Abstract
The American Heart Association’s Life’s Simple 7 initiative allows individuals to assess health factors (BP, cholesterol, and glucose) and health behaviors (cigarette smoking, physical activity, diet, and body mass index) to promote improved cardiovascular health. Because several cardiovascular risk factors also associate with progressive kidney disease, Life’s Simple 7 may also inform an individual’s risk for ESRD. Here, we investigated the association of Life’s Simple 7 components with both ESRD incidence and all-cause mortality among 3093 participants with an estimated GFR (eGFR) <60 ml/min per 1.73 m2 from the population-based Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. During a median 4 years of follow-up, 160 participants developed ESRD, and 610 participants died. Compared with individuals who had zero or one of the Life’s Simple 7 components in the ideal range, those individuals with two, three, and four ideal factors had progressively lower risks for ESRD; furthermore, no participant with five to seven ideal factors developed ESRD. The risk for all-cause mortality exhibited a similar trend. Adjusting for eGFR and albuminuria, however, completely attenuated the associations between the number of ideal factors and the risks for both ESRD and all-cause mortality. In conclusion, a favorable cardiovascular risk profile among individuals with CKD associates with a reduced risk for ESRD and mortality, but whether the severity of kidney disease confounds or mediates this association requires additional investigation.
Over 15 million US adults have moderate-to-severe CKD, defined as an estimated GFR (eGFR) <60 ml/min per 1.73 m2.1,2 Individuals with moderate-to-severe CKD have a high risk for ESRD,3,4 which is associated with poor quality of life and high mortality. Identifying modifiable risk factors for ESRD is an important public health priority.5,6
In 2010, the American Heart Association developed a metric, Life’s Simple 7, for defining ideal cardiovascular health.7 This metric includes four health behaviors (body mass index, physical activity, a healthy diet, and cigarette smoking) and three health factors (BP, cholesterol, and glucose) for a total of seven components. In addition to defining ideal cardiovascular health, this metric is intended to capture the entire spectrum of cardiovascular health.
The burden of cardiovascular disease risk factors is high among individuals with CKD.8,9 Although diabetes and hypertension are well established risk factors for ESRD, there are few data on the association between other health behaviors and health factors and the incidence of ESRD.10,11 Also, few data are available on the relationship between having an ideal risk factor profile using a composite measure and reduced ESRD risk. Therefore, we determined the association between the individual factors that make up the Life’s Simple 7 and the incidence of ESRD among participants with CKD in a large population-based cohort, the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study. We also determined the relationship between composite levels of the four health behaviors, three health factors, and all seven factors and ESRD risk. In secondary analyses, the association between Life’s Simple 7 and all-cause mortality was assessed.
Results
Participant Characteristics
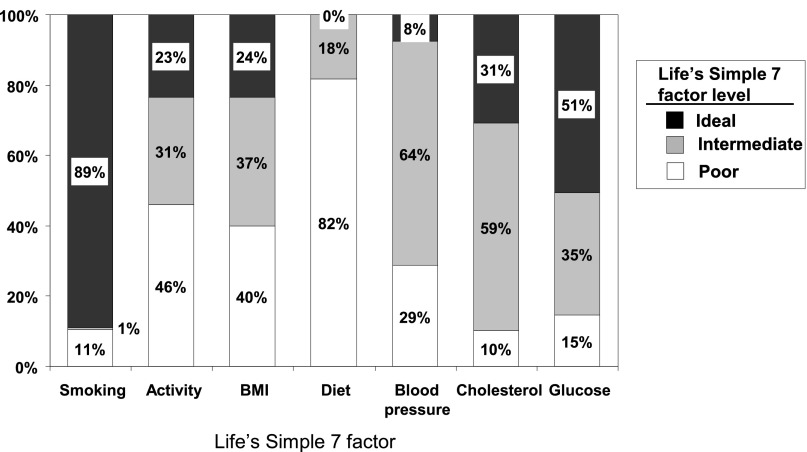
Sociodemographic factors and levels of health behaviors, health factors, eGFR, and albuminuria at baseline are presented in Table 1. The mean age of the study sample was 72.2 years, 42% were African American, and 45% were men. Mean eGFR and geometric mean albuminuria are provided by each Life Simple 7 health behavior and health factor level in Supplemental Table 1. The distribution of poor, intermediate, and ideal Life’s Simple 7 factors is presented in Figure 1. Ideal levels of the Life’s Simple 7 factors were 89% for smoking, 23% for physical activity, 24% for body mass index, 0% for diet, 8% for BP, 31% for cholesterol, and 51% for glucose. Overall, 2%, 25%, 34%, 25%, 11%, and 3% of the population had zero, one, two, three, four, and five or more ideal Life’s Simple 7 factors, respectively.
