Abstract
Whether early glomerular, tubulointerstitial, vascular, and global glomerulosclerotic lesions can predict progression of diabetic nephropathy is not well defined. Here, we sought to determine whether renal structural parameters predict the development of proteinuria or ESRD after long-term follow-up. We measured several renal structures in kidney biopsies from 94 normoalbuminuric patients with longstanding type 1 diabetes using unbiased morphometric methods. Greater width of the glomerular basement membrane and higher levels of glycated hemoglobin were independent predictors of progression to diabetic nephropathy in this normoalbuminuric cohort. Moreover, none of these patients with type 1 diabetes who had glomerular basement membrane widths within the normal range developed proteinuria and/or ESRD. In conclusion, careful quantitative assessment of kidney biopsies in normoalbuminuric patients with type 1 diabetes adds substantially to the prediction of progression to clinical diabetic nephropathy.
Despite recent advances in diabetes management, changes in clinical practice after the publication of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and other landmark studies, and knowledge of the benefits of better BP management and renin angiotensin system (RAS) blockade for patients with overt diabetic nephropathy (DN) and reduced GFR,1–3 DN remains the single most common cause of ESRD in the United States4 and other developed countries. In fact, in the United States, the annual incidence of ESRD caused by DN continues to increase.4
Identifying patients who are vulnerable to or protected from DN could allow preventative strategies to be directed to those patients at increased risk. Although microalbuminuria identifies a subset of patients at greater likelihood of DN progression, microalbuminuria is neither an early nor a sufficiently precise marker by itself of DN risk.5 Thus, we have previously shown that severe glomerular lesions may be present in normoalbuminuric patients with type 1 diabetes (T1D)6,7 and also, that normoalbuminuric patients with decreased GFR have more advanced glomerular lesions.8 A study of young normoalbuminuric T1D patients found that both metabolic (hemoglobin A1c [A1c]) and renal structural parameters (glomerular basement membrane [GBM] width) were predictive of the subsequent development of microalbuminuria.9 Glomerular structural lesions were also predictors of subsequent changes in urinary albumin excretion rate (AER) in a study evaluating a small number of microalbuminuric T1D patients.10 However, there are no long-term follow-up studies in normoalbuminuric T1D patients of the predictive value of renal lesions on harder clinical end points of overt proteinuria, ESRD, and death.
Results
After 11.0±7.2 years of follow-up, 74 patients were alive, and 20 patients were deceased. Follow-up information in regards to patients’ progressor, nonprogressor, and/or survival status was available in 82 (87.2%) patients, whereas 4 (4.3%) patients were lost to follow-up, 5 (5.3%) patients did not return the consent forms after multiple attempts, and 3 (3.2%) patients refused participation. Among 82 patients for whom we had follow-up information, 59 (72%) patients remained normoalbuminuric and were classified as nonprogressors, and 12 (14.6%) patients progressed to the composite end point of proteinuria and/or ESRD. Four (4.9%) subjects were microalbuminuric at follow-up and excluded from the final analyses. Also excluded from the analyses were seven subjects who, deceased at time of follow-up, had inadequate data for classification as progressors or nonprogressors. These subjects were not listed on the US Renal Data System (USRDS).
Twenty patients died during the follow-up period; nine patients were progressors, four patients were nonprogressors, and seven patients (see above) could not be classified. Causes of death among progressors included cardiovascular disease (n=4), renal insufficiency (n=3), complications after pancreas transplant (n=1), and unknown (n=1). One nonprogressor died of accidental poisoning, two nonprogressors died from diabetes, and one nonprogressor died from an unknown cause. Of seven unclassifiable patients, two patients died of complications after pancreas transplant less than 1 year after the kidney biopsy and thus, were not followed long enough before death to be classified in regards to kidney progression, one patient committed suicide, one patient died from a metastatic melanoma, one patient died from a motor vehicle accident, and two patients had diabetes listed as their cause of death.
There were no differences in baseline demographic, clinical, or glomerular structural parameters in 82 subjects for whom we had follow-up information and 12 subjects who were alive and unlisted in the USRDS in whom we could not access renal status at follow-up (data not shown). All 11 patients who were on RAS blocking agents at follow-up agreed to the washout studies. There were no complications related to discontinuation of RAS blocking agents and/or use of alternative antihypertensive agents during the washout period.
