved, as seen in Europe.15 However, we used a higher incidence of eye lesions for South American cases and for 61% of North American cases (Table 1). There is considerable parasite polymorphism in South America.16 Non-type-2 genotypes, which are common there, appear to have more frequent and more severe sequelae. Two thirds of Brazilian children with CT had chorioretinitis by the age of 4 years, compared with 1 in 6 European children with CT.17 Brazilian CT patients with eye lesions showed more severe visual impairment. In other studies, 80% of CT patients in Brazil were found to have chorioretinitis, with 63% of them suffering bilateral lesions.18 Consequently, we assumed that chorioretinitis was present in 90% of CT cases from South America (80% in the first year of life), with bilateral involvement by 6 years of age in 70% of the affected cases. According to recent research in North America, 39% of CT cases (in a sample of 166) were exclusively of type 2 genotype, but the remaining 61% were not.19 Thus, for North American CT cases we assigned a 39% frequency of chorioretinitis, as in Europe, and to the remaining 61% of patients we assigned the same frequency of sequelae as observed in South American patients. We used the same disease model as reported in a previous paper.10 Our stochastic techniques for estimating the incidence of sequelae were similar to the techniques used to calculate disease incidence. Only the most severe disability weight was given to any one case. Thus, an infant with both chorioretinits and severe central nervous system abnormalities was only given the weight for the latter. This adjustment was important, for example, for South American cases, since most CT patients there appear to have chorioretinitis.
Table 1. Disease weights for the various syndromes associated with congenital toxoplasmosis, together with the estimated incidence of sequelae.
| Syndrome | Disability weight | Incidencea (95% CI) |
|---|---|---|
| Fetal loss (> 24 weeks gestation) | 1 | 2.4 (2.3–6.3) |
| Neonatal death | 1 | 0.7 (0.4–1.2) |
| Chorioretinitis in first year of life | 0.033 | 13 (12–15) |
| Chorioretinitis later in life | 0.033 | 16 (5–52) |
| Chorioretinitis in first year of life (NA and SA) | 0.033 | 80 (70–90)b |
| Chorioretinitis later in life (NA and SA) | 0.033 | 10 (5–15)b |
| Intracranial calcification | 0.01 | 11 (7.9–12)b |
| Hydrocephalus | 0.36 | 2.0 (1.0–3.0) |
| CNS abnormalities | 0.36 | 2.9 (1.0–6.0) |
CNS, central nervous system; CI, credible interval; NA, North America; SA, South America.
a Per 100 cases.
b To avoid having the incidence of sequelae being greater than the total incidence, in cases from South America these incidences were applied to the residual incidence once sequelae with higher disability weights had been extracted.
Data were obtained from Kortbeek et al.,10 Havelaar et al.14 and Salomon et al.20
In the most recent GBD study the disability weight for impaired vision ranged from 0.004 for mild distance vision impairment, to 0.195 for distance vision blindness.20 We assumed that cases with chorioretinitis had, on average, moderately impaired distance vision (disability weight [DW] = 0.033). However, Appendix F contains estimates of YLDs and DALYs per case, with DW ranging from 0 to 0.2. This covers the range of vision deficits reported in the GBD 2010 study. Similarly, the DWs we used for neurological disorders seem consistent with the range of values suggested in that study.20 The disability weights we used for the various syndromes associated with CT and the incidence of these syndromes are displayed in Table 1.
Spreadsheet model
We also looked for potentially biased data by examining the targeted populations reported in each study and checking for non-representative sampling (such as sampling in an unrepresentative geographic region of a given country). Bias was assessed on a scale from 1 to 4 (Appendix E). For data with a score of 1 we modelled uncertainty on the sample size of the data. We modelled point estimates of prevalences by using a β distribution, which depended on the sample size reported (e.g. the number of pregnant women serologically investigated for IgM). Data with higher bias scores were given progressively wider uncertainty limits. As for all data, β distributions were used for modelling adjustments based on diagnostic uncertainties. Appropriate PERT distributions were used for parameters not bound between 0 and 1. For countries without data, we used uniform distributions, with the highest and lowest estimates representing the extremes of estimates for neighbouring countries. We then drew random samples from the distributions representing each component of the model for calculating incidence, YLDs and YLLs and used the Excel add-in PopTools21 to obtain incidence and DALY estimates. The process was repeated for 10 000 iterations; the median was reported, together with 95% credible intervals (CIs). Sensitivity estimates for the potential influence of countries with missing data were also undertaken on the assumption that these countries’ incidences were similar to either the highest or the lowest incidence in their corresponding WHO subregions.