Table 1.
Baseline characteristics of REGARDS study participants with an eGFR<60 ml/min per 1.73 m2 by number of ideal Life’s Simple 7 factors
| Characteristic | Overall Population (n=3093) | Number of Ideal Life’s Simple 7 Factors | ||||
|---|---|---|---|---|---|---|
| 0–1 (n=845) | 2 (n=1062) | 3 (n=767) | 4 (n=337) | 5–7 (n=82) | ||
| Age, yr | 72.2 (8.7) | 69.8 (8.2) | 72.2 (8.6) | 73.6 (8.8) | 74.7 (8.6) | 75.3 (9.3) |
| African American, % | 42.3 | 54.4 | 41.1 | 37.9 | 30.0 | 23.2 |
| Men, % | 45.1 | 38.5 | 44.8 | 48.2 | 53.7 | 51.2 |
| Geographic region, % | ||||||
| Stroke belt | 32.6 | 34.1 | 33.4 | 30.1 | 34.1 | 23.2 |
| Stroke buckle | 20.8 | 23.0 | 18.6 | 22.4 | 19.6 | 17.1 |
| Other | 46.6 | 43.0 | 47.9 | 47.5 | 46.3 | 59.8 |
| Household income<$20,000/yr, % | 24.3 | 36.3 | 26.9 | 23.7 | 24.7 | 14.3 |
| Less than high school education, % | 19.2 | 23.1 | 18.6 | 16.8 | 18.1 | 14.6 |
| Physically active, % | 54.0 | 36.8 | 51.0 | 64.2 | 74.5 | 90.2 |
| Current smoker, % | 10.5 | 21.4 | 8.9 | 5.1 | 3.6 | 0.0 |
| Diet factors | ||||||
| Fish consumption, g/wk | 22.0 (29.0) | 24.0 (34.6) | 21.8 (25.7) | 21.0 (28.0) | 19.5 (22.8) | 26.8 (37.7) |
| Sodium intake, mg/d | 2140 (1015) | 2121 (1074) | 2202 (1053) | 2060 (939) | 2175 (981) | 2170 (758) |
| Sugar, mg/d | 249 (203.4) | 226 (197) | 257 (207) | 249 (199) | 273 (219) | 261 (177) |
| Fruits and vegetables, cups/wk | 4.1 (2.3) | 3.9 (2.3) | 4.1 (2.3) | 4.0 (2.3) | 4.2 (2.3) | 5.2 (2.4) |
| Fiber/carbohydrate intake ratio | 0.08 (0.03) | 0.08 (0.03) | 0.08 (0.03) | 0.08 (0.03) | 0.08 (0.03) | 0.09 (0.03) |
| Body mass index, kg/m2 | 29.6 (6.4) | 32.5 (6.0) | 30.4 (6.1) | 27.8 (5.9) | 25.0 (4.5) | 23.6 (4.3) |
| Fasting serum glucose, mg/dl | 105 (37) | 123 (50) | 103 (33) | 96 (22) | 91 (13) | 87 (8) |
| Antiglycemic medication use, % | 31.2 | 65.9 | 31.7 | 14.6 | 3.7 | 1.3 |
| Diabetes mellitus, % | 35.1 | 70.1 | 33.7 | 15.7 | 4.3 | 1.3 |
| Total cholesterol, mg/dl | 186 (43) | 190 (48) | 189 (43) | 184 (42) | 180 (33) | 169 (31) |
| Lipid-lowering medication use, % | 46.3 | 72.4 | 49.0 | 33.8 | 16.2 | 6.1 |
| Systolic BP, mmHg | 130.8 (18) | 133 (18) | 132 (18) | 130 (18) | 128 (17) | 115 (14) |
| Diastolic BP, mmHg | 75 (11) | 75 (11) | 75 (10) | 75 (11) | 73 (10) | 69 (10) |
| Antihypertensive medication use, % | 76.1 | 90.8 | 82.7 | 71.0 | 58.7 | 32.1 |
| Hypertension, % | 80.8 | 90.4 | 85.2 | 76.3 | 65.0 | 31.7 |
| Renin angiotensin system blockers, % | 58.0 | 69.7 | 61.5 | 49.2 | 43.3 | 35.4 |
| Coronary heart disease, % | 31.7 | 38.3 | 32.0 | 27.8 | 25.2 | 23.2 |
| Stroke, % | 13.0 | 14.7 | 13.1 | 12.5 | 10.1 | 9.8 |
| eGFR, ml/min per 1.73 m2 | 47.1 (10.9) | 45.1 (12.0) | 46.9 (10.5) | 48.4 (10.4) | 48.5 (9.8) | 50.6 (8.2) |
| Geometric mean (95% CI) ACR, mg/g | 23.8 (22.3 to 25.4) | 39.4 (34.2 to 45.4) | 23.5 (21.0 to 26.3) | 18.5 (16.4 to 20.8) | 14.4 (12.4 to 16.8) | 13.7 (10.3 to 18.2) |
Supplemental Appendix has listing and definitions of ideal levels for each Life’s Simple 7 factor. ACR, albumin-to-creatinine ratio.
