Abstract
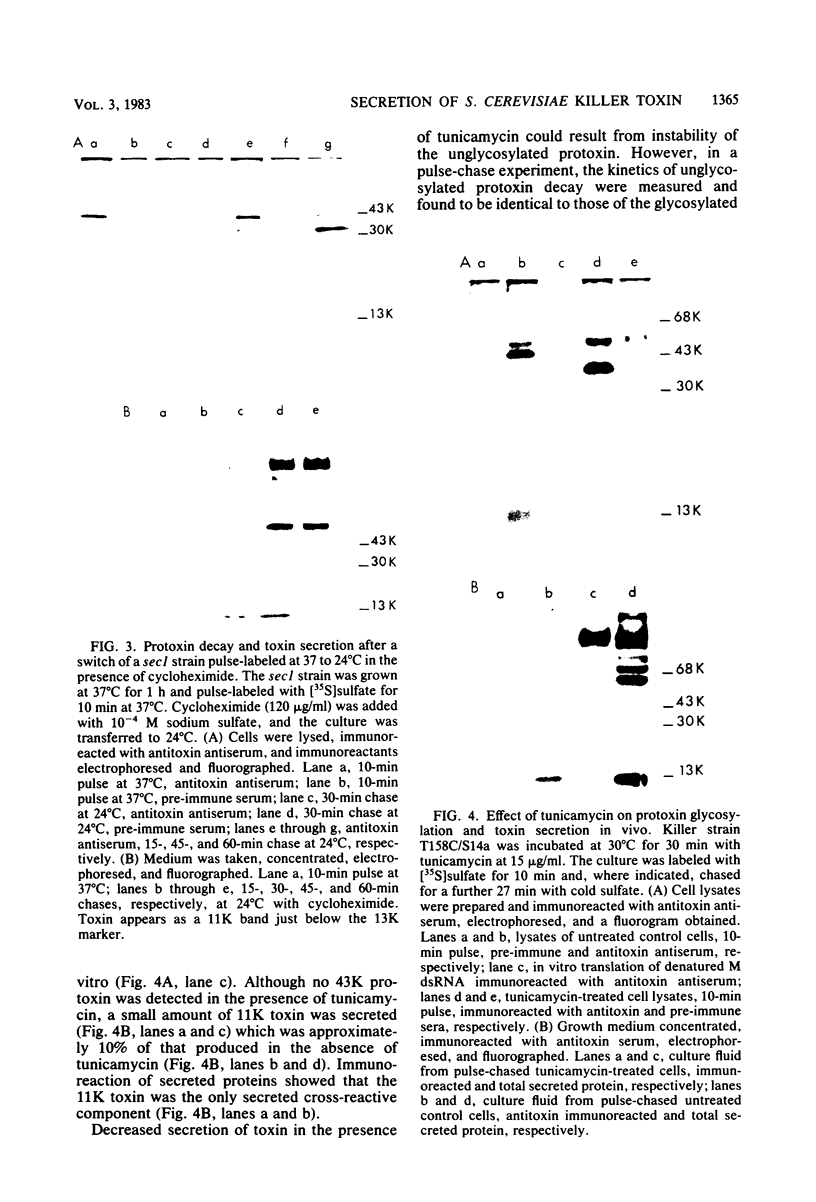
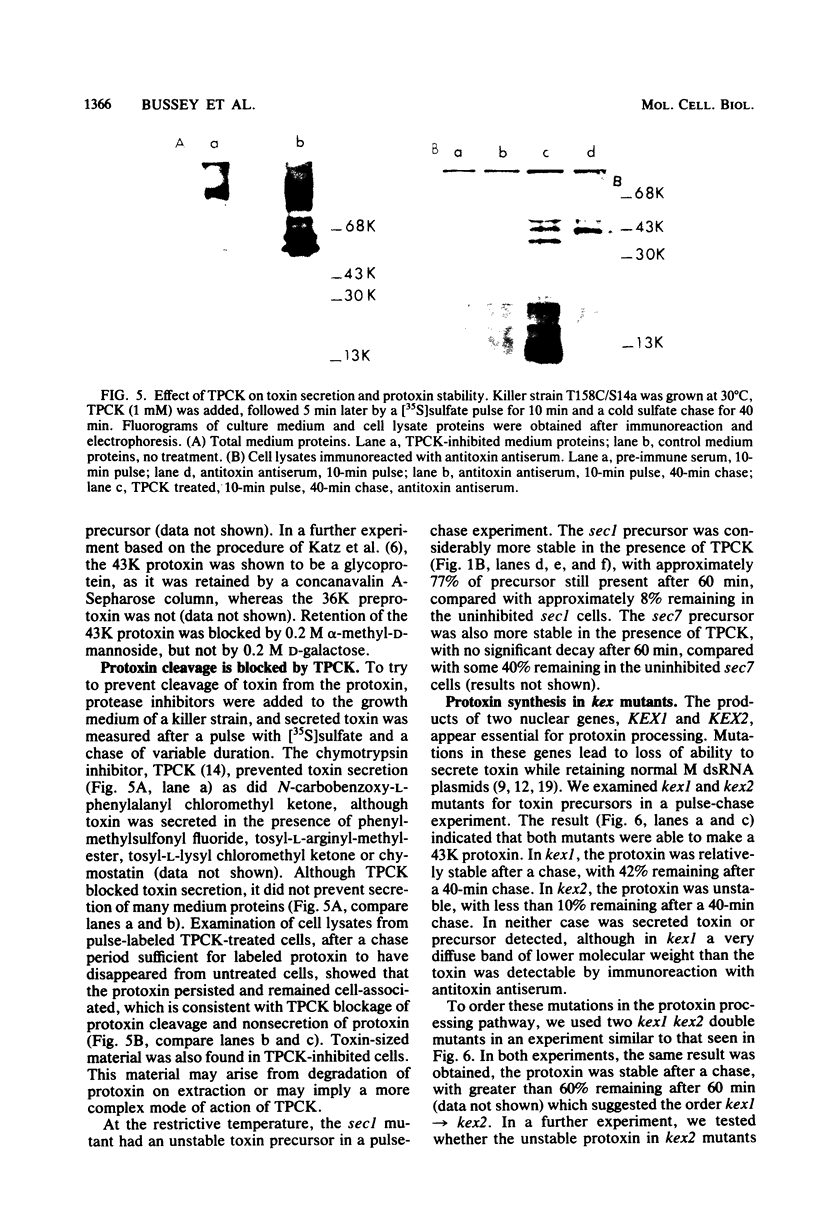
Killer toxin secretion was blocked at the restrictive temperature in Saccharomyces cerevisiae sec mutants with conditional defects in the S. cerevisiae secretory pathway leading to accumulation of endoplasmic reticulum (sec18), Golgi (sec7), or secretory vesicles (sec1). A 43,000-molecular-weight (43K) glycosylated protoxin was found by pulse-labeling in all sec mutants at the restrictive temperature. In sec18 the protoxin was stable after a chase; but in sec7 and sec1 the protoxin was unstable, and in sec1 11K toxin was detected in cell lysates. The chymotrypsin inhibitor tosyl-l-phenylalanyl chloromethyl ketone (TPCK) blocked toxin secretion in vivo in wild-type cells by inhibiting protoxin cleavage. The unstable protoxin in wild-type and in sec7 and sec1 cells at the restrictive temperature was stabilized by TPCK, suggesting that the protoxin cleavage was post-sec18 and was mediated by a TPCK-inhibitable protease. Protoxin glycosylation was inhibited by tunicamycin, and a 36K protoxin was detected in inhibited cells. This 36K protoxin was processed, but toxin secretion was reduced 10-fold. We examined two kex mutants defective in toxin secretion; both synthesized a 43K protoxin, which was stable in kex1 but unstable in kex2. Protoxin stability in kex1 kex2 double mutants indicated the order kex1 → kex2 in the protoxin processing pathway. TPCK did not block protoxin instability in kex2 mutants. This suggested that the KEX1- and KEX2-dependent steps preceded the sec7 Golgi block. We attempted to localize the protoxin in S. cerevisiae cells. Use of an in vitro rabbit reticulocyte-dog pancreas microsomal membrane system indicated that protoxin synthesized in vitro could be inserted into and glycosylated by the microsomal