Abstract
DLG1 (discs-large homolog 1) and CASK (calcium/calmodulin-dependent serine protein kinase) interact at membrane-cytoskeleton interfaces and function as scaffolding proteins that link signaling molecules, receptors, and other scaffolding proteins at intercellular and synaptic junctions. Dlg1-null mice exhibit hydronephrosis, hydroureter, and occasionally hypoplastic kidneys, whereas Cask-null mice do not. To investigate whether DLG1 and CASK cooperate in the developing urogenital system, we generated mice deficient in both DLG1 and CASK either 1) globally, 2) in metanephric mesenchyme, or 3) in nephron progenitors. With each approach, Dlg1;Cask double-knockout (DKO) kidneys were severely hypoplastic and dysplastic and demonstrated rapid, premature depletion of nephron progenitors/stem cells. Several cellular and molecular defects were observed in the DKO kidneys, including reduced proliferation and increased apoptosis of cells in the nephrogenic zone and a progressive decrease in the number of cells expressing SIX2, a transcription factor essential for maintaining nephron progenitors. Fgf8 expression was reduced in early-stage DKO metanephric mesenchyme, accompanied by reduced levels of components of the Ras pathway, which is activated by fibroblast growth factor (FGF) signaling. Moreover, Dlg1+/−;Cask−/− (het/null) kidneys were moderately hypoplastic and demonstrated impaired aggregation of SIX2-positive cells around the ureteric bud tips. Nephron progenitor-specific het/null mice survived with small kidneys but developed glomerulocystic kidney disease and renal failure. Taken together, these results suggest that DLG1 and CASK play critical cooperative roles in maintaining the nephron progenitor population, potentially via a mechanism involving effects on FGF signaling.
In mice, kidney development begins on embryonic day (E)10.5 when signals from the metanephric mesenchyme (MM) induce the ureteric bud (UB) to grow out from the Wolffian duct and invade the MM.1–3 Reciprocal interactions between the UB and MM ensue; this induces the UB to branch and form the collecting system and a subset of the MM to differentiate into all cells of the nephron. The stromal component of the MM gives rise to mesangial cells and portions of the tubulointerstitium.
For proper kidney development to occur, a subpopulation of the MM needs to be maintained in a proliferating, nondifferentiating, stem cell–like state as part of it undergoes a mesenchyme to epithelium transition (MET) to form nephrons. The maintenance of this nephron progenitor cell population is regulated by the concerted action of several genes and signaling pathways. SIX2, a transcription factor expressed by all nephron progenitors, is required for their self-renewal; its inactivation results in depletion of the progenitors by premature MET.4 BMP7 promotes proliferation of nephron progenitors via JNK signaling, and its absence results in their premature depletion.5,6 Fibroblast growth factor (FGF) signaling has also been shown to be crucial for survival of nephron progenitors;7,8 absence of FGF receptors 1 and 2 in the MM leads to renal agenesis.9 Conversely, ectopic Notch signaling in progenitors can induce premature MET and stem cell depletion.10
DLG1 (discs-large homolog 1), a mouse ortholog of the Drosophila discs-large tumor suppressor protein, is a member of the PDZ (postsynaptic density-95/discs-large/zonula occludens-1) and MAGUK (membrane-associated guanylate kinase) families of scaffolding proteins.11 DLG1 plays a vital role in establishing epithelial cell polarity and maintaining neuronal synaptic function.11,12 Its absence in mice has been shown to result in several developmental defects, including impaired palate fusion.13 In the urogenital tract, DLG1 deletion causes severe misalignment of the ureteric smooth muscle cells, resulting in impaired urinary transport and hence hydronephrosis.14 Occasional unilateral renal agenesis and hypoplasia have also been observed in Dlg1 knockout mice.14–16
DLG1 interacts with several proteins at membrane-cytoskeleton interfaces, including calcium/calmodulin-dependent serine protein kinase (CASK). CASK, also a member of the MAGUK family, is a scaffolding protein that uses multiple protein-protein interaction domains to cluster receptors, adhesion molecules, and signaling molecules at intercellular junctions and synapses17 to regulate neuronal and epithelial cell polarity.18,19
Here we report a critical function for DLG1 and CASK in maintaining nephron progenitors: Dlg1;Cask double-knockout (DKO) kidneys were severely hypoplastic and dysplastic and demonstrated a striking premature depletion of nephron progenitors. Furthermore, Dlg1+/−;Cask−/− (het/null) kidneys were moderately hypoplastic and functional but developed glomerular cysts and eventually failed in adults. DKO kidneys exhibited reduced expression of Fgf8, as well as reduced expression and phosphorylation of components of the Ras pathway that mediate FGF signaling. Taken together, these results show that DLG1 and CASK play important cooperative roles in maintaining the nephron progenitor population, potentially through the FGF signaling pathway.
