Abstract
Inherited and acquired disorders that enhance the activity of transporters mediating renal tubular Na+ reabsorption are well established causes of hypertension. It is unclear, however, whether primary activation of an Na+-independent chloride transporter in the kidney can also play a pathogenic role in this disease. Here, mice overexpressing the chloride transporter pendrin in intercalated cells of the distal nephron (TgB1-hPDS mice) displayed increased renal absorption of chloride. Compared with normal mice, these transgenic mice exhibited a delayed increase in urinary NaCl and ultimately, developed hypertension when exposed to a high-salt diet. Administering the same sodium intake as NaHCO3 instead of NaCl did not significantly alter BP, indicating that the hypertension in the transgenic mice was chloride-sensitive. Moreover, excessive chloride absorption by pendrin drove parallel absorption of sodium through the epithelial sodium channel ENaC and the sodium-driven chloride/bicarbonate exchanger (Ndcbe), despite an appropriate downregulation of these sodium transporters in response to the expanded vascular volume and hypertension. In summary, chloride transport in the distal nephron can play a primary role in driving NaCl transport in this part of the kidney, and a primary abnormality in renal chloride transport can provoke arterial hypertension. Thus, we conclude that the chloride/bicarbonate exchanger pendrin plays a major role in controlling net NaCl absorption, thereby influencing BP under conditions of high salt intake.
Hypertension is one of the most common human diseases in industrialized countries. Among the different environmental and genetic factors predisposing individuals to hypertension, chronic exposure to high dietary Na+ intake has emerged as one of the most important risk factors. However, the molecular mechanisms underlying salt-sensitive hypertension are still poorly understood in the majority of patients. Although it is widely assumed that all pressor effects of dietary NaCl depend on its Na+ content, several studies have drawn attention to the fact that the sodium has to be in the form of NaCl to produce an increase in BP. In 1929, Berghoff and Geraci1 reported that BP increased in seven individuals on a high NaCl intake but not a high NaHCO3 intake, an observation subsequently confirmed by Morgan.2 In humans or animals with salt-sensitive hypertension, selective dietary loading of Na+ without Cl− has repeatedly failed to induce an increase in BP.3–7 Likewise, in hypertensive and normotensive subjects, substitution of dietary NaCl with equimolar NaHCO3 leads to a reduction of BP, further suggesting a modulating effect of dietary chloride on BP.8,9 Surprisingly, although it is widely accepted that excessive sodium reabsorption can lead to hypertension, the potential role of primary activation of renal chloride transport in the pathogenesis of hypertension is poorly understood.
It was recently shown that Cl− absorption in the collecting duct occurs through intercalated cells and requires the presence of the Cl−/HCO3− exchanger pendrin (Pds/Slc26a4).10,11 We subsequently showed that the functional coupling of pendrin together with the Na+-dependent Cl−/HCO3− exchanger (Ndcbe/Slc4a8) results in electroneutral, thiazide-sensitive NaCl absorption, and hence, mimicks the NaCl cotransporter (NCC) of the distal convoluted tubule.12 Although the importance of this system in vascular volume regulation is suspected based on the observation that pendrin disruption impairs normal renal NaCl balance11 or protects against mineralocorticoid-induced hypertension,13 its potential pathogenic role as a primary determinant of hypertension is unknown.
Thus, to investigate the potential primary role of enhanced pendrin activity in the pathogenesis of salt-sensitive hypertension, we have created a mouse model overexpressing pendrin in the intercalated cells of the aldosterone-sensitive distal nephron. We show that pendrin overexpression results in increased chloride absorption that confers on mice markedly salt-sensitive elevation of BP. Interestingly, the pressor effect of high salt intake was strictly chloride-dependent and occurred despite appropriate downregulation of the sodium transporters in the aldosterone-sensitive distal nephron. We conclude from these experiments that a primary abnormality of renal chloride reabsorption can lead to NaCl-sensitive hypertension.
Results
Generation of a Mouse Model Overexpressing Pendrin in the Intercalated Cells
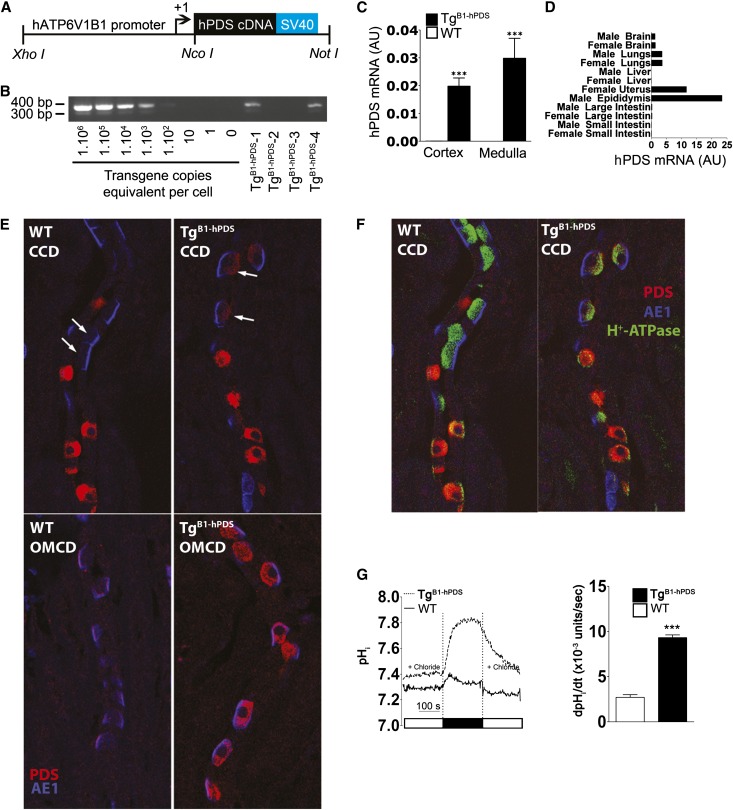
A 6.9-kb fragment corresponding to the human V-ATPase B1-subunit gene (ATP6V1B1 gene; GenBank accession no. NM_039350) has been successfully used to drive expression of both enhanced green fluorescent protein (EGFP) and the Cre-recombinase (Cre) into renal intercalated cells.14,15 Importantly, preliminary experiments revealed that the promoter is likely to be insensitive to a high-salt diet (Supplemental Figure 1). We used this promoter fragment to drive the expression of human pendrin (hPDS/SLC26A4) cDNA in intercalated cells of the connecting tubule and the cortical collecting duct (CCD; detailed methods of the transgene construction and genotyping can be found in Supplemental Material and Supplemental Table 1). The transgene includes the 5′-flanking region of the ATP6V1B1 gene extending to but excluding the endogenous translational start codon, the human SLC26A4 cDNA with its own translational start site, and the SV40 late region polyadenylation signal, and it is referred to as B1-hPDS (Figure 1A). The rationale for using the human instead of the mouse cDNA is to be able to distinguish the expression of the transgene from the expression of the endogenous pendrin gene. Approximately 110 live pups were analyzed for the presence of the B1-hPDS transgene by performing PCR analysis of tail DNA (Figure 1B), and 63 pups were found to be positive for transgene integration. However, only one founder transmitted the transgene in a Mendelian fashion and therefore, was used to generate animals for this study. The mice that were used in the present study were F6. Mendelian transmission of the transgene and Southern blot analyses (Supplemental Figure 2) were compatible with a single site of integration. Figure 1C shows that high levels of the human pendrin transcript were detected by RT-PCR in the renal cortex and medulla of transgenic B1-hPDS (TgB1-hPDS) mice, whereas it was not present in the wild-type littermates. We previously reported that, beyond expression in renal intercalated cells, our ATP6V1B1 promoter fragment was able to drive expression of EGFP in epididymis, uterus, small and large intestines, and nonciliated airway epithelial cells15 and Cre-recombinase in the brain.14 Thus, we tested expression of the transgene in these different tissues (Figure 1D). No hPDS transcript was detected in tissues isolated from wild-type mice, whereas in tissues isolated from TgB1-hPDS mice, hPDS transcript was detected at high levels in uterus and epididymis. hPDS transcript was also detectable in lungs and brain, but no significant level of hPDS transcript was detected in small or large intestine or liver.