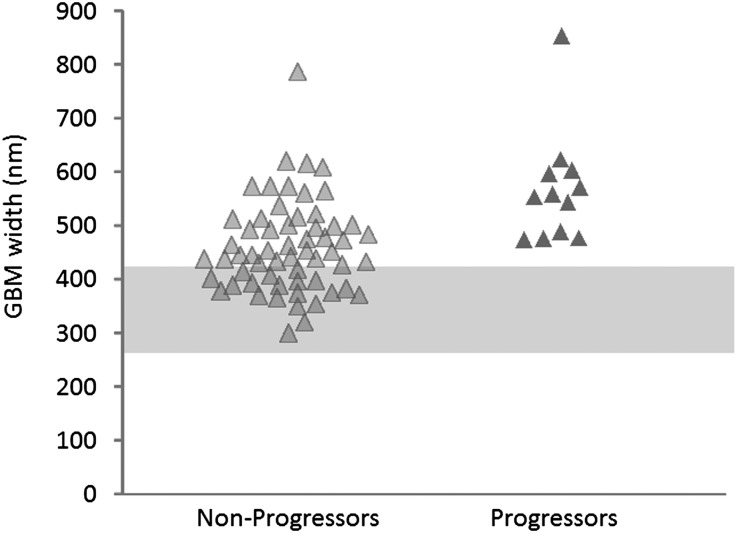
There were no differences between progressors and nonprogressors in man/woman ratio, baseline diabetes duration, length of follow-up, AER, GFR, BP levels, and frequency of hypertension or diabetic retinopathy (Table 1). As expected, as a group, these normoalbuminuric patients, whether later classified as progressors or nonprogressors, had abnormal baseline glomerular structural parameters compared with normal controls (Table 2). At baseline, progressors were, on average, 8 years younger at the time of the initial studies (P=0.007), had higher A1c (P=0.01) (Table 1), and had greater GBM width (P<0.001) (Figure 1 and Table 2) than nonprogressors. The overall risk of progression in these normoalbuminuric subjects was 17%. In those patients with GBM width in the same range as the progressors, the risk was nearly doubled (33.3%). All 21 subjects with GBM width in the normal range were nonprogressors, despite equivalent duration of T1D in the nonprogressors with normal GBM width (20.4±9.7 years) versus the nonprogressors with abnormal GBM width (19.5±11.5 years; P=0.74). No other glomerular structural parameter, including mesangial parameters (mesangial fractional volume per glomerulus [Vv(Mes/glom)], mesangial matrix fractional volume per glomerulus [Vv(MM/glom)], and mesangial cell fractional volume per glomerulus [Vv(MC/glom)]) and glomerular filtration surface density (filtration surface density per glomerulus [Sv(PGBM/glom)]), was statistically significantly different between progressors and nonprogressors.
Table 1.
Baseline demographic and clinical characteristics of progressors and nonprogressors
| Progressors (n=12) | Nonprogressors (n=59) | P Value | |
|---|---|---|---|
| Sex (men/women) | 4/8 | 24/35 | NS |
| Age (yr) | 26.7±7.3 | 35.0±9.9 | 0.007 |
| Age at onset (yr) | 11.1±6.2 | 15.1±8.7 | NS |
| Diabetes duration (yr) | 15.6±7.4 | 19.8±10.2 | NS |
| Follow-up (yr) | 13.9±7.6 | 12.4±5.4 | NS |
| Albumin excretion rate (µg/min) | 7.0±5.2 | 7.5±4.7 | NS |
| GFR (ml/min per 1.73 m2) | 105.4±25.4 | 108.7±21.3 | NS |
| Systolic BP (mmHg) | 113.0±9.9 | 114.2±10.8 | NS |
| Diastolic BP (mmHg) | 74.2±7.5 | 70.5±7.8 | NS |
| Hypertension (yes/no) | 3/9 | 12/47 | NS |
| Retinopathy (yes/no/unknown) | 5/5/2 | 14/29/16 | NS |
| A1c (%) | 10.4±2.5 | 8.0±1.2 | 0.01 |
Data are mean ± SD or number of subjects with a given characteristic.
Table 2.
