Abstract
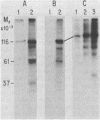
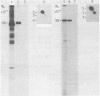
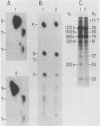
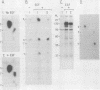
Protein kinases that phosphorylate the hydroxyl group of tyrosine residues of proteins have been implicated in cell transformation by some retroviruses and in regulation of normal cell growth by some polypeptide growth factors. To facilitate the identification of tyrosine kinase substrates, we developed monoclonal antibodies to the hapten azobenzylphosphonate. One of these antibodies, MA-2G8, proved to be especially attractive in that it bound a derivative of aminophenylphosphate, a close phosphotyrosine analog, with higher affinity than it bound the corresponding derivative of aminobenzylphosphonate; however, its affinity for phosphoserine was negligible. In this paper we describe the optimal conditions for using this antibody to isolate phosphotyrosine proteins, emphasizing particularly that its interaction with phosphotyrosyl proteins is sensitive to ionic detergents and to antibody density on the immunosorbent matrix. The antibody also bound ATP citrate lyase; this enzyme lacks phosphotyrosine but contains phosphohistidine, which is similar structurally to phosphotyrosine. By attaching the antibody at high density to Sepharose beads and omitting ionic detergents from the buffers, it was possible by microbatch immunoadsorption (followed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) to isolate the 120,000-dalton transforming protein and several other phosphotyrosyl proteins from cells transformed by Abelson murine leukemia virus. Under the same conditions, phosphotyrosyl proteins were also isolated from human epidermal carcinoma cells (A431) that had been stimulated with epidermal growth factor; most prominent among these proteins was the 170,000-dalton receptor for epidermal growth factor.
Full text
PDF



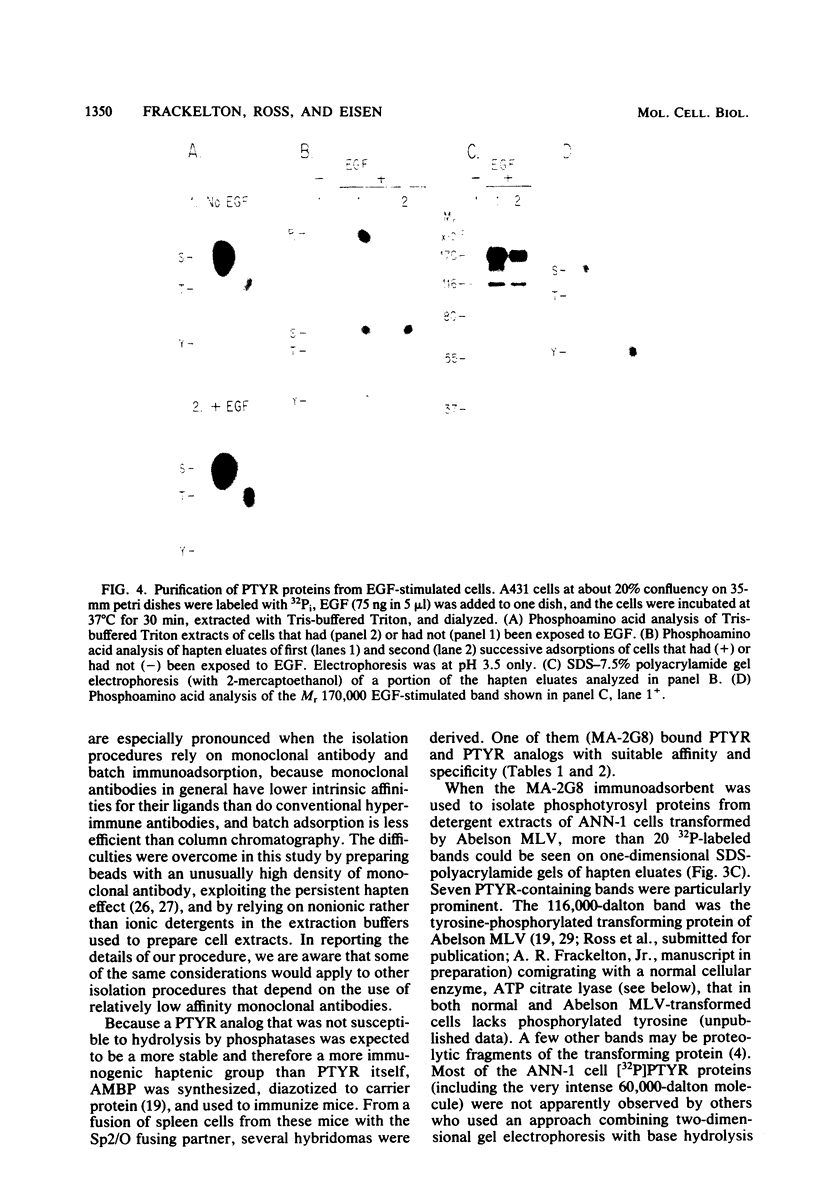


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander M. C., Palmer J. L., Pointer R. H., Kowaloff E. M., Koumjian L. L., Avruch J. Insulin-stimulated phosphorylation of ATP-citrate lyase in isolated hepatocytes. Stoichiometry and relation to the phosphoenzyme intermediate. J Biol Chem. 1982 Feb 25;257(4):2049–2055. [PubMed] [Google Scholar]