Figure 1.
Generation and characterization of transgenic mouse model overexpressing human pendrin in the renal intercalated cells. (A) Schematic of the transgene (B1-hPDS) containing ∼6.9 kb from the 5′-flanking region of the ATP6V1B1 gene extending to but excluding the endogenous translational start codon, the human SLC26A4 cDNA (with its own translational start site; indicated by arrow), and the SV40 late region polyadenylation signal. Positions of the primers used for genotyping or RNA expression (quantitative PCR) are shown. (B) Normal mouse DNA spiked with 0, 1, 10, 102, 103,104, 105, and 106 copies per cell equivalent of the purified transgene DNA (left) was amplified by PCR using primers specific for the hB1-EGFP transgene. PCR genotyping (right) of tail DNA from different pups (TgB1-hPDS-1 to -4); in this example, mice 1 and 4 are hemizygous for the B1-hPDS transgene, whereas mice 2 and 3 do not carry the B1-hPDS transgene, (i.e., wild-type littermates). The intensity of the PCR product from mice 1 and 4 compared with normal DNA spiked with variable amounts of the purified transgene indicates that the transgenics have an almost 1000 copies per cell equivalent of the transgene. (C) Quantitative RT-PCR analyses of the human pendrin transcript in the renal cortex and medulla from TgB1-hPDS and wild-type (WT) mice. (D) Quantitative RT-PCR analyses of the human pendrin transcript in different tissue. (E) Colocalization studies of pendrin (red) and AE1 (blue) on mouse kidney sections reveal the presence of transgenic hPDS in α-intercalated cells (arrows) in TgB1-hPDS but not WT mice. (F) Colocalization studies of pendrin (red), AE1 (blue), and the H+-ATPase reveal the absence of pendrin staining in principle cells and a normal pattern of expression of AE1 and the proton pump. CCD, cortical collecting duct; OMCD, outer medullary collecting duct. (G) Apical Cl−/HCO3− exchange activity in intercalated cells of CCD isolated from either TgB1-hPDS or WT mice. Traces are the average of pHi changes recorded when luminal Cl− is removed and then readded in the presence of extracellular HCO3− (25 mM) and Na+-free solutions. The initial rate of intracellular Cl−-dependent alcalinization (right), reflecting apical Cl−/HCO3− exchange, is much higher in TgB1-hPDS than WT mice. Statistical significance is assessed by two-tailed unpaired t test; n=3 tubules in each group. ***P<0.001 versus control group.
We next examined the expression of the hPDS protein by immunohistochemistry. However, all the different antipendrin antibodies available to us recognize only the rodent orthologs of pendrin. Thus, a new rabbit polyclonal antibody was raised against the synthetic peptide APGGRSEPPQLPEYS (corresponding to amino acids 3–18 of the N-terminal end of human pendrin protein) (Supplemental Material). Preliminary experiments showed that, although this antibody was recognizing human pendrin, it also crossreacted with the murine ortholog (Supplemental Figure 3). We used this antibody to localize both endogenous (murine) and transgenic (human) pendrin on kidney sections from TgB1-hPDS and control mice (Figure 1, E and F). As previously shown by others,16 pendrin expression in wild-type mice was detected exclusively in β- or non-α, non-β intercalated cells, which were identified by the coexpression of the V H+-ATPase but not the anion exchanger 1 (AE1). By contrast, pendrin staining was also detected in α-intercalated cells (V H+-ATPase and AE1-positive) of the renal cortex and medulla in TgB1-hPDS mice. No staining was detected in principle cells of the collecting duct or the connecting tubule. Ectopic expression of hPDS in α-intercalated cells was expected, because our B1 promoter fragment was shown to drive expression of EGFP and Cre in both intercalated cell subtypes.14,15 Noteworthy, the expression of the transgene had no particular impact on AE1 or the V H+-ATPase cellular or subcellular localization.
In our first B1-EGFP transgenic mouse model, we noted that EGFP expression was slightly stronger in the medulla than in the cortex.15 In the present study, the transgene expression also looked stronger in the renal medulla than in the cortex (Figure 1, E and F). However, immunohistochemistry is not a good technique for quantitation, and we were not able to check the level of expression of the trangene by Western blot, because our antibody was not suitable for this technique. Thus, we next checked pendrin activity in the renal cortex by measuring intracellular pHi changes consecutive to manipulation of luminal Cl− concentration in intercalated cells in cortical collecting ducts isolated from TgB1-hPDS and control mice. Basolateral Cl−/HCO3− activity was not assessed in these experiments. Figure 1G shows that apical Cl−/HCO3− exchange activity was much higher in intercalated cells from TgB1-hPDS transgenic mice than wild-type littermates, showing that our transgenic strategy for overexpressing pendrin is effective in cortical intercalated cells.