Results
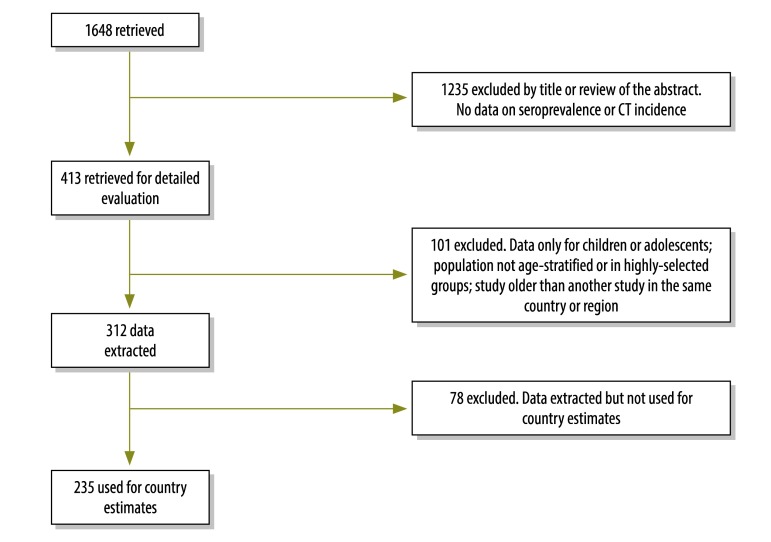
Our search generated 1648 unique citations, including those found in the reference lists of papers found through the systematic review and additional materials supplied by the authors who were contacted. Of this total, we retrieved 413 publications and we used data from 235 of these publications to estimate the incidence of CT in 108 countries (Fig. 2). This included 41 publications – 30 published before 2000 and 11 after 2010 – that were identified when the search criteria were extended for specific countries with no data. All the citations we used are listed in the appendices. We were unable to retrieve data for 87 countries. These countries accounted for approximately 14 million births out of a global total of approximately 131 million in 2008 (11% of the total).
Fig. 2.
Flowchart showing study selection for the review of the evidence surrounding congenital toxoplasmosis (CT)
The global estimated incidence of CT is 190 100 annual cases (95% CI: 179 300–206 300). This amounted to an incidence rate of approximately 1.5 cases of CT per 1000 live births (Table 2). If we were to assume that all countries with missing data had the CT incidences per 1000 births of WHO’s European subregion A or Americas subregion C (the subregions with the lowest and highest CT incidences per 1000 births, respectively), the global estimated annual incidence would be 179 000 and 209 000 cases of CT, respectively. Table 2 shows the global distribution of CT cases by WHO epidemiological regions, which are further subdivided into subregions based on mortality strata (A to E). Appendix E gives the estimated CT incidence and burden (in DALYs) for each country, along with estimates by WHO epidemiological region, uncertainty limits, and the methods for arriving at the estimates. From the annual incidence, the global burden of CT was calculated to be 1.20 million DALYs (95% CI: 0.76–1.90) per annum.
Table 2. Global incidence and burden of congenital toxoplasmosis, by region of the World Health Organization.