Glomerular structural parameters in progressors and nonprogressors
| Progressors (n=12) | Nonprogressors (n=59) | P Value | |
|---|---|---|---|
| GBM width (nm) | 567.3±104.1 | 459.5±85.7 | <0.001 |
| Vv(Mes/glom) | 0.26±0.07 | 0.28±0.07 | NS |
| Vv(MM/glom) | 0.14±0.05 | 0.16±0.05 | NS |
| Vv(MC/glom) | 0.07±0.03 | 0.08±0.02 | NS |
| Sv(PGBM/glom) (µm2/µm3) | 0.12±0.02 | 0.12±0.03 | NS |
Data are mean ± SD. GBM normal values (mean ± SD): 322±46 nm; Vv(Mes/glom) normal values: 0.20±0.03; Vv(MM/glom) normal values: 0.09±0.02; Vv(MC/glom) normal values: 0.08±0.02; Sv(PGBM/glom) normal values: 0.13±0.02 µm2/µm3.
Figure 1.
GBM width in normoalbuminuric T1D patients who remained normoalbuminuric (nonprogressors) or progressed to proteinuria and/or ESRD (progressors) during follow-up. The shaded area represents the normal range (mean ±2 SD) of GBM width.
There were also group differences in the percent of global glomerular sclerosis (GS; P=0.005) (Table 3). Progressors had a higher percentage of GS (4.6 [0%–40%]) than nonprogressors (0.0 [0%–10%]; P=0.04) and controls (0.0 [0.0]; P=0.003), whereas there were no statistically significant differences between nonprogressors and controls (P=0.06). There were also differences in the index of arteriolar hyalinosis (IAH) among groups (P=0.001). Both progressors (1.63 [1.0–2.7]; P<0.001) and nonprogressors (1.04 [1.0–1.5]; P=0.02) had higher IAH than controls (1.0 [1.0–1.0]), and progressors’ IAH values were greater in progressors than nonprogressors (P=0.01). There were no group differences in interstitium fractional volume per cortex [Vv(Int/cortex)] or atrophic tubuli fractional volume per cortex [Vv(AT/cortex)]. There were no statistically significant differences in baseline renal structural parameters between seven patients who progressed to ESRD and five patients who progressed to proteinuria.
Table 3.
Renal structural parameters in progressors, nonprogressors, and controls
| Progressors (n=12) | Nonprogressors (n=11) | Controls (n=8) | P Value | |
|---|---|---|---|---|
| GS (%) | 4.6 (0–40) | 0.0 (0–10) | 0.0 (0–0) | 0.005 |
| IAH | 1.63 (1.0–2.7) | 1.04 (1.0–1.5) | 1.0 (1.0–1.0) | 0.001 |
| Vv(Int/cortex) | 0.15±0.04 | 0.16±0.05 | 0.13±0.03a | NS |
| Vv(AT/cortex) | 0.0 (0.0–0.0) | 0.0 (0.0–0.01) | 0.0 (0.0–0.01) | NS |
Data are mean ± SD or median (minimum to maximum).
Vv(Int/cortex) was measured in six control subjects. Two subjects did not have enough points hitting the interstitium for a precise estimate of this parameter.
Multiple logistic regression analyses indicated that only GBM width (P=0.008) and A1c (P=0.003) were independent predictors of progression. Seven deceased patients who could not be classified by their kidney status before death were followed for a shorter period of time (4.8±4.2 years; P=0.001 versus progressors and P=0.001 versus nonprogressors) and had higher diastolic BP (78.0±7.0 mmHg) and A1c (9.3±1.6%) than nonprogressors (P=0.02 and P=0.05, respectively). Glomerular structural parameters in these subjects were intermediate between progressors and nonprogressors but not different from either group (Supplemental Table 1). When baseline glomerular structural parameters from these deceased subjects were compared with those parameters of progressors or nonprogressors, all differences between progressors and nonprogressors reported above remained significant (Supplemental Table 1).
Discussion
This study is the only study to date of normoalbuminuric T1D patients who had baseline research kidney biopsies and long-term (≥5 years) follow-up looking for early predictors of progression to proteinuria and/or ESRD. We acquired follow-up information in 87% of the original cohort, which argues against major ascertainment bias. However, because 12 patients, for whom we could not obtain follow-up information, were not found in ESRD or death registries, it is possible that the incidence of progression was slightly overestimated.