Pendrin Overexpression Stimulates Chloride Absorption by the Renal Tubule
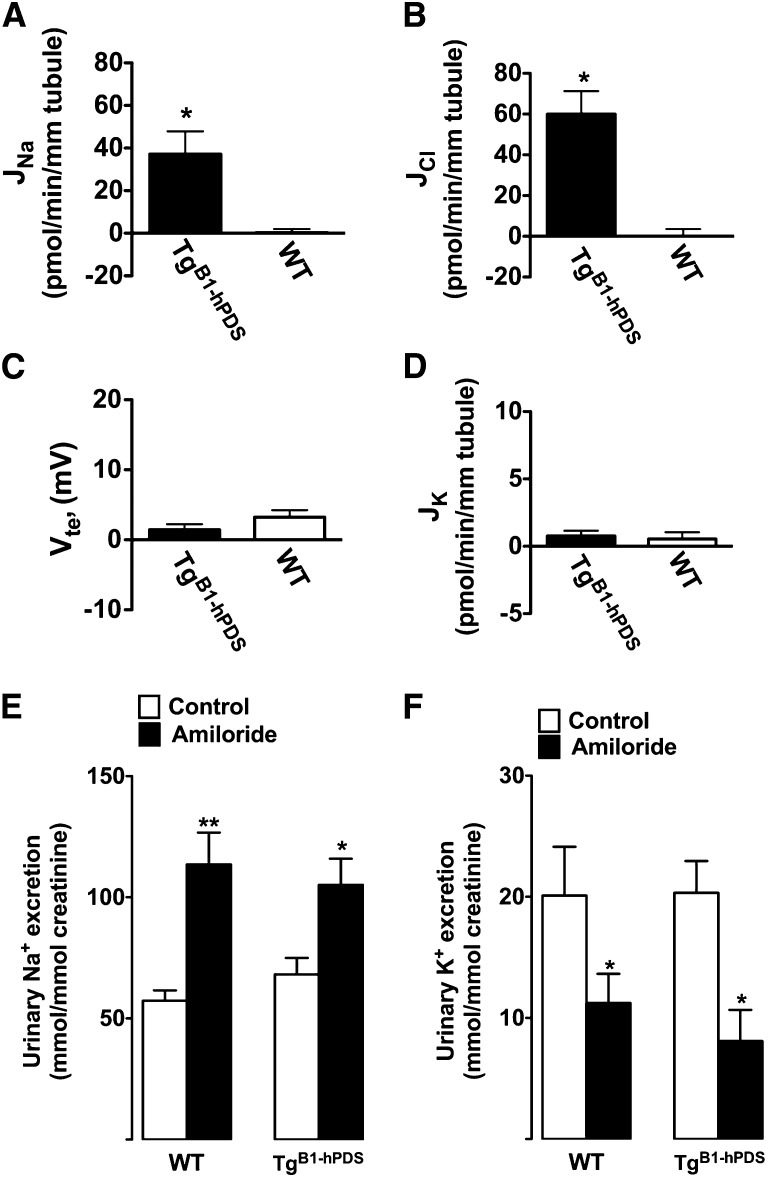
To determine whether the B1-hPDS transgene expression promotes excessive chloride absorption, cortical collecting ducts were isolated from either TgB1-hPDS transgenics or wild-type littermates. All animals were fed a normal salt diet (i.e., 0.3% Na+ administered as NaCl). Transepithelial fluxes of Na+ (JNa), Cl− (JCl), and K+ (JK) and transepithelial voltage (Vte) were measured as described previously.12 Figure 2, A and B shows that, although no transport activity was detectable in CCDs isolated from control mice, tubules from TgB1-hPDS mice exhibited net NaCl absorption. The magnitudes of Na+ and Cl− fluxes were very similar to those magnitudes observed in CCDs isolated from mice maintained on a low-salt diet (figure 1 in ref. 12). CCDs from TgB1-hPDS mice did not develop a net negative Vte and did not secrete K+ (Figure 2, C and D). The latter findings suggested that epithelial Na+ channel (ENaC) activity is impaired as a consequence of excessive electroneutral NaCl absorption. However, under normal conditions, ENaC is mostly active in the connecting tubule, which is not accessible to the microperfusion. Thus, to assess the effects of the transgene on ENaC activity, we next tested whether ENaC-dependent Na+ absorption and K+ secretion are inhibited in TgB1-hPDS mice by monitoring the effects of an acute injection of amiloride (1.45 mg/kg body wt) on urinary excretion of Na+ and K+. As shown in Figure 2, E and F, amiloride injection increased urine Na+ excretion and decreased K+ excretion similarly in the TgB1-hPDS mice and wild-type littermates. Taken together, these results indicate that, although the B1-hPDS transgene promotes electroneutral NaCl absorption, it does not inhibit ENaC.
Figure 2.
Characterization of ion transport activities in the aldosterone-sensitive distal nephron in TgB1-hPDS and WT mice. Analyses of Na+, Cl−, and K+ transepithelial fluxes and transepithelial voltage in CCDs isolated from TgB1-hPDS and WT mice fed a normal salt diet. (A) JNa is the rate of Na+ absorption. (B) JCl is the rate of Cl− absorption. (C) Vte is the transepithelial voltage. (D) JK is the rate of K+ secretion. Statistical significance is assessed by two-tailed unpaired t test; n=7 in each group. *P<0.05 versus control group. Effect of amiloride injection on (E) urinary Na+ excretion and (F) urinary K+ excretion in TgB1-hPDS and WT mice. Mice kept under normal Na+ diet (0.3% Na+ in food) were subcutaneously injected with amiloride (1.45 mg/kg body wt). Urines from TgB1-hPDS and WT mice were collected over 2 days starting at 9 AM and ending at 3 PM the next day. Vehicle was injected on day 1, whereas amiloride was injected at 9 AM on the second day (black bars). Excreted ion concentrations (millimolar) were normalized to the urinary concentration of creatinine (millimolar) to minimize the effects of incomplete urine sampling over such short periods. Values are the mean ± SEM from seven determinations in each group. Statistical significance was tested versus time controls by paired t test. *P<0.05, **P<0.01.
Increased Electroneutral NaCl Absorption in the Distal Nephron Does Not Lead to High Serum K+ Concentration or Metabolic Acidosis but Provokes Chloride-Sensitive Hypertension
Phenotypical analyses of TgB1-hPDS mice and wild-type littermates are summarized in Supplemental Table 2. Increased electrogenic chloride absorption in the CCD, also known as a chloride shunt, has been proposed to decrease the transepithelial voltage; thereby, it impairs tubular K+ secretion and provokes metabolic acidosis and hyperkalemia.17,18 However, TgB1-hPDS mice exhibit net NaCl absorption in the CCD, but in the absence of any Vte, they did not exhibit high serum K+ concentration. Another question was whether overexpression of pendrin can favor the development of acidosis. In fact, we initially anticipated that ectopic expression of hPDS in α-intercalated cells would short circuit acid secretion and thereby, lead to distal tubular acidosis. Thus, to determine whether TgB1-hPDS mice display hyperchloremic metabolic acidosis, we performed blood gas analyses in TgB1-hPDS mice and wild-type littermates. These analyses showed that both TgB1-hPDS mice and wild-type littermates fed a normal salt diet had normal blood acid–base parameters and did not display an alkaline urine pH, indicating the absence of significant bicarbonaturia.
Because the effects of the transgene might have been, at least partly, masked by some compensatory mechanisms, the same analyses were again performed after feeding the mice for 2 weeks with a high-salt (3% Na+ administrated as NaCl) diet. This maneuver is expected to increase urinary chloride delivery to the CCD and hence, accelerate Cl−/HCO3− exchange through the transgene. However, as shown in Supplemental Table 2, serum K+ concentration and acid–base status of TgB1-hPDS mice remained normal and identical to those levels observed in wild-type littermates.