| Region | Incident cases (95% CI) | Incidencea (95% CI) | DALYs (95% CI) | DALYsa (95% CI) |
|---|---|---|---|---|
| AFR D | 26 500 (24 300–30 100) | 2.0 (1.8–2.3) | 171 500 (92 300–294 500) | 13 (6.9–22) |
| AFR E | 37 000 (33 900–41 000) | 2.4 (2.2–2.5) | 235 900 (129 600–379 000) | 15 (8.3–24) |
| AMR A | 2940 (2360–3540) | 0.6 (0.5–0.8) | 19 700 (14 100–26 700) | 4.2 (3.0–5.7) |
| AMR B | 15 300 (13 100–17 800) | 1.8 (1.5–2.0) | 105 300 (82 500–127 500) | 12 (9.4–15) |
| AMR C | 5077 (4225–6792) | 3.4 (2.5–4.1) | 35 000 (24 400–41 200) | 19 (13–22) |
| EMR B | 8450 (6950–9530) | 2.5 (2.1–2.9) | 53 900 (27 800–84 800) | 17 (8.5–26) |
| EMR D | 26 300 (21 200–31 200) | 2.2 (1.7–2.6) | 164 900 (84 600–277 800) | 14 (6.9–23) |
| EUR A | 2170 (1900–2896) | 0.5 (0.4–0.6) | 13 600 (7 508–23 400) | 2.8 (1.3–4.3) |
| EUR B | 5200 (4500–6090) | 1.5 (1.3–1.7) | 32 200 (17 500–54 700) | 9.2 (5.0–16) |
| EUR C | 4200 (3700–4800) | 1.6 (1.4–1.8) | 26 400 (14 400–42 700) | 10 (5.4–16) |
| SEAR B | 6430 (4240–8600) | 1.3 (0.9–1.7) | 40 300 (18 700–71 800) | 8.1 (3.8–14) |
| SEAR D | 25 400 (20 700–30 700) | 0.8 (0.7–1.0) | 158 300 (85 900–275 400) | 5.1 (2.8–8.9) |
| WPR A | 960 (720–1200) | 0.6 (0.5–0.8) | 5950 (2900–10 100) | 3.9 (1.9–6.6) |
| WPR B | 24 200 (20 500–28100) | 1.1 (0.9–1.3) | 154 700 (81 200–253 000) | 7.1 (3.7–12) |
| Total | 190 100 (179 300–206 300) | 1.5 (1.4–1.6) | 1 200 000 (760 000–1 900 000) | 9.6 (5.8–15) |
AFR, African Region; AMR, Region of the Americas; CI, credible interval; DALY, disability-adjusted life year; EMR, Eastern Mediterranean Region; EUR, European Region; SEAR, South-East Asia Region; WPR, Western Pacific Region.
a Per 1000 live births.
Discussion
We have used all available evidence and various techniques to generate our data. Hence, we believe that our estimates of the global burden of CT are robust. Most commonly we obtained CT incidence estimates from age-stratified seroprevalence data, which were the most widely available. Although this approach has been used before,8,22–25 it has at least two major disadvantages. First, infection pressure, or probability of exposure to T. gondii, varies with age. In particular, children and adults can have different rates of seroconversion.8,9 We minimized this potential error by modelling the age-stratified data only in adults of reproductive age and using logistic regression to discount the cumulative incidence acquired during childhood. Second, the evidence suggests that seroprevalence has decreased over the last few decades in high-income countries.1 This could artificially inflate the differences between younger and older women and upwardly bias the estimated rates of seroconversion during pregnancy. However, we ameliorated this potential bias, since there was substantially more data from other sources in high-income countries, such as birth surveillance data. In lower-income countries, on the other hand, there is less certain evidence of decreases in seroprevalence over time. Indeed, in Malaysia and the Russian Federation, seroprevalence appears to be increasing in the general population.26,27 In such cases, using age-stratified data to estimate incidence could lead to underestimates of the risk of CT. Furthermore, in countries undergoing rapid industrialization, such as China, demand for meat has increased enormously, and this might increase the risk of exposure to T. gondii over time.
We adjusted the data in accordance with the known performance of the diagnostic tests used in the studies, while taking into account any secondary or confirmatory tests used. However, diagnostic tests can perform with varying sensitivity and specificity in different populations, so this may have introduced further uncertainty in our estimates. This uncertainty is impossible to adjust for because we could only use the test performance values data that were available and extrapolated them to other populations.
The incidence of CT changes much less than population seroprevalence. When infection pressure is high, a small proportion of women are susceptible to infection when they reach child-bearing age, since most have already been exposed. Therefore, although seronegative women are at greater risk, the at-risk population is low overall. When infection pressure is low, the opposite is the case: a large population is at risk but the probability of exposure is low. From a theoretical perspective, the largest number of children is born with CT when the incidence of seroconversion is about 4% per annum. At this incidence, approximately 60% of pregnant women are immune and 1% might be affected. However, if the incidence of seroconversion halves to 2% per annum, the number of affected pregnancies is reduced to approximately 0.8%8 – a decrease of only 20%. Consequently, very small changes in the expected number of CT cases occur even in the presence of large fluctuations in population seroprevalence. We modelled this phenomenon using Monte-Carlo methods. Therefore, the relative stability of the incidence of CT despite wider fluctuations in population seroprevalence makes our estimates more robust. This is reflected in the relatively narrow CIs of our estimates for the global incidence of CT, independent of wide variations in the quality of the data from different countries and the wide variations in population seroprevalence. Furthermore, simple sensitivity analysis suggests that countries with missing data are not likely to have a large influence on the estimated global burden of CT.