Baseline glycemic control and kidney structural lesions were independent predictors of progression to the composite end point of proteinuria and/or ESRD in these normoalbuminuric T1D patients. All cardiovascular deaths occurred in patients who had already reached one of these renal outcomes. Worse glycemic control has long been implicated with increased risk of chronic diabetic complications in both T1D11 and type 212 diabetic persons. It is, therefore, not surprising that A1c was a predictor of progression in this cohort. Progressors were also younger than nonprogressors at baseline, whereas there was no statistical difference in their age at diabetes onset. However, the relationships between age of onset, sex, and microvascular complications are apparently quite complex,13 and our cohort is too small to be analyzed in this regard.
We have previously implicated greater baseline GBM as a predictor of increased likelihood of developing microalbuminuria over the next 5 years in young normoalbuminuric T1D research subjects.14 However, because microalbuminuria is not a precise predictor of hard clinical end points,5 we asked whether baseline GBM width and/or other renal structural parameters on longer-term follow-up were independent predictors of progression to proteinuria and/or ESRD.
It is important to note that our baseline renal biopsy findings reflect the wide range of severity of glomerular structural lesions that we have previously shown in normoalbuminuric longstanding T1D patients.7 Thus, a substantial proportion of normoalbuminuric T1D patients with long diabetes duration have glomerular lesions, including increased GBM width, mesangial expansion, and reduced glomerular filtration surface density, that, in fact, overlap in severity with lesions seen in microalbuminuric and even proteinuric patients.7 Moreover, we have previously shown strong relationships between renal structure and function in T1D patients6,7,15 and the fact that normoalbuminuric T1D patients with reduced GFR have more severe underlying glomerular lesions than those patients with normal GFR.8 Thus, it may not be surprising that the patients progressing to serious end points were those patients with more severe lesions at initial evaluation. It was, perhaps, more surprising that greater GBM width was the only renal structural parameter that independently predicted the progression to proteinuria and ESRD.
Although mesangial expansion and its reciprocal decrease in glomerular filtration surface density7,16,17 are stronger correlates of albuminuria and GFR decline than GBM width in more advanced stages of DN,6,7,15,16 there was no signal that these other glomerular parameters were early predictors of overt DN and ESRD in the present study. Across the broad spectrum of DN lesions, GBM width and mesangial expansion are quite highly correlated; however, this finding, in fact, is largely driven by patients with more advanced lesions.7 We have previously shown in a study of younger patients with average T1D duration of about 9 years that, earlier in the natural history of DN, GBM width has a linear relationship with diabetes duration, whereas the relationship of duration with mesangial changes is better described as curvilinear, which initially steady, may accelerate later.18 Because ultimately, GBM width and mesangial fractional volume do become closely correlated, earlier increases in GBM width may, in fact, represent an important predictor of other glomerular structural parameters and thereby, a predictor of DN risk.
Arteriolar hyalinosis can be an early lesion of DN,19 and its greater severity is associated with increased percent global GS in T1D patients.20 Although both these structural parameters were increased in progressors versus nonprogressors, they did not emerge as independent predictors of DN risk in multiple regression analyses. However, this finding in no way reduces their potential contribution to the emergence of clinical DN.20
Cortical interstitial fractional volume and the fraction of cortical tubules that were atrophic were unrelated to subsequent DN risk in these patients who were all normoalbuminuric at baseline. This finding is consistent with our previous studies that indicated that, in T1D patients with GFRs above 45 ml/min per 1.73 m2, these parameters added little to the explanation of GFR loss.15,21 Thus, although these lesions may be critical to clinical progression in the later stages of DN, when serum creatinine levels are above ∼2.5 mg/dl, their contribution to renal dysfunction at early stage is likely relatively limited.
GBM width has been directly correlated with A1c in a number of studies,19,22–24 with this single clinical variable explaining between 9% and 36% of the variability in this structural parameter. However, glycemia cannot explain all of the variability in the risk of microvascular complications. It is known that there is familial concordance for DN risk.25–27 We have previously shown that glomerular structural parameters, including GBM width, are concordant in sibling pairs where both have T1D and that this concordance remains statistically significant after adjusting for glycemia. Thus, at least in part, the glycemia-independent predictive value of GBM width on the hard end points in the present study may represent the genetic propensity to and/or protection from DN risk. This finding suggests that GBM width may be a useful genetic marker of DN risk and that those genes and pathways that regulate GBM production and removal are appropriate candidates for understanding how genetic predisposition to DN operates. Having GBM width in the range of the progressors doubled the risk of progression from 17% to 33.3%. Perhaps even more striking is the observation that none of the T1D patients with GBM width within the normal range, despite longstanding diabetes, progressed to proteinuria and/or ESRD.