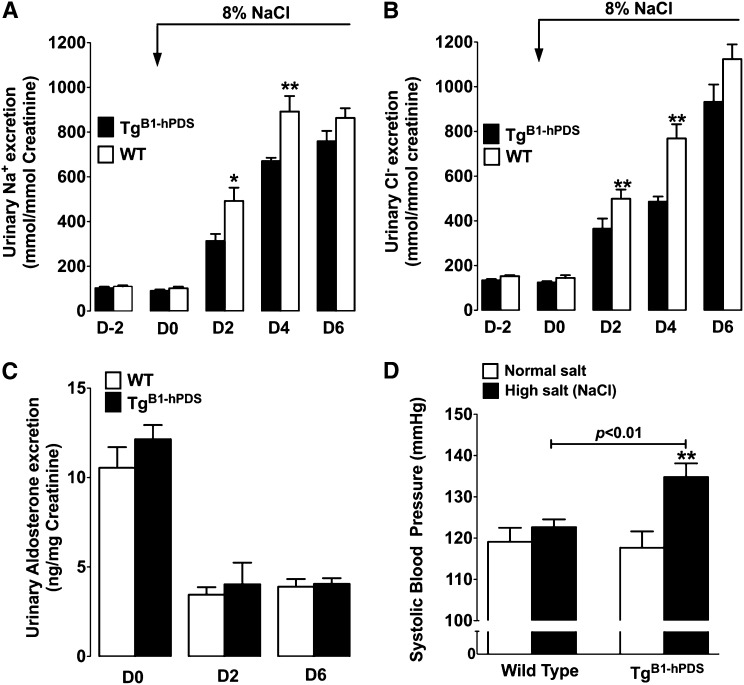
We next tested the effects of pendrin overexpression on renal adaptation to a high-salt diet. Figure 3, A and B shows that animals from both genotypes exhibited the same systolic BP and urinary excretion of Na+ and Cl− when fed a normal salt diet (0.3% Na+). When animals were switched to a high-salt diet (3% Na+), urinary excretion of both Na+ and Cl− dramatically increased in both groups. However, these adaptive changes were significantly delayed in TgB1-hPDS mice compared with wild-type littermates. Urinary aldosterone was not different between genotypes in animals fed a normal salt diet, and they decreased similarly to nearly undetectable levels when animals were fed a high-salt diet (Figure 3C). The high-salt diet led to a significant rise in systolic BP in TgB1-hPDS mice, whereas the wild-type littermates experienced no change in BP (Figure 3D). Almost the same values of BP were obtained when recorded by radiotelemetry (Supplemental Figure 4); accordingly, all subsequent BP measurements were performed using the tail-cuff method. Monitoring of heart rate detected a significant decrease in heart rate in both genotypes fed a high-salt diet but did not reveal any difference between genotype (Supplemental Figure 4).
Figure 3.
Effects of dietary NaCl loading in TgB1-hPDS mice. Time course of renal excretion of (A) Na+ and (B) Cl− in TgB1-hPDS and WT mice fed a normal salt diet and then switched to a high NaCl diet. (C) Urine aldosterone concentration was measured under a normal salt diet or after 2 or 6 days of high NaCl diet. Data are presented as the mean ± SEM; n=7 for each genotype. Statistical significance versus WT was assessed by unpaired t test. *P<0.05, **P<0.01. (D) Systolic BP measured by tail-cuff under normal salt diet (0.3% Na+/0.8% NaCl) or after 2 weeks of high-salt (3% Na+/8% NaCl) diet. Data are presented as the mean ± SEM; n=10 for each genotype. The difference in systolic BP was assessed by ANOVA followed by Bonferroni’s posthoc test. **P<0.01 versus normal salt diet.
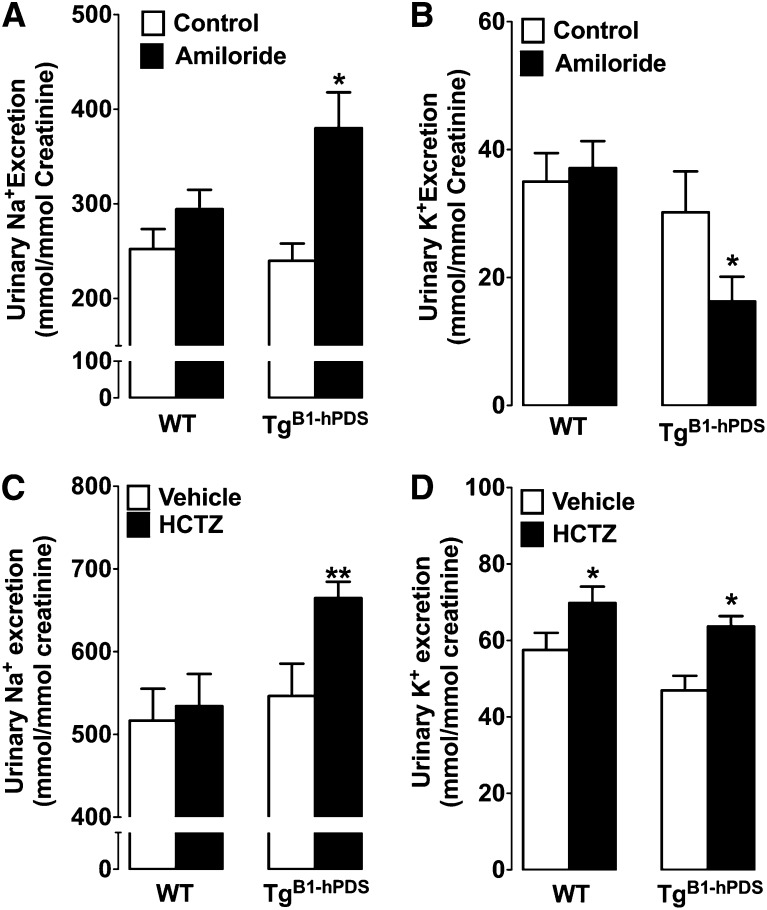
We recently described that pendrin works in tandem with Ndcbe to mediate electroneutral and thiazide-sensitive NaCl absorption.12 Pech et al.19 reported that pendrin is required for normal ENaC activity.19 Thus, to determine whether pendrin overexpression secondarily drives Na+ absorption by Ndcbe, ENaC, or both molecules, we tested the effects of acute injections of either amiloride (1.45 mg/kg) or hydrochlorothiazide (50 mg/kg). These experiments were performed in mice fed a high-salt diet, because the hypertensive phenotype observed in the TgB1-hPDS mice was only apparent in this situation. Figure 4 shows that the response to amiloride was increased in TgB1-hPDS mice compared with wild-type littermates, indicating that ENaC activity is increased in this model; the response to hydrochlorothiazide (HCTZ) was also greater in TgB1-hPDS mice. The NCC and pendrin/Ndcbe transporters are targeted by HCTZ in vivo.12 However, it is unlikely that higher sensitivity to HCTZ in the TgB1-hPDS mice reflects increased activity of NCC, because we directly observed the presence of electroneutral NaCl absorption in the collecting duct (Figure 2), a nephron segment devoid of NCC. Moreover, in our model, expression of human pendrin is driven by the B1 promoter; it is not expressed in the distal convoluted tubule but exclusively expressed in the connecting tubule and collecting duct, which is evident from two previous studies using the exact same promoter to drive expression of either EGFP or Cre.14,15 Thus, these results indicate that Cl− absorption in the distal nephron is paralleled by Na+ absorption occurring by either ENaC or Ndcbe.