Figure 1.
Prevalence of health behaviors and health factors among REGARDS study participants with an eGFR<60 ml/min per 1.73 m2.
ESRD Incidence
Over a median of 4 years of follow-up, 160 participants developed ESRD. The number of ESRD cases by each Life’s Simple 7 factor is presented in Supplemental Table 2. For each Life’s Simple 7 factor except cholesterol, the incidence of ESRD was highest for individuals with poor levels and lowest among participants with an ideal level (Table 2). After adjustment for age, race, sex, geographic region of residence, income, education, and history of stroke and coronary heart disease, ideal levels for physical activity, BP, and glucose were associated with a lower hazard ratio (HR) for ESRD.
Table 2.
Incidence rates and adjusted HRs for ESRD associated with each Life’s Simple 7 factor
| Life Simple 7 Factor | Life Simple 7 Factor Level | ||
|---|---|---|---|
| Poor | Intermediate | Ideal | |
| ESRD incidence rates (95% CI)a | |||
| Cigarette smoking | 19.7 (13.1 to 29.6) | —b | 12.4 (10.5 to 14.7) |
| Physical activity | 16.4 (13.3 to 20.2) | 11.8 (8.8 to 15.8) | 8.7 (5.9 to 12.7) |
| Body mass index | 17.0 (13.7 to 21.1) | 10.7 (8.1 to 14.1) | 10.4 (7.3 to 14.9) |
| Diet | 11.3 (9.0 to 14.1) | 8.8 (5.1 to 15.1) | — |
| BP | 23.3 (18.8 to 28.9) | 9.5 (7.6 to 11.9) | 4.3 (1.6 to 11.4) |
| Cholesterol | 18.2 (12.1 to 27.4) | 11.4 (9.2 to 14.2) | 14.5 (11.1 to 18.9) |
| Glucose | 22.0 (16.1 to 30.1) | 20.1 (16.2 to 25.0) | 6.1 (4.5 to8.3) |
| HR (95% CI)c | |||
| Cigarette smoking | 1 (reference) | — | 0.79 (0.50 to 1.23) |
| Physical activity | 1 (reference) | 0.66 (0.46 to 0.94) | 0.50 (0.32 to 0.78) |
| Body mass index | 1 (reference) | 0.80 (0.55 to 1.14) | 0.94 (0.61 to 1.44) |
| Diet | 1 (reference) | 1.01 (0.50 to 2.04) | — |
| BP | 1 (reference) | 0.43 (0.32 to 0.60) | 0.26 (0.09 to 0.70) |
| Cholesterol | 1 (reference) | 0.66 (0.41 to 1.05) | 0.83 (0.50 to 1.36) |
| Glucose | 1 (reference) | 1.07 (0.73 to 1.56) | 0.38 (0.24 to 0.59) |
Supplemental Appendix has definitions of poor, intermediate, and ideal levels.
Incidence rates per 1,000 person-years.
Too few individuals in these groups to produce incidence rates or HRs.
HRs are adjusted for age, race, sex, geographic region of residence, income, education, and history of stroke and coronary heart disease.