The deceased patients who could not be classified in regards to renal outcomes had GBM width values between the values of progressors and nonprogressors. This result suggests that some of these patients, if they had survived longer, would have progressed to proteinuria and/or ESRD, whereas others would have remained normoalbuminuric.
In summary, GBM width after long T1D duration is a strong independent predictor of DN risk in normoalbuminuric T1D patients. Severity of arteriolar hyalinosis and percent global GS were also greater in the progressors and although not independent of other variables in multiple regression analyses, could nonetheless be important contributors to renal dysfunction in these patients. Better understanding of how these earlier DN lesions come about could lead to new treatment targets aimed at preventing important diabetic complications. Clearly, there is a need for less invasive biomarkers given the practicality challenges of the adoption of renal biopsies in routine patient care. However, GBM thickening as an early risk predictor may facilitate early DN biomarker discovery compared with waiting an additional decade or more for serious clinical renal events to develop.
Concise Methods
Patients
All normoalbuminuric T1D patients who had volunteered for research kidney biopsies and kidney function studies between 1982 and 2005 who were 18 years or older, had T1D for at least 5 years, were not on RAS blocking agents at the time of the initial studies, had adequate biopsy tissue available for study, had not received a pancreas transplant that functioned for more than 3 months, and were followed for at least 5 years postbiopsy were included in this study. Ninety-four subjects met these inclusion criteria. These studies were approved by the Institutional Review Board at the University of Minnesota, and informed consent was obtained from all living participants. Relatives of deceased patients were also contacted.
Baseline Studies
Patients were admitted to the General Clinical Research Center (GCRC) at the University of Minnesota for these studies.
AER was measured by a fluorescent immunoassay28 in at least two 24-hour urine collections at baseline and at least two overnight urine collections at follow-up. Normoalbuminuria was defined as AER<20 μg/min, microalbuminuria was defined as 20–200 μg/min, and proteinuria was defined as >200 μg/min in at least two of three consecutive urine samples.
Creatinine was measured by Jaffe’s reaction. GFR was measured by iohexol plasma disappearance29 or iothalamate clearance,30 or it was estimated by the average of three carefully GCRC-collected creatinine clearances. The mean of these multiple GCRC creatinine clearances has previously been shown to be strongly correlated with inulin clearance (r=0.92; P<0.001) performed during the same GCRC admission.17
Glycated hemoglobin was measured by a nonautomated ion exchange chromatography microcolumn from 1982 to 1986 (A1 reference range=5.5%–8.5%), an automated HPLC (Biorad) from 1986 to 1992 (A1 reference range=5.1%–7.3%; A1c reference range=4.3%–6.1%), and the same method standardized to Diabetes Control and Complications Trial values since 1992 (A1 reference range=5.4%–7.4%; A1c reference rang=4.3%–6.0%). Results from before 1992 were converted to current A1c standards using published equations.31
BP values are the average of multiple (>15) standardized Dinamap measurements performed by trained GCRC nursing staff. Hypertension was defined as BP>130/85 mmHg or use of antihypertensive agents. Retinopathy was assessed by fundoscopy and classified as absent, background, or proliferative.
For renal biopsy measurements, kidney biopsy tissues were processed for light (LM) and electron microscopy (EM) as we have previously detailed.7 Briefly, EM kidney tissue was fixed in 2.5% glutaraldehyde in Millonig’s buffer and embedded in Polybed 812. Ultrathin sections were examined with a JEOL 100CX electron microscope. Micrographs using a systematic uniform random sampling protocol at 11,000× were obtained for measurement of GBM width and mesangium composition. Micrographs at 3900× were constructed into a montage of the entire glomerular profile for measurements of Vv(Mes/glom) and Sv(PGBM/glom). GBM width was estimated by the orthogonal intercept method.32 Vv(Mes/glom), Vv(MM/glom), and Vv(MC/glom) were estimated by point counting,33 and Sv(PGBM/glom) was estimated by the intercept method.6,7 Glomerular profiles were screened by a single masked observer (M.M.) for compression artifacts. An adequate number of glomeruli (at least two nonsclerosed glomeruli) per biopsy were required for the EM morphometric studies.30 Reference values for EM glomerular structural parameters were derived from intraoperative kidney biopsies in 76 normal living kidney transplant donors. These subjects (33 men) were 37.6±12.1 years of age (19–64 years) as previously published.7
Tissue for LM was fixed in Zenker’s solution, embedded in paraffin, sectioned at 2-μm thickness, and stained with periodic acid-Schiff. LM tissues were used for estimates of Vv(Int/cortex), Vv(AT/cortex), percentage of global GS, and IAH. LM readings were done in all progressors in whom LM tissue was available as well as a subset of nonprogressors matched to the progressors for sex and diabetes duration at baseline biopsy. We also examined LM in a subset of sex- and age-matched living kidney donor tissues. The number of sclerosed glomeruli was estimated at 150×; all other LM studies were done at a magnification of 300×.