Figure 4.
Effects of amiloride and hydrochlorothiazide injections in TgB1-hPDS and WT mice fed a high-salt diet. Urinary (A) Na+ and (B) K+ excretion after amiloride injection in TgB1-hPDS and WT mice. Urinary (C) Na+ and (D) K+ excretion after HCTZ injection in TgB1-hPDS and WT mice. Mice on a high-salt diet (3% Na+/8% NaCl in food) for 2 weeks were subcutaneously injected with amiloride (1.45 mg/kg body wt), HCTZ (50 mg/kg body wt), or vehicle. Urines from TgB1-hPDS and WT mice were collected over 2 days from 9 AM to 3 PM; the first-day injections were in vehicle only (white bars), whereas the diuretics were injected at 9 AM on the following day (black bars). The concentration of excreted Na+ and K+ (millimolar) were normalized to the urinary concentration of creatinine (millimolar) to minimize the effects of incomplete urine sampling over such short periods. Values are the mean ± SEM from seven determinations in each group. Statistical significance was tested versus time controls by paired t test. *P<0.05, **P<0.01.
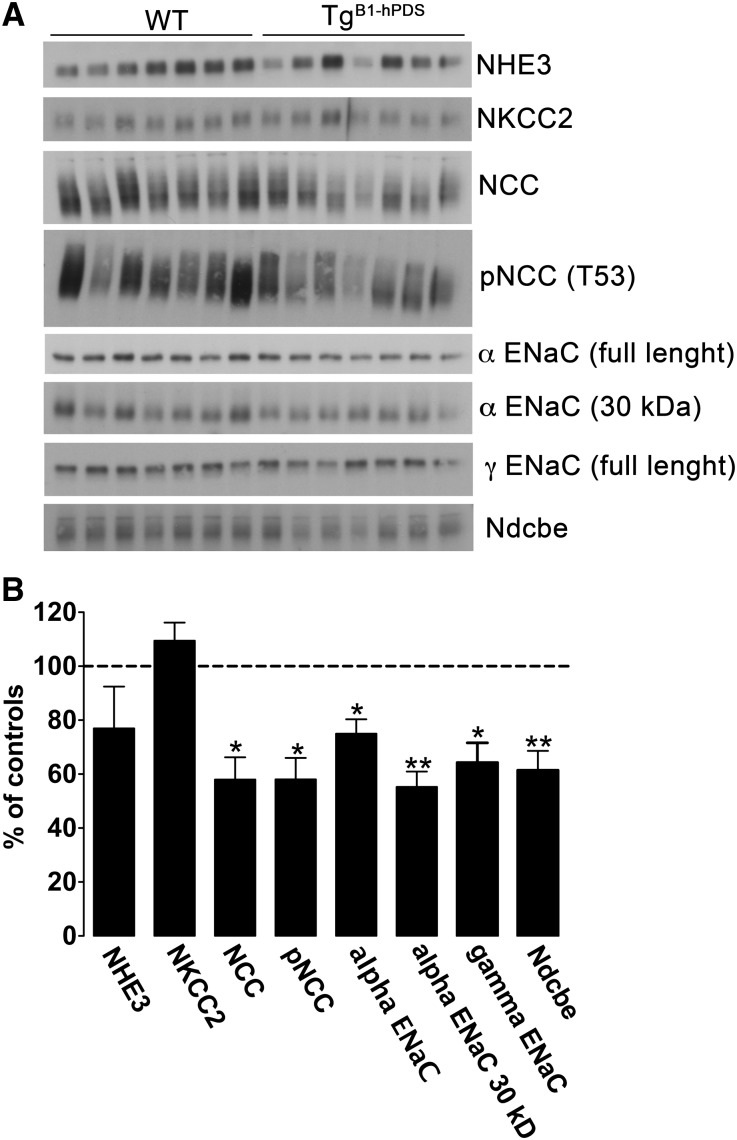
Pech et al.19 have recently reported that extracellular bicarbonate stimulates ENaC activity by increasing β- and γ-ENaC protein abundance and more importantly, promoting γ-ENaC proteolytic cleavage, a process associated with an increase in the channel activity.20 Conversely, pendrin knockout mice exhibit decreased ENaC activity associated with decreased β- and γ-ENaC activity and decreased γ-ENaC proteolytic activation. In the experiments shown in Figure 4, we detected increased ENaC activity in TgB1-hPDS mice fed a high-salt diet; therefore, we next assessed the profile of expression of different sodium transporters, including ENaC, along the nephron. Unexpectedly, expression of NCC, p(T55)NCC, ENaC (both α- and γ-subunits), and Ndcbe assessed by immunoblotting of membrane fractions isolated from the renal cortex was decreased in TgB1-hPDS mice compared with wild-type littermates (Figure 5 and Supplemental Table 3). This downregulation of sodium transporters in the aldosterone-sensitive distal nephron in TgB1-hPDS mice is expected in response to the hypoaldosteronism and the salt-sensitive hypertension observed under these conditions. The proteolytic product of γ-ENaC subunit was also undetectable, which was expected in salt-loaded animals. Taken together, these results indicate that excessive chloride absorption by the B1-hPDS transgene is able to accelerate Na+ absorption through ENaC and Ndcbe, although their expression levels as assayed by immunoblotting were downregulated. This result suggests that primary activation of chloride reabsorption by pendrin stimulates Na reabsorption through these transporters sufficiently to overcome the observed downregulation at the level of protein expression. Possible mechanisms include changes in transporter surface expression, phosphorylation, activation state, and/or driving force.
Figure 5.
Protein abundance of critical sodium transporters along the nephron in TgB1-hPDS and WT mice fed a high-salt diet. (A) Immunoblots of membrane fractions from the renal cortex extracted from seven TgB1-hPDS and seven WT mice fed a high-salt diet for 2 weeks. Each lane was loaded with 10–15 µg protein. Immunoblots were probed with the different primary antibodies as indicated. (B) Densitometric analyses of data were expressed as percentages of control (i.e., percent of control). Mean control values and SEMs for each respective immunoblot are provided in Supplemental Table 3. Bars represent mean ± SEM. Statistical significance is assessed by two-tailed unpaired t test; n=7 in each group. *P<0.05, **P<0.01 versus controls.