Results
Dlg1;Cask DKO Kidneys Are Small and Dysplastic
Dlg1−/− mice demonstrate multiple developmental defects, the nature of which indicates that DLG1 plays diverse cellular roles that depend on the cell type. For example, Dlg1−/− mice exhibit craniofacial defects, including impaired palate closure,13 suggesting a role in epithelial cell sheet fusion. On the other hand, these mice also show misalignment of ureteric smooth muscle cells,14 suggesting a role in cell polarity or organization. The enhanced synaptic transmission detected with overexpression of DLG1 during development indicates an additional role in trafficking glutamate receptors.20
It is possible that the diverse roles for DLG1 are related to distinct repertoires of interacting proteins in different cell types. We therefore investigated the cell-specific functions of DLG1 as they relate to its interactions with CASK, another scaffolding protein involved in synapse function and palate fusion, by mutating both proteins. DKO mice lacking DLG1 and CASK appeared grossly similar to Dlg1−/− mice; they died at birth with cleft palate and craniofacial defects. But unlike most Dlg1 mutants, all Dlg1−/−;Cask−/− mice exhibited strikingly small and dysplastic kidneys, nearly equivalent in size to the adrenal glands and approximately one tenth the size of control kidneys at E18.5 (not shown). This is in contrast to Cask−/− mice, which died at birth with apparently normal kidneys (see below), and Dlg1−/− mice, which occasionally manifest unilateral agenesis or hypoplasia but commonly show hydronephrosis.14,15
DLG1 and CASK could be detected in both the MM and the UB (Supplemental Figure 1). To investigate the basis for the kidney growth defects in double-mutant mice, we applied the Cre-loxP system to mutate Dlg1 and Cask in different compartments of the developing kidney (Figure 1A). We used the Pax3-Cre transgene21 to delete both genes in the entire MM,22,23 which includes both stromal cell and nephron progenitors.24 Deleting Dlg1 and Cask via Pax3-Cre resulted in tiny kidneys that were similar in size to the constitutive Dlg1−/−;Cask−/− kidneys, but embryo size and weight and development of other organs appeared normal (Figure 1, B and C, and data not shown).