Interstitium was defined as the portion of the cortex not composed of glomeruli, tubules, vessels (with a diameter greater than the diameter of an average tubule), or adventia. Vv(Int/cortex) was determined by a single masked observer by point counting LM on random, systematic, unbiased digital images at an actual magnification of 730× as previously published.34 The digital stereology grid used in point counting consisted of coarse points (60 mm apart), each surrounded by four fine points (30 mm apart). Nonoverlapping digital images of cortex were taken of one tissue section on a given microscope slide. Additional tissue sections were imaged at least 100 μm apart if needed to obtain adequate numbers of coarse points.34 Vv(Int/cortex) was calculated using the following formula:
where FPI is the number of fine points hitting interstitium and CPC is the number of coarse points hitting cortex. A minimum of 65 FPI was required, because this number provides a stable estimate of Vv(Int/cortex).34 An average of 262 coarse points (range=83–423) and 159 fine points (range=65–253) was counted.
Vv(AT/cortex) was estimated by point counting on the fields used for Vv(Int/cortex). Atrophic tubules were defined by the presence of thickened or reduplicated tubular basement membranes surrounding tubules of reduced diameters containing shortened or flat tubular epithelial cells; in the absence of thickened tubular basement membranes, atrophy was defined as a tubular diameter <50% of normal as determined by comparison with adjacent tubules.34
Percent global GS was obtained by two masked observers (M.L.C and M.M.). A mean of 26 (range=9–72) glomeruli was examined per biopsy. Values in the group of sex- and age-matched normal kidney donor controls were 0%. IAH was also obtained by these same masked observers who, reading slides together, agreed on the subjective estimate of the fraction of each arteriolar wall replaced by hyaline in one complete LM section. Because higher scores for IAH are associated with more global GS, greater weighting was given to arterioles with higher scores according to the previously published formula19:
 |
The denominator is the total number of arterioles counted.
We examined 26 (range=7–59) vessels per biopsy. Normal value in this group of sex- and age-matched controls was one.
Follow-Up Studies
Patients who were alive were contacted and asked to complete a questionnaire accessing their current clinical status. Patients who were on dialysis or had received a kidney transplant were classified as progressors. All remaining patients were asked to provide at least two overnight urine samples for AER measurements. Patients who were proteinuric were also classified as progressors.
Patients who were on angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) at follow-up and were normoalbuminuric or microalbuminuric underwent a 2-month ACEi/ARB drug washout and then had AER measured again using the same protocol. Patients who developed hypertension BP levels>130/85 mmHg during the washout period had their BP levels controlled by the study investigators (M.L.C. and M.M.) or their primary physician according to our study protocol. Briefly, ACEi or ARB therapy was discontinued, and patients were started on a calcium channel blocker (amlodipine: 2.5 mg). BP was measured before discontinuation of therapy and 2 weeks after the alternative therapy began. The dose of antihypertensive agents was adjusted as need. If BP still exceeded 130/85 mmHg, a diuretic (indapamide: 1.25 mg one time daily) was added and increased, if necessary, up to 2.5 mg one time daily. If BP levels still exceeded 130/85 mmHg, an α-blocker, terazosin (1 mg at bedtime), was added and adjusted up to 8 mg one time daily as needed. The protocol specified that, if BP levels were not controlled with these agents or there were side effects of these drugs (such as dizziness or rash), these medications were to be discontinued, and patient’s usual medications (ACEi or ARB) were resumed. In fact, resumption of these drugs was never necessary. AER values at the end of the washout period were used to classify these patients as progressors or nonprogressors.