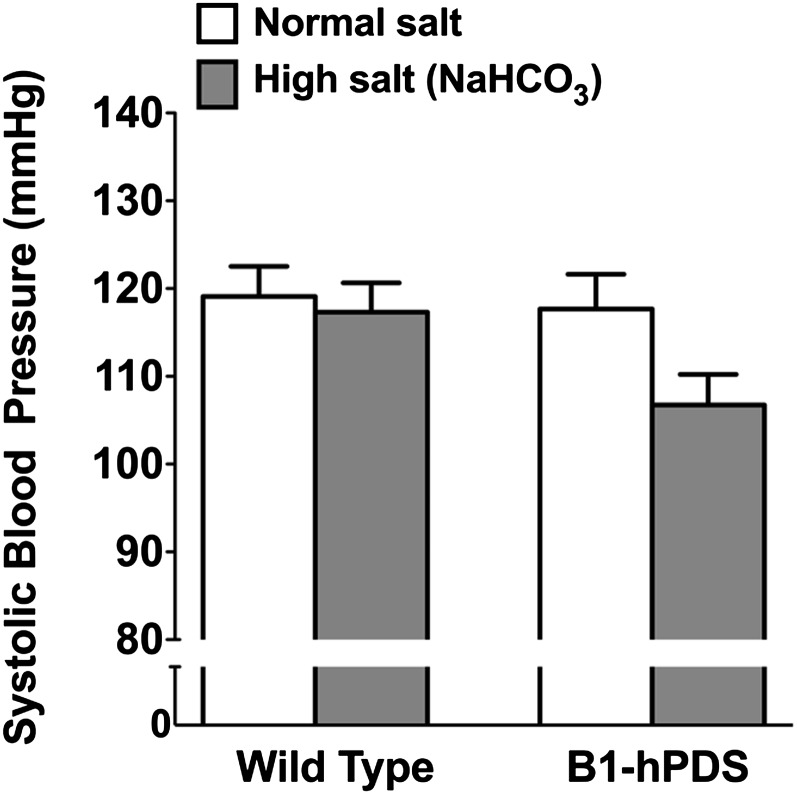
To further show the critical role of chloride in the pathogenesis of salt-sensitive hypertension in this model, we examined the effects of chloride on BP by administering NaHCO3 instead of NaCl as previously described.4,7 Contrasting with the marked pressor effects of high NaCl intake observed in the preceding experiments, high NaHCO3 intake did not alter systolic BP in the TgB1-hPDS mice or wild-type littermates, which confirms that the abnormal renal handling of chloride is the primary cause of salt-sensitive hypertension in TgB1-hPDS mice (Figure 6).
Figure 6.
Effect of NaHCO3 loading on systolic BP in TgB1-hPDS and WT mice. Systolic BP measured by tail-cuff on normal salt diet (0.3% Na+) or after 2 weeks of high-salt (3% Na+ administrated as NaHCO3) diet. Data are presented as the mean ± SEM; n=8 for each genotype. The difference in systolic BP was tested by ANOVA.
Discussion
In most human populations, sodium is almost invariably ingested as a chloride salt, and sodium ion has no pressor effects when chloride is substituted with another anion like sulfate or bicarbonate.3,8,9 The latter observation has led several groups to conclude that salt-sensitive hypertension is chloride-dependent.3,4,7 However, renal transporters that have been shown to have a clear impact on BP, like the Na/K/2Cl cotransporter type 2 or NCC,21 transport equimolar amounts of sodium and chloride. In the collecting duct, it was thought until recently that chloride is mostly absorbed passively because of the lumen-negative transepithelial potential difference that results from ENaC-dependent electrogenic absorption of Na+.22,23 As a consequence, most efforts have been made to dissect the mechanisms accounting for renal sodium absorption and its regulation, whereas the pathogenic effects of abnormal renal chloride transport have not been studied as extensively.
Here, we show that overexpression of pendrin leads to chloride-dependent hypertension. Because the trangene hPDS was detectable by PCR in the brain, we cannot rule out the possibility that overexpression of pendrin in the central nervous system did also alter central regulation of BP. However, the expression of hPDS in the brain was marginal compared with the kidney. We also did not detect differences in heart rate between TgB1-hPDS and control mice that might have revealed some dysregulation of the autonomous nervous system. Importantly, we show that renal overexpression of pendrin drives excessive renal chloride reclamation, which in turn, is responsible for chloride-sensitive hypertension. Moreover, we show that, despite normal downregulation of all the different sodium transporters in the aldosterone-sensitive distal nephron, excessive chloride absorption by transgenic pendrin is still able to drive parallel sodium absorption. This important finding challenges the classic paradigm assuming that sodium transport is always primary and secondarily drives chloride as the accompanying anion. Rather, these studies support the possibility that a primary increase in chloride transport can serve as the primary event driving NaCl absorption in the distal nephron. It was previously shown that mice with pendrin disruption do not increase vascular volume and do not raise BP in response to administration of deoxycorticosterone pivalate,13 a mineralocorticoid that is expected to maximally stimulate sodium absorption by ENaC. We now show that a primary increase in pendrin activity can cause hypertension, even when aldosterone is decreased. The fact that primary changes in chloride transport can drive NaCl absorption also has important therapeutic implications, because our results suggest that pendrin might be a very interesting target to modulate NaCl absorption by the distal nephron; pendrin blockers could, therefore, represent a new class of antihypertensive compounds. This possibility is further supported by a recent study showing that double deletion of NCC and pendrin leads to massive sodium wasting, hypovolemia, and hypotension.24
From the study of two independent mouse models of pseudohypoaldosteronism type II,25,26 it has been concluded that increased electroneutral NaCl absorption by NCC is the main mechanism leading to this syndrome. Functional coupling of pendrin and Ndcbe mediates NCC-like activity.12 Moreover, this transport system is expressed in the nephron segments where ENaC-dependent K+ and H+ secretion occurs. Thus, we hypothesized that upregulation of this transport system might decrease ENaC activity and hence, might impair K+ and H+ secretion even more than NCC upregulation. However, our present results clearly indicate that this hypothesis is not the case. The absence of effects on potassium and acid–base homeostasis cannot be attributed to a failure of our transgenic strategy, because we clearly show that the B1-hPDS transgene was able to increase apical Cl−/HCO3− exchange activity in the cortical collecting duct. Moreover, we were able to show that the transgene accounts for excessive renal NaCl absorption, and our TgB1-hPDS mice displayed salt-sensitive hypertension. Nevertheless, our results are in line with two recent studies that reported that increased NCC activity is not sufficient to cause Gordon’s syndrome.27,28
In summary, our study solidifies the concept of chloride-sensitive hypertension by showing that a primary enhancement of renal chloride transport by overexpression of pendrin can lead to salt-sensitive hypertension. Thus, we conclude that the chloride/bicarbonate exchanger pendrin has a major role in controlling net NaCl absorption and hence, BP in the setting of high salt intake.
Concise Methods
Animals
All the experimental procedures have been reviewed and approved by the local ethics committee from the University Pierre et Marie Curie, and they were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health publication No.93–23, revised 1985).
Metabolic Studies
All experiments were performed using age- and sex-matched Tg(B1-hPDS) mice and wild-type littermate (3–5 months). For urine collection, mice were housed in metabolic cages (Techniplast, France). Mice were given deionized water ad libitum and pair-fed with standard laboratory chow containing 0.3% sodium (INRA, France). They were allowed to adapt for 3–5 days in the cages. At steady state, urine collection was performed daily under mineral oil in the urine collector for electrolyte measurements. Mice were then switched to a high-salt diet (3% Na+), either as NaCl or NaHCO3 (INRA, France). After the switch, urine was collected every 24 hours.