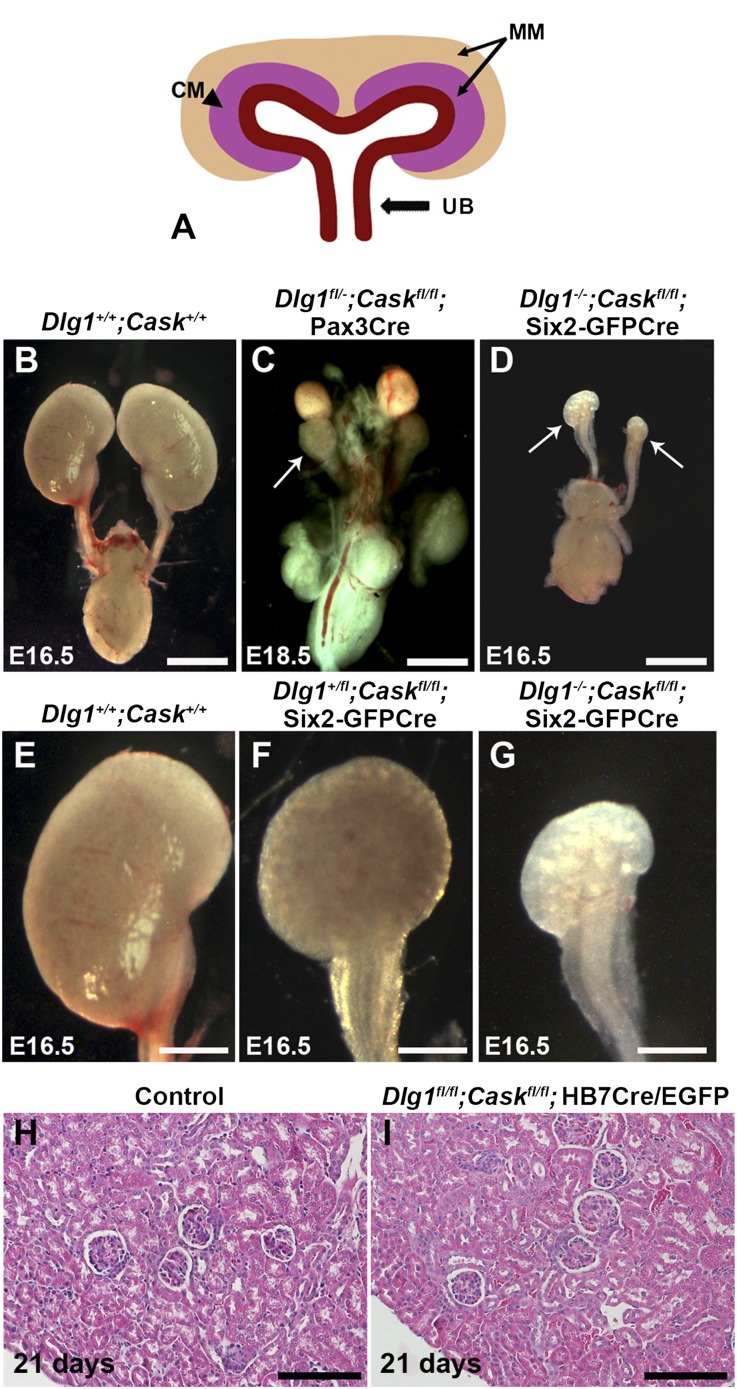
Figure 1.
Renal hypoplasia in the absence of DLG1 and CASK in nephron progenitors. (A) Diagram showing cell populations deleted by Cre: Pax3-Cre, MM; Six2-EGFP/Cre, the cap mesenchyme (CM) subset of the MM; and HoxB7-Cre/EGFP, UB. (B–D) Comparison of kidneys from control (B); Dlg1fl/-;Caskfl/fl;Pax-3Cre (C), and Dlg1−/−;Caskfl/fl;Six2-EGFP/Cre (D) kidneys. Arrows point to the severely hypoplastic kidneys. (E–G) Comparison of control (E; high magnification of B), Dlg1+/fl;Caskfl/fl;Six2-EGFP/Cre (F), and Dlg1−/−;Caskfl/fl;Six2-EGFP/Cre (G; high magnification of D) kidneys. Dlg1+/fl;Caskfl/fl;Six2-EGFP/Cre kidneys were smaller than controls, but not as small as the Dlg1−/−;Caskfl/fl;Six2-EGFP/Cre kidneys. (H and I) Hematoxylin-eosin staining of kidneys from control and Dlg1fl/fl;Caskfl/fl;Hoxb7Cre/EGFP mice at postnatal day 21 revealed no histopathology. Scale bars: B–D, 1 mm; E–G, 0.5 mm; H and I, 100 µm.