A graded association was present between a higher number of ideal Life’s Simple 7 factors and a lower incidence rate of ESRD (Table 3). The association between the number of ideal Life’s Simple 7 factors and ESRD risk was partially attenuated by adjustment for eGFR and no longer present after adjustment for log albumin-to-creatinine ratio (Table 4).
Table 3.
Incidence rates and adjusted HRs for ESRD associated with number of ideal Life’s Simple 7 factors
| Number of Ideal Life’s Simple 7 Factors | Participants (%) | N (%) Developing ESRD | Incidence Rate (95% CI) per 1,000 person-yr | HR (95% CI) |
|---|---|---|---|---|
| 0 or 1 | 27.3 | 72 (8.5) | 22.5 (17.9 to 28.4) | 1 (reference) |
| 2 | 34.3 | 48 (4.5) | 11.4 (9.6 to 15.1) | 0.66 (0.46 to 0.96) |
| 3 | 24.8 | 29 (3.8) | 9.5 (6.6 to 13.7) | 0.59 (0.38 to 0.92) |
| 4 | 10.9 | 11 (3.3) | 7.5 (4.2 to 13.6) | 0.52 (0.27 to 0.98) |
| ≥5 | 2.7 | 0 (0.0) | 0 (0 to 0) | a |
HRs are adjusted for age, race, sex, geographic region of residence, income, education, and history of stroke and coronary heart disease. Supplemental Appendix has definitions of ideal levels for each Life Simple 7 factor.
HR could not be estimated, because no incident ESRD events occurred in this group.
Table 4.
Adjusted HRs for ESRD associated with number of ideal Life’s Simple 7 factors
| Number of Ideal Life’s Simple 7 Factors | HR (95% CI) | |||
|---|---|---|---|---|
| Multivariable Adjusted | Multivariable + eGFR | Multivariable + Log ACR | Multivariable + eGFR and Log ACR | |
| 0 or 1 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 2 | 0.66 (0.46 to 0.96) | 0.82 (0.56 to 1.20) | 1.00 (0.68 to 1.47) | 1.16 (0.78 to 1.73) |
| 3 | 0.59 (0.38 to 0.92) | 0.84 (0.54 to 1.32) | 1.10 (0.68 to 1.77) | 1.26 (0.78 to 2.05) |
| 4 | 0.52 (0.27 to 0.98) | 0.88 (0.46 to 1.68) | 1.28 (0.65 to 2.52) | 1.31 (0.67 to 2.59) |
| ≥5 | a | a | a | a |
Multivariable adjusted includes age, race, sex, geographic region of residence, income, education, and history of stroke and coronary heart disease. Supplemental Appendix has definitions of ideal levels for each Life Simple 7 factor. ACR, albumin-to-creatinine ratio.
HR could not be estimated, because no incident ESRD events occurred in this group.
A graded association was present between having more ideal health behaviors and a lower ESRD incidence rate (Table 5, health behaviors). This association remained present after multivariable adjustment for age, race, sex, geographic region of residence, income, education, and history of stroke and coronary heart disease. A graded association was also present between a higher number of ideal health factors and a lower ESRD incidence rate before and after multivariable adjustment (Table 5, health factors). The associations between having more ideal health behaviors and health factors and lower ESRD risk were completely attenuated by adjustment for eGFR and albuminuria.
Table 5.
ESRD incidence rates and adjusted HRs for ESRD associated with number of ideal health behaviors and health factors
| Number | Participants (%) | N (%) Developing ESRD | Incidence Rate (95% CI) per 1000 person-yr | Model 1 HR (95% CI) | Model 2 HR (95% CI) |
|---|---|---|---|---|---|
| Ideal health behaviors | |||||
| 0 | 6.3 | 16 (8.2) | 23.1 (14.0 to 37.8) | 1 (reference) | 1 (reference) |
| 1 | 57.4 | 100 (5.6) | 14.4 (11.8 to 17.5) | 0.77 (0.45 to 1.31) | 0.62 (0.34 to 1.12) |
| 2 | 30.4 | 39 (4.2) | 10.1 (7.4 to 13.9) | 0.65 (0.36 to 1.17) | 0.72 (0.38 to 1.37) |
| 3 or 4 | 5.9 | 5 (2.7) | 6.6 (2.7 to 15.8) | 0.51 (0.18 to 1.41) | 1.01 (0.35 to 2.88) |
| Ideal health factors | |||||
| 0 | 33.5 | 78 (7.5) | 19.7 (15.8 to 24.6) | 1 (reference) | 1 (reference) |
| 1 | 46.0 | 66 (4.6) | 11.6 (9.1 to 14.7) | 0.73 (0.52 to 1.01) | 1.40 (0.98 to 2.02) |
| 2 | 18.4 | 16 (2.8) | 6.8 (4.2 to 11.2) | 0.49 (0.29 to 0.85) | 0.99 (0.53 to 1.86) |
| 3 | 2.1 | 0 (0) | 0 (0 to 0) | a | a |
Model 1 includes adjustment for age, race, sex, geographic region of residence, income, education, and history of stroke and coronary heart disease. Model 2 includes adjustment for variables in model 1, eGFR, and albuminuria (log transformed). Health behaviors include body mass index, cigarette smoking, physical activity, and diet. Health factors include serum glucose, total cholesterol, and BP.