Patients in whom follow-up information was not available were searched for in the USRDS and the National Death Index. Date of beginning of renal replacement therapy (dialysis or renal transplant) and date and cause of death were recorded for all subjects for whom we had a perfect match in these registries.
Statistical Analyses
Patients remaining normoalbuminuric on no RAS blockade were classified as nonprogressors. Patients progressing to the composite outcomes of proteinuria and ESRD were defined as progressors. Baseline demographic and clinical characteristics of progressors and nonprogressors were compared by unpaired t test or Pearson chi-squared test. Data not normally distributed were analyzed by nonparametric tests. Values of P<0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Mr. Thomas Groppoli, Ms. Ann Palmer, and Ms. Frida Maiers, Department of Pediatrics, University of Minnesota, for technical assistance; Ms. Ashley Kinneberg, Department of Medicine and Pediatrics, University of Minnesota, for patient recruitment; and Ms. Tanya Doble, Department of Medicine, University of Minnesota and Ms. Patricia L. Erickson, Department of Pediatrics, University of Minnesota, for helping with manuscript preparation. We are especially grateful to the patients who volunteered for these research renal biopsy studies.
This study was funded by research grants from the National Institutes of Health (NIH), National Institute of Diabetes and Digestive and Kidney Diseases Grant DK13083-41, and funds from the Pennock Professorship and the Minnesota Lions Diabetes Foundation (M.L.C., principal investigator). M.L.C. was a recipient of a Career Development Award from the Juvenile Diabetes Research Foundation (JDRF) and is the guarantor of this work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012070739/-/DCSupplemental.
References
- 1.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD, The Collaborative Study Group : The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med 329: 1456–1462, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators : Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345: 861–869, 2001 [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study Group : Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317: 703–713, 1998 [PMC free article] [PubMed] [Google Scholar]
- 4.USRDS : 2011 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2011 [Google Scholar]
- 5.Caramori ML, Fioretto P, Mauer M: The need for early predictors of diabetic nephropathy risk: Is albumin excretion rate sufficient? Diabetes 49: 1399–1408, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Fioretto P, Steffes MW, Mauer M: Glomerular structure in nonproteinuric IDDM patients with various levels of albuminuria. Diabetes 43: 1358–1364, 1994 [DOI] [PubMed] [Google Scholar]
- 7.Caramori ML, Kim Y, Huang C, Fish AJ, Rich SS, Miller ME, Russell G, Mauer M: Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes 51: 506–513, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Caramori ML, Fioretto P, Mauer M: Low glomerular filtration rate in normoalbuminuric type 1 diabetic patients: An indicator of more advanced glomerular lesions. Diabetes 52: 1036–1040, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Perrin NE, Torbjörnsdotter T, Jaremko GA, Berg UB: Risk markers of future microalbuminuria and hypertension based on clinical and morphological parameters in young type 1 diabetes patients. Pediatr Diabetes 11: 305–313, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Bangstad HJ, Osterby R, Hartmann A, Berg TJ, Hanssen KF: Severity of glomerulopathy predicts long-term urinary albumin excretion rate in patients with type 1 diabetes and microalbuminuria. Diabetes Care 22: 314–319, 1999 [DOI] [PubMed] [Google Scholar]
- 11.The Diabetes Control and Complications Trial Research Group : The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329: 977–986, 1993 [DOI] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study (UKPDS) Group : Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 13.Harjutsalo V, Maric C, Forsblom C, Thorn L, Wadén J, Groop PH, FinnDiane Study Group : Sex-related differences in the long-term risk of microvascular complications by age at onset of type 1 diabetes. Diabetologia 54: 1992–1999, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Steinke JM, Sinaiko AR, Kramer MS, Suissa S, Chavers BM, Mauer M, International Diabetic Nephopathy Study Group : The early natural history of nephropathy in Type 1 Diabetes: III. Predictors of 