We did not utilize DisMod (World Health Organization, Geneva, Switzerland),28a software tool that can be used to check the consistency of estimates of disease incidence, prevalence, duration and case-fatality rates. The latest generally available version of DisMod software (version 2) is not suitable for infectious diseases that confer immunity28 and the incidence of CT is strongly regulated by immunity in women of child-bearing age. When possible, we checked for consistency by basing estimates on different types of data. For example, in Brazil we estimated the expected incidence of CT from age-stratified prevalence rates in women and from the incidence of IgM-positive blood tests in neonates (the latter adjusted for sensitivity and specificity). Both techniques gave estimates ranging from 6000 to 9000 cases of CT per year.
The highest burden of CT is clearly in South America and it is driven by the more pathogenic genotypes that circulate in that part of the world. The regions with the highest incidence of CT (as opposed to the greatest number of DALYs) include parts of the Middle East and some low-income countries in Africa.
Our estimates of the incidence and burden of CT point to a very large global burden of toxoplasmosis. The burden is even greater when various conditions and problems related to toxoplasmosis are considered. For example, toxoplasmosis is known to cause chorioretinitis in healthy adults29 and especially in people who have immunosuppression.30 Encephalitis due to toxoplasmosis is a well-known complication of HIV infection. There are also increasing reports of neurological, psychiatric or psychomotor disorders related to “latent” toxoplasmosis,31–34and of a higher frequency of road traffic accidents among seropositive individuals.35 Even if toxoplasmosis accounts for only a small fraction of these problems in the population, the global burden of disease attributable to toxoplasmosis is considerably greater than suggested by our CT data. In addition, since much toxoplasmosis can be prevented, at least in theory, by observing appropriate food hygiene,36 the disease deserves a higher profile in GDB studies and should not be subsumed into other disease categories, such as “congenital disorders”.
In the GBD 2010 study,37 age weighting and disability weights were not used, a practise we have also followed. However, we calculated YLDs as DW × d × i, where d is disease duration and i is disease incidence. In the GBD 2010 study, YLDs were calculated as the number of cases multiplied by DW. The two techniques should give similar estimates, since most sequelae of CT are lifelong, if one assumes, of course, that the incidence of CT does not change over time and that the population size is stable. However, with a growing population, which is characteristic of most low-income countries, our method yields a better estimate of YLDs, since there will be more cases among the young than among older people, simply because of the shape of the population pyramid.
Acknowledgements
We thank Xiao-Nong Zhou of the Chinese Centers for Disease Control and Prevention, Shanghai, for his generous assistance.
Funding:
This study was commissioned by the WHO Food Borne Epidemiology Burden Reference Group which provided small amounts of funding for expenses. The best evidence review of toxoplasmosis prevalence in pregnancy was undertaken with the University of Utah, Subaward # 10008325-01, Prime award # U36CCU319276.
Competing interests:
PT is a member of WHO’s Foodborne Disease Epidemiology Reference Group, which commissioned this work.
References
- 1.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39:1385–94. doi: 10.1016/j.ijpara.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363:1965–76. doi: 10.1016/S0140-6736(04)16412-X. [DOI] [PubMed] [Google Scholar]
- 3.Jones JL, Lopez A, Wilson M, Schulkin J, Gibbs R. Congenital toxoplasmosis: a review. Obstet Gynecol Surv. 2001;56:296–305. doi: 10.1097/00006254-200105000-00025. [DOI] [PubMed] [Google Scholar]
- 4.Flint JA, Van Duynhoven YT, Angulo FJ, DeLong SM, Braun P, Kirk M, et al. Estimating the burden of acute gastroenteritis, foodborne disease, and pathogens commonly transmitted by food: an international review. Clin Infect Dis. 2005;41:698–704. doi: 10.1086/432064. [DOI] [PubMed] [Google Scholar]
- 5.Stein C, Kuchenmüller T, Hendrickx S, Prüss-Ustün A, Wolfson L, Engels D, et al. The Global Burden of Disease assessments–WHO is responsible? PLoS Negl Trop Dis. 2007;1:e161. doi: 10.1371/journal.pntd.0000161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gras L, Gilbert RE, Wallon M, Peyron F, Cortina-Borja M. Duration of the IgM response in women acquiring Toxoplasma gondii during pregnancy: implications for clinical practice and cross-sectional incidence studies. Epidemiol Infect. 2004;132:541–8. doi: 10.1017/S0950268803001948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.US Census Bureau [Internet]. International Programs, International Data Base. Washington: USCB; 2013. Available from: http://www.census.gov/population/international/data/idb/region.php [accessed 1 April 2013].