HR could not be estimated, because no incident ESRD events occurred in this group.
All-Cause Mortality
During a median 4 years of follow-up, 610 deaths occurred. After multivariable adjustment, the HRs for all-cause mortality were lower for participants with intermediate levels of physical activity, diet, BP, and glucose and ideal levels of cigarette smoking, physical activity, and glucose (Supplemental Table 3). Compared with individuals with zero or one ideal Life’s Simple 7 factors, the multivariable adjusted HRs (95% confidence interval [95% CI]) for all-cause mortality associated with having two, three, four, and five or more ideal Life’s Simple 7 factors were 0.85 (95% CI=0.69 to 1.04), 0.74 (95% CI=0.59 to 0.92), 0.56 (95% CI=0.41 to 0.77), and 0.70 (95% CI=0.40 to 1.22), respectively (Supplemental Table 4). The association between having more ideal Life’s Simple 7 factors and reduced all-cause mortality was no longer present after additional adjustment for albuminuria and eGFR (Supplemental Table 5).
Discussion
Patients with ESRD on dialysis have an extraordinarily high mortality risk.6 The incidence of ESRD has increased markedly over the past several decades, and identifying modifiable risk factors for ESRD has important public health implications.6 In the current study, using the American Heart Association’s Life’s Simple 7 metric, individuals with a better cardiovascular disease health profile had a lower incidence of ESRD. Both a better health behavior profile and a better health factor profile were associated with a lower ESRD risk. A better cardiovascular disease profile was also associated with a lower risk of all-cause mortality among participants with CKD. These associations were attenuated and no longer present after adjustment for eGFR and albuminuria.
The American Heart Association published its Life’s Simple 7 metric as part of a process for defining ideal cardiovascular health and developing a strategic goal for cardiovascular health promotion.7 In defining cardiovascular health, the seven health behaviors and health factors each met several criteria considered by an expert panel, including being associated with a reduced risk for cardiovascular disease and being consistent with current clinical and public health guidelines. Also, the seven health factors can be used to define and monitor the prevalence of ideal cardiovascular health in the US population. Given the low rate of ideal cardiovascular health among US adults, the panel decided to focus on improvements in cardiovascular health in general. They set the goal for a 20% overall improvement in the cardiovascular health of all Americans by 2020.
Although the components of Life’s Simple 7 were chosen for their association with cardiovascular health, many of the individual components are associated with ESRD incidence. Diabetes and elevated BP are the two leading causes of ESRD among US adults.11–13 Additionally, in prior studies, current smoking, higher cholesterol, and higher body mass index levels have been associated with a more rapid decline in kidney function and a higher risk for ESRD.14–16 The Life’s Simple 7 metric does not consider cumulative exposure to cigarette smoking and ESRD risk. Future studies are needed to evaluate the association between pack-years of smoking and its effect on ESRD. Being physically active has been associated with better renal function and lower mortality among individuals with CKD.17 Also, in a recent study, a Western pattern diet (higher intake of red and processed meats, saturated fats, and sweets) was associated with an increased risk for albuminuria and a rapid decline in eGFR.18 However, few data are available on physical activity and diet patterns and the incidence of ESRD. In the current study, higher levels of physical activity were associated with lower ESRD incidence rates, but no association was present between diet and ESRD. Also, the current study extends the prior data by combining individual factors and showing that a better overall cardiovascular disease health profile is associated with a substantially reduced ESRD risk. Although the association between health behaviors and health factors and ESRD risk was strongest for physical activity, BP, and glucose, the population attributable risk may be larger for more prevalent conditions.