membranes. This membrane-associated protoxin was protected from trypsin proteolysis. Pulse-chased cells or spheroplasts, with or without TPCK, failed to secrete protoxin. The protoxin may not be secreted into the lumen of the endoplasmic reticulum, but may remain membrane associated and may require endoproteolytic cleavage for toxin secretion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bostian K. A., Hopper J. E., Rogers D. T., Tipper D. J. Translational analysis of the killer-associated virus-like particle dsRNA genome of S. cerevisiae: M dsRNA encodes toxin. Cell. 1980 Feb;19(2):403–414. doi: 10.1016/0092-8674(80)90514-0. [DOI] [PubMed] [Google Scholar]
- Bostian K. A., Jayachandran S., Tipper D. J. A glycosylated protoxin in killer yeast: models for its structure and maturation. Cell. 1983 Jan;32(1):169–180. doi: 10.1016/0092-8674(83)90507-x. [DOI] [PubMed] [Google Scholar]
- Bussey H. Physiology of killer factor in yeast. Adv Microb Physiol. 1981;22:93–122. doi: 10.1016/s0065-2911(08)60326-4. [DOI] [PubMed] [Google Scholar]
- Bussey H., Sacks W., Galley D., Saville D. Yeast killer plasmid mutations affecting toxin secretion and activity and toxin immunity function. Mol Cell Biol. 1982 Apr;2(4):346–354. doi: 10.1128/mcb.2.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E., Thorner J. Recovery of S. cerevisiae a cells from G1 arrest by alpha factor pheromone requires endopeptidase action. Cell. 1979 Nov;18(3):623–635. doi: 10.1016/0092-8674(79)90117-x. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Tunicamycin--an inhibitor of yeast glycoprotein synthesis. Biochem Biophys Res Commun. 1974 May 7;58(1):287–295. doi: 10.1016/0006-291x(74)90925-5. [DOI] [PubMed] [Google Scholar]