- 8.Larsen SO, Lebech M. Models for prediction of the frequency of toxoplasmosis in pregnancy in situations of changing infection rates. Int J Epidemiol. 1994;23:1309–14. doi: 10.1093/ije/23.6.1309. [DOI] [PubMed] [Google Scholar]
- 9.Welton NJ, Ades AE. A model of toxoplasmosis incidence in the UK: evidence synthesis and consistency of evidence. J R Stat Soc Ser C Appl Stat. 2005;54:385–404. doi: 10.1111/j.1467-9876.2005.00490.x. [DOI] [Google Scholar]
- 10.Kortbeek LM, Hofhuis A, Nijhuis CDM, Havelaar AH. Congenital toxoplasmosis and DALYs in the Netherlands. Mem Inst Oswaldo Cruz. 2009;104:370–3. doi: 10.1590/S0074-02762009000200034. [DOI] [PubMed] [Google Scholar]
- 11.Murray CJ, Acharya AK. Understanding DALYs (disability-adjusted life years). J Health Econ. 1997;16:703–30. doi: 10.1016/S0167-6296(97)00004-0. [DOI] [PubMed] [Google Scholar]
- 12.Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet. 1997;349:1436–42. doi: 10.1016/S0140-6736(96)07495-8. [DOI] [PubMed] [Google Scholar]
- 13.Murray CJ, Ezzati M, Flaxman AD, Lim S, Lozano R, Michaud C, et al. GBD 2010: design, definitions, and metrics. Lancet. 2012;380:2063–6. doi: 10.1016/S0140-6736(12)61899-6. [DOI] [PubMed] [Google Scholar]
- 14.Havelaar AH, Kemmeren JM, Kortbeek LM. Disease burden of congenital toxoplasmosis. Clin Infect Dis. 2007;44:1467–74. doi: 10.1086/517511. [DOI] [PubMed] [Google Scholar]
- 15.Ajzenberg D, Cogné N, Paris L, Bessières M-H, Thulliez P, Filisetti D, et al. Genotype of 86 Toxoplasma gondii isolates associated with human congenital toxoplasmosis, and correlation with clinical findings. J Infect Dis. 2002;186:684–9. doi: 10.1086/342663. [DOI] [PubMed] [Google Scholar]
- 16.Ferreira A de M, Vitor RWA, Gazzinelli RT, Melo MN. Genetic analysis of natural recombinant Brazilian Toxoplasma gondii strains by multilocus PCR-RFLP. Infect Genet Evol. 2006;6:22–31. doi: 10.1016/j.meegid.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert RE, Freeman K, Lago EG, Bahia-Oliveira LMG, Tan HK, Wallon M, et al. European Multicentre Study on Congenital Toxoplasmosis (EMSCOT). Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl Trop Dis. 2008;2:e277. doi: 10.1371/journal.pntd.0000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasconcelos-Santos DV, Machado Azevedo DO, Campos WR, Oréfice F, Queiroz-Andrade GM, Carellos EV et al.; UFMG Congenital Toxoplasmosis Brazilian Group. Congenital toxoplasmosis in southeastern Brazil: results of early ophthalmologic examination of a large cohort of neonates. Ophthalmology 2009;116:2199-205, e1. [DOI] [PubMed]
- 19.McLeod R, Boyer KM, Lee D, Mui E, Wroblewski K, Karrison T, et al. Toxoplasmosis Study Group Prematurity and severity are associated with Toxoplasma gondii alleles (NCCCTS, 1981–2009). Clin Infect Dis. 2012;54:1595–605. doi: 10.1093/cid/cis258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomon JA, Vos T, Hogan DR, Gagnon M, Naghavi M, Mokdad A, et al. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–43. doi: 10.1016/S0140-6736(12)61680-8. http://dx.doi.org/10.1016/S0140-6736(12)61680-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood GM. Pop Tools version 3.2.5, released September 2011. PopTools; 2011. Available from: http://www.poptools.org [accessed 5 April 2013]. [Google Scholar]