All biochemical and hormonal analyses were performed using standard methods as detailed in Supplemental Material.
BP Measurements in Conscious Mice
Mice were fed normal or high-salt diet for at least 2 weeks. Systolic BP was measured using a computerized tail-cuff system after 2 weeks of daily training, which has been described elsewhere.29 Then, at least 10 measurements were performed every day for at least 7 consecutive days. Only the last 4 days were kept for analyses. If the variability of the measurements made in a single day exceeded the SD by more than 20%, the day was discarded and replaced by an additional day of measurement. Moreover, to ensure that it was the case in our study, BPs were also measured by radiotelemetry in mice fed either a normal or high-salt diet in a pilot experiment (Supplemental Material). Similar values were obtained using both techniques (compare Figure 4 with Supplemental Figure 3).
Immunofluorescence Studies and Immunoblot Analyses
Kidney sections were triple labeled with a rabbit polyclonal human pendrin antibody (diluted 1:200), a guinea pig anti-AE130 (diluted 1:5000), and a chicken anti-Atp6v1e1,31 which detects the V H+-ATPase (diluted 1:500), using the standard technique as previously described in detail.32 For Western blot analyses, 10–60 μg membrane-enriched protein fraction were separated on reducing 7.5% SDS polyacrylamide gels. Protein loading was assessed on gels ran in parallel and stained with Coomassie blue.33 Blots were probed with anti α-ENaC (dilution 1:10,000), anti γ-ENaC (1:30,000),34 anti-Ndcbe (1:250),12 anti-NHE3 the Na/H exchanger type 3 (1:1000),35 anti-NKCC2 the Na/K/2Cl cotransporter type 2 (1:5000),35 anti-NCC (1:50,000),36 and anti-NCC phospho-Thr55 antibody (residues 41–60 of human NCC phosphorylated at Thr55, HPSHLTHSSTFCMRpTFGYNT, S908B; 1:275).37 Proteins were detected by chemiluminescence (ECL Kit; Amersham Biosciences). Antibodies against α- and γ-subunits of ENaC were generously donated by J. Loffing (University of Zurich, Zurich, Switzerland). Antibody against α-ENaC was raised against the N terminus of mouse α-ENaC (MLDHTRAPELNLDLDLDVSNC).
RT-PCR
Total RNA was extracted using the RNeasy Kit (Qiagen). Reverse transcription was performed by using a first-strand cDNA synthesis kit for RT-PCR (Roche Diagnostics). Real-time PCR was performed on a LightCycler (Roche Diagnostics) with a LightCycler 480 SYBR Green I Master qPCR Kit (Roche Diagnostics). No amplification was observed in the absence of reverse transcription, which confirmed that samples were free of genomic DNA. Primer sequences and a methodology for the PCR amplification are provided in detail in Supplemental Material. Results are expressed as the mean ± SEM from six mice.
Transgene expression in different organs was assessed in one male mouse and one female mouse as described in Figure 1D.
In Vitro Microperfusion of Isolated Collecting Ducts
CCDs were isolated and microperfused in vitro as described by Burg et al.38 Intracellular pH was monitored by using the pH-sensitive dye BCECF.12 [Na+], [K+], and [creatinine] measurements were performed by HPLC12; transepithelial voltage (Vte) was measured continuously between Ag-AgCl electrodes connected to 0.15 M NaCl–agar bridges inserted in the perfusion pipette and bathing solutions as described previously12 to calculate the rate of K+ (JK) and Na+ (JNa) transepithelial transport, which is described in detail in Supplemental Material.
Disclosures
None.
Supplementary Material
Acknowledgments
R.C. and D.E. are funded by Institut National de la Santé et de la Recherche Médicale, Centre National de la Recherche Scientifique, the Transatlantic Network for Hypertension from the Fondation Leducq, Grant subvention de recherche 2010 AMGEN from the Société de Néphrologie (to R.C.), Grant subvention de recherche 2010 from the association pour l’information et la recherche sur les maladies rénales génétiques AIRG (to R.C.), and Grant HYPERCLO programm BLANC 2010-R10164DD from l'Agence Nationale de la Recherche (to D.E.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012080787/-/DCSupplemental.
References
- 1.Berghoff RS.Geraci AS: The influence of sodium chloride on blood pressure. Intern Med J 56: 395–397, 1929 [Google Scholar]
- 2.Morgan TO: The effect of potassium and bicarbonate ions on the rise in blood pressure caused by sodium chloride. Clin Sci 63: 407s–409s, 1982 [Google Scholar]
- 3.Kurtz TW, Al-Bander HA, Morris RC, Jr: “Salt-sensitive” essential hypertension in men. Is the sodium ion alone important? N Engl J Med 317: 1043–1048, 1987 [DOI] [PubMed] [Google Scholar]
- 4.Kurtz TW, Morris RC, Jr: Dietary chloride as a determinant of “sodium-dependent” hypertension. Science 222: 1139–1141, 1983 [DOI] [PubMed] [Google Scholar]
- 5.Schmidlin O, Tanaka M, Bollen AW, Yi SL, Morris RC, Jr: Chloride-dominant salt sensitivity in the stroke-prone spontaneously hypertensive rat. Hypertension 45: 867–873, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M, Schmidlin O, Yi SL, Bollen AW, Morris RC, Jr: Genetically determined chloride-sensitive hypertension and stroke. Proc Natl Acad Sci U S A 94: 14748–14752, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitescarver SA, Ott CE, Jackson BA, Guthrie GP, Jr, Kotchen TA: Salt-sensitive hypertension: Contribution of chloride. Science 223: 1430–1432, 1984 [DOI] [PubMed] [Google Scholar]
- 8.Schorr U, Distler A, Sharma AM: Effect of sodium chloride- and sodium bicarbonate-rich mineral water on blood pressure and metabolic parameters in elderly normotensive individuals: A randomized double-blind crossover trial. J Hypertens 14: 131–135, 1996 [PubMed] [Google Scholar]
- 9.Luft FC, Zemel MB, Sowers JA, Fineberg NS, Weinberger MH: Sodium bicarbonate and sodium chloride: Effects on blood pressure and electrolyte homeostasis in normal and hypertensive man. J Hypertens 8: 663–670, 1990 [DOI] [PubMed] [Google Scholar]