The relationship between ideal levels of Life’s Simple 7 components and ESRD and mortality risk was explained by lower eGFR and higher albuminuria. This result suggests that kidney disease may be either a confounder or a mediator between health behaviors and health factors and ESRD risk. Prior studies have shown that many of the factors in Life’s Simple 7 are associated with incident CKD.19,20 Therefore, eGFR and albuminuria may be intermediates between health behaviors and health factors and ESRD risk. However, given the data available in the current study, we cannot exclude the possibility that they are confounders. Future studies with repeated measures of Life’s Simple 7, eGFR, and albuminuria with follow-up for ESRD risk are needed to explore this question further.
Data from the current study were not available to assess whether changes in cardiovascular health actually reduce ESRD risk. A prior trial of patients with diabetes compared conventional care with an intensive multifactorial intervention aimed at reducing total and saturated fat intake, smoking cessation, increasing exercise, achieving lower BP, and achieving lipid treatment goals, angiotensin converting enzyme inhibitor use, and aspirin use for individuals with peripheral vascular disease on the development of nephropathy and incidence and progression of retinopathy and neuropathy.21 Over a mean follow-up of 7.8 years, the multifactorial intervention was associated with a substantially lower incidence of nephropathy (odds ratio=0.27, 95% CI=0.10 to 0.75), progression of retinopathy (odds ratio=0.45, 95% CI=0.21 to 0.95), and progression of neuropathy (odds ratio=0.31, 95% CI=0.12 to 0.78). Over an additional 5 years of observational follow-up, all-cause mortality, cardiovascular disease, and ESRD were each lower in patients randomized to the multifactorial intervention.22
The findings from the present analyses should be interpreted within the context of known and potential limitations. Approximately one third of REGARDS Study participants did not return a Food Frequency Questionnaire (FFQ). We relied on multiple imputation to fill in data for these participants. Excluding these individuals yielded similar results. We had insufficient information in our data to precisely replicate Life’s Simple 7 metrics for physical activity and diet score. Therefore, we used a modified definition of these health behaviors. Only 160 ESRD cases occurred during follow-up. This finding prevented us from evaluating the joint effect of health behaviors and health factors on ESRD risk. Despite these limitations, the current study has many strengths, including a broad collection of baseline data by standardized questionnaire administration, a validated dietary questionnaire, anthropometrics, BP measurements, and blood collection. Additional strengths include the large population-based sample of US white and black adults enrolled in the REGARDS Study, prospective follow-up for ESRD, and linkage with the US Renal Data System (USRDS) to identify ESRD cases. Over 95% of ESRD cases among US adults are captured through the USRDS.6
In conclusion, in this large population-based sample of US adults with CKD, a healthier cardiovascular disease risk factor profile as defined by the American Heart Association’s Life’s Simple 7 metric was associated with a reduced risk for ESRD. The reduced risk for ESRD was present for a better health behavior profile and a better health factor profile. Furthermore, a better cardiovascular health profile was associated with lower risk for all-cause mortality among this population with CKD. Randomized controlled trials are needed to determine the actual risk reduction benefit that can be achieved through improvements in health behaviors and health factors. In the interim, patients with CKD should be encouraged to improve their health profile.
Concise Methods
Study Participants
The REGARDS Study is a population-based cohort study of stroke incidence and cognitive decline among US adults≥45 years of age.23 The study was designed to oversample African Americans and residents from the Southern US states, commonly referred to as the stroke buckle and stroke belt. Overall, 30,239 African-American and white adults were enrolled between January of 2003 and October of 2007. Of the ESRD cases that were eligible for this analysis, 82% (n=160 of 195 cases) had an eGFR<60 ml/min per 1.73 m2. Therefore, a priori, we limited this analysis to participants with an eGFR<60 ml/min per 1.73 m2 at the baseline study visit who were not receiving renal replacement therapy at baseline (n=3093). The REGARDS Study protocol was approved by the Institutional Review Boards at the participating centers, and all participants provided informed consent.