- Kurjan J., Herskowitz I. Structure of a yeast pheromone gene (MF alpha): a putative alpha-factor precursor contains four tandem copies of mature alpha-factor. Cell. 1982 Oct;30(3):933–943. doi: 10.1016/0092-8674(82)90298-7. [DOI] [PubMed] [Google Scholar]
- Leibowitz M. J., Wickner R. B. A chromosomal gene required for killer plasmid expression, mating, and spore maturation in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2061–2065. doi: 10.1073/pnas.73.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick P., Ferro S., Schekman R. Order of events in the yeast secretory pathway. Cell. 1981 Aug;25(2):461–469. doi: 10.1016/0092-8674(81)90064-7. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Secretion and cell-surface growth are blocked in a temperature-sensitive mutant of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1858–1862. doi: 10.1073/pnas.76.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. T., Saville D., Bussey H. Saccharomyces cerevisiae killer expression mutant kex2 has altered secretory proteins and glycoproteins. Biochem Biophys Res Commun. 1979 Sep 12;90(1):187–193. doi: 10.1016/0006-291x(79)91607-3. [DOI] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Scheele G., Jacoby R., Carne T. Mechanism of compartmentation of secretory proteins: transport of exocrine pancreatic proteins across the microsomal membrane. J Cell Biol. 1980 Dec;87(3 Pt 1):611–628. doi: 10.1083/jcb.87.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Steiner D. F., Quinn P. S., Chan S. J., Marsh J., Tager H. S. Processing mechanisms in the biosynthesis of proteins. Ann N Y Acad Sci. 1980;343:1–16. doi: 10.1111/j.1749-6632.1980.tb47238.x. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Leibowitz M. J. Two chromosomal genes required for killing expression in killer strains of Saccharomyces cerevisiae. Genetics. 1976 Mar 25;82(3):429–442. doi: 10.1093/genetics/82.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]