5-year urinary albumin excretion rate patterns in initially normoalbuminuric patients. Diabetes 54: 2164–2171, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Najafian B, Kim Y, Crosson JT, Mauer M: Atubular glomeruli and glomerulotubular junction abnormalities in diabetic nephropathy. J Am Soc Nephrol 14: 908–917, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Mauer SM, Steffes MW, Ellis EN, Sutherland DE, Brown DM, Goetz FC: Structural-functional relationships in diabetic nephropathy. J Clin Invest 74: 1143–1155, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis EN, Steffes MW, Goetz FC, Sutherland DE, Mauer SM: Glomerular filtration surface in type I diabetes mellitus. Kidney Int 29: 889–894, 1986 [DOI] [PubMed] [Google Scholar]
- 18.Drummond KN, Kramer MS, Suissa S, Lévy-Marchal C, Dell’Aniello S, Sinaiko A, Mauer M, International Diabetic Nephropathy Study Group : Effects of duration and age at onset of type 1 diabetes on preclinical manifestations of nephropathy. Diabetes 52: 1818–1824, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Drummond K, Mauer M, International Diabetic Nephropathy Study Group : The early natural history of nephropathy in type 1 diabetes: II. Early renal structural changes in type 1 diabetes. Diabetes 51: 1580–1587, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Harris RD, Steffes MW, Bilous RW, Sutherland DE, Mauer SM: Global glomerular sclerosis and glomerular arteriolar hyalinosis in insulin dependent diabetes. Kidney Int 40: 107–114, 1991 [DOI] [PubMed] [Google Scholar]
- 21.Najafian B, Mauer M: Morphologic features of declining renal function in type 1 diabetes. Semin Nephrol 32: 415–422, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis EN, Warady BA, Wood EG, Hassanein R, Richardson WP, Lane PH, Howard C, Kemp SF, Aceto T, Garibaldi L, Wiegmann TB, Savin VJ: Renal structural-functional relationships in early diabetes mellitus. Pediatr Nephrol 11: 584–591, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Rudberg S, Osterby R, Dahlquist G, Nyberg G, Persson B: Predictors of renal morphological changes in the early stage of microalbuminuria in adolescents with IDDM. Diabetes Care 20: 265–271, 1997 [DOI] [PubMed] [Google Scholar]
- 24.Berg UB, Torbjörnsdotter TB, Jaremko G, Thalme B: Kidney morphological changes in relation to long-term renal function and metabolic control in adolescents with IDDM. Diabetologia 41: 1047–1056, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Seaquist ER, Goetz FC, Rich S, Barbosa J: Familial clustering of diabetic kidney disease. Evidence for genetic susceptibility to diabetic nephropathy. N Engl J Med 320: 1161–1165, 1989 [DOI] [PubMed] [Google Scholar]
- 26.Borch-Johnsen K, Nørgaard K, Hommel E, Mathiesen ER, Jensen JS, Deckert T, Parving HH: Is diabetic nephropathy an inherited complication? Kidney Int 41: 719–722, 1992 [DOI] [PubMed] [Google Scholar]
- 27.Quinn M, Angelico MC, Warram JH, Krolewski AS: Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 39: 940–945, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Chavers BM, Simonson J, Michael AF: A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int 25: 576–578, 1984 [DOI] [PubMed] [Google Scholar]
- 29.Gaspari F, Perico N, Matalone M, Signorini O, Azzollini N, Mister M, Remuzzi G: Precision of plasma clearance of iohexol for estimation of GFR in patients with renal disease. J Am Soc Nephrol 9: 310–313, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Mauer M, Drummond K: The early natural history of nephropathy in type 1 diabetes: I. Study design and baseline characteristics of the study participants. Diabetes 51: 1572–1579, 2002 [DOI] [PubMed] [Google Scholar]
- 31.Camargo JL, Zelmanovitz T, Paggi A, Friedman R, Gross JL: Accuracy of conversion formulae for estimation of glycohaemoglobin. Scand J Clin Lab Invest 58: 521–528, 1998 [DOI] [PubMed] [Google Scholar]
- 32.Jensen EB, Gundersen HJ, Osterby R: Determination of membrane thickness distribution from orthogonal intercepts. J Microsc 115: 19–33, 1979 [DOI] [PubMed] [Google Scholar]
- 33.Steffes MW, Bilous RW, Sutherland DE, Mauer SM: Cell and matrix components of the glomerular mesangium in type I diabetes. Diabetes 41: 679–684, 1992 [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim HN, Jackson S, Connaire J, Matas A, Ney A, Najafian B, West A, Lentsch N, Ericksen J, Bodner J, Kasiske B, Mauer M: Angiotensin II blockade in kidney transplant recipients. J Am Soc Nephrol 24: 320–327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.