- 10.Pech V, Kim YH, Weinstein AM, Everett LA, Pham TD, Wall SM: Angiotensin II increases chloride absorption in the cortical collecting duct in mice through a pendrin-dependent mechanism. Am J Physiol Renal Physiol 292: F914–F920, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, Verlander JW: NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: Role in Cl- conservation. Hypertension 44: 982–987, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Leviel F, Hübner CA, Houillier P, Morla L, El Moghrabi S, Brideau G, Hassan H, Parker MD, Kurth I, Kougioumtzes A, Sinning A, Pech V, Riemondy KA, Miller RL, Hummler E, Shull GE, Aronson PS, Doucet A, Wall SM, Chambrey R, Eladari D: The Na+-dependent chloride-bicarbonate exchanger SLC4A8 mediates an electroneutral Na+ reabsorption process in the renal cortical collecting ducts of mice. J Clin Invest 120: 1627–1635, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, Green ED, Wall SM: Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, Nelson RD: The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller RL, Zhang P, Smith M, Beaulieu V, Paunescu TG, Brown D, Breton S, Nelson RD: V-ATPase B1-subunit promoter drives expression of EGFP in intercalated cells of kidney, clear cells of epididymis and airway cells of lung in transgenic mice. Am J Physiol Cell Physiol 288: C1134–C1144, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kim YH, Kwon TH, Frische S, Kim J, Tisher CC, Madsen KM, Nielsen S: Immunocytochemical localization of pendrin in intercalated cell subtypes in rat and mouse kidney. Am J Physiol Renal Physiol 283: F744–F754, 2002 [DOI] [PubMed] [Google Scholar]
- 17.Schambelan M, Sebastian A, Rector FC, Jr: Mineralocorticoid-resistant renal hyperkalemia without salt wasting (type II pseudohypoaldosteronism): Role of increased renal chloride reabsorption. Kidney Int 19: 716–727, 1981 [DOI] [PubMed] [Google Scholar]
- 18.Take C, Ikeda K, Kurasawa T, Kurokawa K: Increased chloride reabsorption as an inherited renal tubular defect in familial type II pseudohypoaldosteronism. N Engl J Med 324: 472–476, 1991 [DOI] [PubMed] [Google Scholar]
- 19.Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, Walp E, Kim YH, Sutliff RL, Bao HF, Eaton DC, Wall SM: Pendrin modulates ENaC function by changing luminal HCO3-. J Am Soc Nephrol 21: 1928–1941, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carattino MD, Hughey RP, Kleyman TR: Proteolytic processing of the epithelial sodium channel gamma subunit has a dominant role in channel activation. J Biol Chem 283: 25290–25295, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji W, Foo JN, O’Roak BJ, Zhao H, Larson MG, Simon DB, Newton-Cheh C, State MW, Levy D, Lifton RP: Rare independent mutations in renal salt handling genes contribute to blood pressure variation. Nat Genet 40: 592–599, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neil RG, Helman SI: Transport characteristics of renal collecting tubules: Influences of DOCA and diet. Am J Physiol 233: F544–F558, 1977 [DOI] [PubMed] [Google Scholar]
- 23.Stoner LC, Burg MB, Orloff J: Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol 227: 453–459, 1974 [DOI] [PubMed] [Google Scholar]
- 24.Soleimani M, Barone S, Xu J, Shull GE, Siddiqui F, Zahedi K, Amlal H: Double knockout of pendrin and Na-Cl cotransporter (NCC) causes severe salt wasting, volume depletion, and renal failure. Proc Natl Acad Sci U S A 109: 13368–13373, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalioti MD, Zhang J, Volkman HM, Kahle KT, Hoffmann KE, Toka HR, Nelson-Williams C, Ellison DH, Flavell R, Booth CJ, Lu Y, Geller DS, Lifton RP: Wnk4 controls blood pressure and potassium homeostasis via regulation of mass and activity of the distal convoluted tubule. Nat Genet 38: 1124–1132, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Yang SS, Morimoto T, Rai T, Chiga M, Sohara E, Ohno M, Uchida K, Lin SH, Moriguchi T, Shibuya H, Kondo Y, Sasaki S, Uchida S: Molecular pathogenesis of pseudohypoaldosteronism type II: Generation and analysis of a Wnk4(D561A/+) knockin mouse model. Cell Metab 5: 331–344, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Hadchouel J, Soukaseum C, Büsst C, Zhou XO, Baudrie V, Zürrer T, Cambillau M, Elghozi JL, Lifton RP, Loffing J, Jeunemaitre X: Decreased ENaC expression compensates the increased NCC activity following inactivation of the kidney-specific isoform of WNK1 and prevents hypertension. Proc Natl Acad Sci U S A 107: 18109–18114, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCormick JA, Nelson JH, Yang CL, Curry JN, Ellison DH: Overexpression of the sodium chloride cotransporter is not sufficient to cause familial hyperkalemic hypertension. Hypertension 58: 888–894, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM: Regulation of blood pressure by the type 1A angiotensin II receptor gene. Proc Natl Acad Sci U S A 92: 3521–3525, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehberger PA, Shmukler BE, Stuart-Tilley AK, Peters LL, Alper SL, Wagner CA: Distal renal tubular acidosis in mice lacking the AE1 (band3) Cl-/HCO3- exchanger (slc4a1). J Am Soc Nephrol 18: 1408–1418, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Breton S, Wiederhold T, Marshansky V, Nsumu NN, Ramesh V, Brown D: The B1 subunit of the H+ATPase is a PDZ domain-binding protein. Colocalization with NHE-RF in renal B-intercalated cells. J Biol Chem 275: 18219–18224, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Eladari D, Cheval L, Quentin F, Bertrand O, Mouro I, Cherif-Zahar B, Cartron JP, Paillard M, Doucet A, Chambrey R: Expression of RhCG, a new putative NH(3)/NH(4)(+) transporter, along the rat nephron. J Am Soc Nephrol 13: 1999–2008, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Quentin F, Chambrey R, Trinh-Trang-Tan MM, Fysekidis M, Cambillau M, Paillard M, Aronson PS, Eladari D: The Cl-/HCO3- exchanger pendrin in the rat kidney is regulated in response to chronic alterations in chloride balance. Am J Physiol Renal Physiol 287: F1179–F1188, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Wagner CA, Loffing-Cueni D, Yan Q, Schulz N, Fakitsas P, Carrel M, Wang T, Verrey F, Geibel JP, Giebisch G, Hebert SC, Loffing J: Mouse model of type II Bartter’s syndrome. II. Altered expression of renal sodium- and water-transporting proteins. Am J Physiol Renal Physiol 294: F1373–F1380, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Kim GH, Ecelbarger C, Knepper MA, Packer RK: Regulation of thick ascending limb ion transporter abundance in response to altered acid/base intake. J Am Soc Nephrol 10: 935–942, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Schmitt R, Ellison DH, Farman N, Rossier BC, Reilly RF, Reeves WB, Oberbäumer I, Tapp R, Bachmann S: Developmental expression of sodium entry pathways in rat nephron. Am J Physiol 276: F367–F381, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Richardson C, Rafiqi FH, Karlsson HK, Moleleki N, Vandewalle A, Campbell DG, Morrice NA, Alessi DR: Activation of the thiazide-sensitive Na+-Cl- cotransporter by the WNK-regulated kinases SPAK and OSR1. J Cell Sci 121: 675–684, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Burg M, Grantham J, Abramow M, Orloff J: Preparation and study of fragments of single rabbit nephrons. Am J Physiol 210: 1293–1298, 1966 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.