Data Collection
Trained interviewers conducted computer-assisted telephone interviews to obtain information on participants’ demographics, cigarette smoking, physical activity, history of stroke and coronary heart disease, and use of antihypertensive, antiglycemic, and cholesterol-lowering medication. Trained and certified health professionals conducted in-home study visits that included a physical examination, electrocardiogram, the collection of a blood sample, and a spot urine sample. Serum creatinine assays were performed at the University of Vermont and calibrated against an isotope dilution mass spectroscopic standard. The Chronic Kidney Disease Epidemiology Collaboration equation was used to calculate eGFR. Urinary albumin and creatinine were measured at the Department of Laboratory Medicine and Pathology at the University of Minnesota using the BN ProSpec Nephelometer from Dade Behring (Marburg, Germany), and this information was used to calculate the albumin-to-creatinine ratio. At the end of the in-home examination, the Block 98 FFQ was left with the participants for self-administration.24,25 Using the FFQ, each participant recorded food intake for 1 year before their in-home visit.
Life’s Simple 7
Components of Life’s Simple 7 include cigarette smoking, physical activity, diet, body mass index, BP, cholesterol, and glucose.7 These components are categorized as being poor, intermediate, or ideal (Supplemental Appendix). The diet score for Life’s Simple 7 was based on fish, fruit and vegetable consumption, and sodium, sugar, and fiber/carbohydrate ratio intake. An ideal diet was defined by meeting four or five of the following criteria: fish consumption≥2 servings/wk, fruit/vegetables≥4.5 cups/d, sodium intake<1500 mg/d, sugar<450 kcal/wk, and fiber/carbohydrate ratio>0.1. Systolic BP and diastolic BP were measured two times using a standardized protocol and averaged for the analyses. Total cholesterol was measured using an enzymatic reaction, and glucose was measured using colorimetric reflectance spectrophotometry. Consistent with the Life’s Simple 7 scoring approach, people on treatment who achieved their goal levels for BP (systolic BP<140 mmHg and diastolic BP<90 mmHg), cholesterol (total cholesterol<200 mg/dl), and glucose (serum glucose<126 mg/dl) were placed in the intermediate health category. The goal for systolic/diastolic BP<130/80 mmHg for people with CKD is controversial and not consistent with the Life’s Simple 7 metric; therefore, it was not used in the current analysis. In addition to using all 7 factors for a composite Life’s Simple 7 score, the seven health factors were grouped into two domains (health behaviors: cigarette smoking, physical activity, diet, and body mass index; health factors: BP, cholesterol, and glucose).
Study Outcomes
ESRD was the primary outcome, and all-cause mortality was evaluated as a secondary outcome. ESRD subsequent to the in-home examination and through August 31, 2009 was assessed by linkage with the USRDS. The USRDS is a registry of ESRD, and it captures over 95% of incident cases in the United States.6 All-cause mortality through August 31, 2009 was assessed through contact with proxies provided by the participant on recruitment or during follow-up.
Statistical Analyses
The distribution of each Life’s Simple 7 component as poor, intermediate, and ideal and the percent of participants with zero through seven ideal factors were calculated. ESRD incidence rates were calculated by level of each Life’s Simple 7 factor. Also, for each factor, the adjusted HRs for ESRD associated with intermediate and ideal versus poor health were calculated. ESRD incidence rates and HRs for ESRD were also calculated for participants with two, three, four, and five or more versus zero or one ideal Life’s Simple 7 factors, one, two, three, or four versus no ideal health behaviors, and one, two, or three versus no ideal health factors. All HRs were calculated using Cox proportional hazards models with adjustment for age, race, sex, geographic region of residence (stroke belt, stroke buckle, or other), income, education, and history of stroke or coronary heart disease. In a secondary analysis, we calculated the HR for ESRD associated with Life’s Simple 7 scores with adjustment for eGFR, albuminuria, and both eGFR and albuminuria. Also, the association between each Life’s Simple 7 component and overall Life’s Simple 7 scores with all-cause mortality was evaluated.
For participants missing information on diet, poor or intermediate scores were imputed using chained equations with five datasets.26 The assumptions of proportionality were met for all Cox models. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC) and Stata version 11 (Stata Incorporated, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
This research project is supported by Cooperative Agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but were not directly involved in the collection, management, analysis, or interpretation of the data. Amgen did not have any role in the design and conduct of the study, the collection, management, data analysis, or interpretation of the data, or the preparation or approval of the manuscript. P.M. and L.G. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070642/-/DCSupplemental.