- 22.Ades AE, Nokes DJ. Modeling age- and time-specific incidence from seroprevalence:toxoplasmosis. Am J Epidemiol. 1993;137:1022–34. doi: 10.1093/oxfordjournals.aje.a116758. [DOI] [PubMed] [Google Scholar]
- 23.Zuber PL, Jacquier P, Hohlfeld P, Walker AM. Toxoplasma infection among pregnant women in Switzerland: a cross-sectional evaluation of regional and age-specific lifetime average annual incidence. Am J Epidemiol. 1995;141:659–66. doi: 10.1093/oxfordjournals.aje.a117482. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Marin JE, Montoya-de-Londono MT, Castano-Osorio JC. A maternal screening program for congenital toxoplasmosis in Quindio, Colombia and application of mathematical models to estimate incidences using age-stratified data. Am J Trop Med Hyg. 1997;57:180–6. doi: 10.4269/ajtmh.1997.57.180. [DOI] [PubMed] [Google Scholar]
- 25.Fernandes GCVR, Azevedo RS, Amaku M, Yu ALF, Massad E. Seroepidemiology of Toxoplasma infection in a metropolitan region of Brazil. Epidemiol Infect. 2009;137:1809–15. doi: 10.1017/S0950268809002799. [DOI] [PubMed] [Google Scholar]
- 26.Kalintin A. Epidemiological and immunological aspects of toxoplasmosis in high risk groups [dissertation]. Omsk: University of Omsk; 2007. Russian. [Google Scholar]
- 27.Nissapatorn V, Noor Azmi MA, Cho SM, Fong MY, Init I, Rohela M, et al. Toxoplasmosis: prevalence and risk factors. J Obstet Gynaecol. 2003;23:618–24. doi: 10.1080/01443610310001604376. [DOI] [PubMed] [Google Scholar]
- 28.Barendregt JJ, Van Oortmarssen GJ, Vos T, Murray CJ. A generic model for the assessment of disease epidemiology: the computational basis of DisMod II. Popul Health Metr. 2003;1:4. doi: 10.1186/1478-7954-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Holland GN. Reconsidering the pathogenesis of ocular toxoplasmosis. Am J Ophthalmol. 1999;128:502–5. doi: 10.1016/S0002-9394(99)00263-9. [DOI] [PubMed] [Google Scholar]
- 30.Liesenfeld O, Wong S, Remington JS. Toxoplasmosis in the setting of AIDS. In: Mergan TC, Bartlett JG, Bolognesi D, editors. Textbook of AIDS medicine. 2nd ed. Baltimore: Williams and Wilkins; 1999. pp. 225–9. [Google Scholar]
- 31.Palmer BS. Meta-analysis of three case controlled studies and an ecological study into the link between cryptogenic epilepsy and chronic toxoplasmosis infection. Seizure. 2007;16:657–63. doi: 10.1016/j.seizure.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Koseoglu E, Yazar S, Koc I. Is Toxoplasma gondii a causal agent in migraine? Am J Med Sci. 2009;338:120–2. doi: 10.1097/MAJ.0b013e31819f8cac. [DOI] [PubMed] [Google Scholar]
- 33.Miman O, Kusbeci OY, Aktepe OC, Cetinkaya Z. The probable relation between Toxoplasma gondii and Parkinson’s disease. Neurosci Lett. 2010;475:129–31. doi: 10.1016/j.neulet.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 34.Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38:642–7. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flegr J, Klose J, Novotná M, Berenreitterová M, Havlícek J. Increased incidence of traffic accidents in Toxoplasma-infected military drivers and protective effect RhD molecule revealed by a large-scale prospective cohort study. BMC Infect Dis. 2009;9:72. doi: 10.1186/1471-2334-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kijlstra A, Jongert E. Toxoplasma-safe meat: close to reality? Trends Parasitol. 2009;25:18–22. doi: 10.1016/j.pt.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]