- BERG P. Acyl adenylates; the synthesis and properties of adenyl acetate. J Biol Chem. 1956 Oct;222(2):1015–1023. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Boss M. A., Dreyfuss G., Baltimore D. Localization of the Abelson murine leukemia virus protein in a detergent-insoluble subcellular matrix: architecture of the protein. J Virol. 1981 Nov;40(2):472–481. doi: 10.1128/jvi.40.2.472-481.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottam G. L., Srere P. A. Nature of the phosphorylated residue in citrate clevage enzyme. Biochem Biophys Res Commun. 1969 Jun 27;35(6):895–900. doi: 10.1016/0006-291x(69)90708-6. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Fabricant R. N., De Larco J. E., Todaro G. J. Nerve growth factor receptors on human melanoma cells in culture. Proc Natl Acad Sci U S A. 1977 Feb;74(2):565–569. doi: 10.1073/pnas.74.2.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Van Zaane D., Bloemers H. P., Bloemendal H. Synthesis of Rauscher murine leukemia virus-specific polypeptides in vitro. Proc Natl Acad Sci U S A. 1976 Feb;73(2):356–360. doi: 10.1073/pnas.73.2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981 Jun;24(3):741–752. doi: 10.1016/0092-8674(81)90100-8. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Varmus H. E., Bishop J. M. The purified product of the transforming gene of avian sarcoma virus phosphorylates tyrosine. J Biol Chem. 1980 Dec 25;255(24):11973–11980. [PubMed] [Google Scholar]
- Ross A. H., Baltimore D., Eisen H. N. Phosphotyrosine-containing proteins isolated by affinity chromatography with antibodies to a synthetic hapten. Nature. 1981 Dec 17;294(5842):654–656. doi: 10.1038/294654a0. [DOI] [PubMed] [Google Scholar]
- Rothberg P. G., Harris T. J., Nomoto A., Wimmer E. O4-(5'-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Beemon K., Eckhart W. Evidence that the phosphorylation of tyrosine is essential for cellular transformation by Rous sarcoma virus. Cell. 1980 Jul;20(3):807–816. doi: 10.1016/0092-8674(80)90327-x. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Hunter T., Raschke W. C. Evidence that the Abelson virus protein functions in vivo as a protein kinase that phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1552–1556. doi: 10.1073/pnas.78.3.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabarova Z. A. Synthetic nucleotide-peptides. Prog Nucleic Acid Res Mol Biol. 1970;10:145–182. doi: 10.1016/s0079-6603(08)60564-4. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Szmelcman S., Boos W., Schwartz M. On the significance of the retention of ligand by protein. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2120–2124. doi: 10.1073/pnas.72.6.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G. T., Eisen H. N., Jones R. H. The problem of hapten persistently bound to antibody. Biochem J. 1970 Jan;116(1):151–153. doi: 10.1042/bj1160151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]