See related editorial, “Ideal Cardiovascular Health and Progression of CKD: Perhaps not so “Simple”,” on pages 1031–1033.
References
- 1.Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levey AS, de Jong PE, Coresh J, Nahas ME, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU: The definition, classification and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Hallan SI, Ritz E, Lydersen S, Romundstad S, Kvenild K, Orth SR: Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J Am Soc Nephrol 20: 1069–1077, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimmel PL: Psychosocial factors in adult end-stage renal disease patients treated with hemodialysis: Correlates and outcomes. Am J Kidney Dis 35[Suppl 1]: S132–S140, 2000 [DOI] [PubMed] [Google Scholar]
- 6.United States Renal Data System (USRDS) : USRDS 2009 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, MD, National Instistutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2010 [Google Scholar]
- 7.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, American Heart Association Strategic Planning Task Force and Statistics Committee : Defining and setting national goals for cardiovascular health promotion and disease reduction: The American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 121: 586–613, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, Hamm LL, Kusek JW, Chen J, Whelton PK, He J: The prevalence of nontraditional risk factors for coronary heart disease in patients with chronic kidney disease. Ann Intern Med 140: 9–17, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Muntner P, He J, Astor BC, Folsom AR, Coresh J: Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: Results from the atherosclerosis risk in communities study. J Am Soc Nephrol 16: 529–538, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Whelton PK, Klag MJ: Hypertension as a risk factor for renal disease. Review of clinical and epidemiological evidence. Hypertension 13[Suppl]: I19–I27, 1989 [DOI] [PubMed] [Google Scholar]
- 11.Perneger TV, Brancati FL, Whelton PK, Klag MJ: End-stage renal disease attributable to diabetes mellitus. Ann Intern Med 121: 912–918, 1994 [DOI] [PubMed] [Google Scholar]
- 12.Perneger TV, Klag MJ, Feldman HI, Whelton PK: Projections of hypertension-related renal disease in middle-aged residents of the United States. JAMA 269: 1272–1277, 1993 [PubMed] [Google Scholar]
- 13.Perneger TV, Whelton PK, Klag MJ: History of hypertension in patients treated for end-stage renal disease. J Hypertens 15: 451–456, 1997 [DOI] [PubMed] [Google Scholar]
- 14.Muntner P, Coresh J, Smith JC, Eckfeldt JH, Klag MJ: Plasma lipids and risk of developing renal dysfunction: The atherosclerosis risk in communities study. Kidney Int 58: 293–301, 2000 [DOI] [PubMed] [Google Scholar]
- 15.Mänttäri M, Tiula E, Alikoski T, Manninen V: Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension 26: 670–675, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Haroun MK, Jaar BG, Hoffman SC, Comstock GW, Klag MJ, Coresh J: Risk factors for chronic kidney disease: A prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 14: 2934–2941, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Beddhu S, Baird BC, Zitterkoph J, Neilson J, Greene T: Physical activity and mortality in chronic kidney disease (NHANES III). Clin J Am Soc Nephrol 4: 1901–1906, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin J, Fung TT, Hu FB, Curhan GC: Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the Nurses’ Health Study. Am J Kidney Dis 57: 245–254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D: Predictors of new-onset kidney disease in a community-based population. JAMA 291: 844–850, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D: Obesity and prevalent and incident CKD: The Hypertension Detection and Follow-Up Program. Am J Kidney Dis 46: 587–594, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Gaede P, Vedel P, Parving HH, Pedersen O: Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: The Steno type 2 randomised study. Lancet 353: 617–622, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Gaede P, Lund-Andersen H, Parving HH, Pedersen O: Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 358: 580–591, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G: The reasons for geographic and racial differences in stroke study: Objectives and design. Neuroepidemiology 25: 135–143, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Block G, Woods M, Potosky A, Clifford C: Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol 43: 1327–1335, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Block G, Thompson FE, Hartman AM, Larkin FA, Guire KE: Comparison of two dietary questionnaires validated against multiple dietary records collected during a 1-year period. J Am Diet Assoc 92: 686–693, 1992 [PubMed] [Google Scholar]
- 26.Rubin DB: Multiple Imputation for Nonresponse in Surveys, New York, John Wiley & Sons, Inc., 1987 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.