To attempt to narrow the primary cellular site of the kidney development defect, we used the Six2-enhanced green fluorescent protein (EGFP)/Cre transgene to generate embryos with a combined deletion of Dlg1 and Cask only in the self-renewing nephron progenitors, a subset of the MM also known as cap mesenchyme (Figure 1A). This resulted in extremely small and dysplastic kidneys, similar to the Pax3-Cre deletion (Figure 1D and data not shown). We then deleted Dlg1 and Cask in the Wolffian duct, ureteric bud, and its collecting duct derivatives using the Hoxb7-Cre/EGFP transgene. This did not result in a significant difference in kidney size compared with controls, and the glomeruli and tubules appeared normal (Figure 1, H and I, and data not shown). Together, our results show that the tiny dysplastic kidneys observed in constitutive Dlg1−/−;Cask−/− mice can be attributed solely to the lack of both DLG1 and CASK in nephron progenitors. For simplicity, mice lacking DLG1 and CASK in nephron progenitors by any genetic means will be referred to as DKOs; exact genotypes are indicated in the figure legends.
Molecular and Cellular Defects in DKO Kidneys
Histologic analysis of DKO kidneys revealed that the nephrogenic zone was markedly depleted at E16.5, with significant reduction of both MM and UB tips compared with controls (Figure 2, A, A’, B, and B’). Very few nascent nephrons were observed in the DKO kidneys, but the presence of tubular structures and glomeruli, some cystic (Figure 2, B and B’), indicated that MET had occurred. The nephrogenic zone appeared to be replaced by interstitium (Figure 2B’) and a population of cells that we later identified as Wilms tumor 1 (WT1)–positive/SIX2-negative cells (Figure 3J).
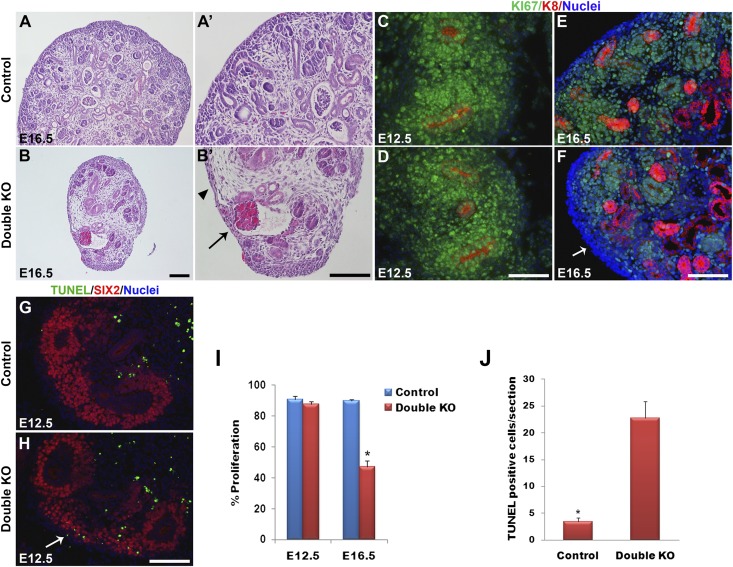
Figure 2.
Defects in renal architecture, cell proliferation, and apoptosis in DKO kidneys. (A and B) Hematoxylin-eosin staining of kidney sections at E16.5. Control kidneys at low (A) and high (A’) magnifications show the MM to be aggregated around the UB tips in the nephrogenic zone in the outer cortex. (B and B’) DKO (Dlg1fl/fl;Caskfl/fl;Pax3-Cre) kidneys showed a striking depletion of MM. Arrowhead points to a zone lacking MM and UB tips. Arrow points to a glomerulus. (C–F) Immunostaining of E12.5 and E16.5 kidneys for Ki67 (green; proliferating cells) and K8 (red; UB derivatives); nuclei are blue. Ki67 staining was similar in E12.5 control (C) and DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) (D) kidneys but was undetectable in some cortical zones in the E16.5 DKO (Dlg1fl/fl;Caskfl/fl;Pax-3Cre) kidneys (arrow in F) compared with control (E). (G and H) TUNEL staining at E12.5 revealed increased apoptosis in the nephrogenic zone of DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) kidneys (H) versus controls (G). (I) The percentage of Ki67-positive cells in DKO versus control kidneys was not different at E12.5 but was significantly reduced in DKO kidneys at E16.5 (*P<0.05). (J) TUNEL-positive cells were significantly increased in the DKO kidneys at E12.5 (*P<0.05). Data represent mean values; bars indicate SEM. Scale bars, 100 µm.
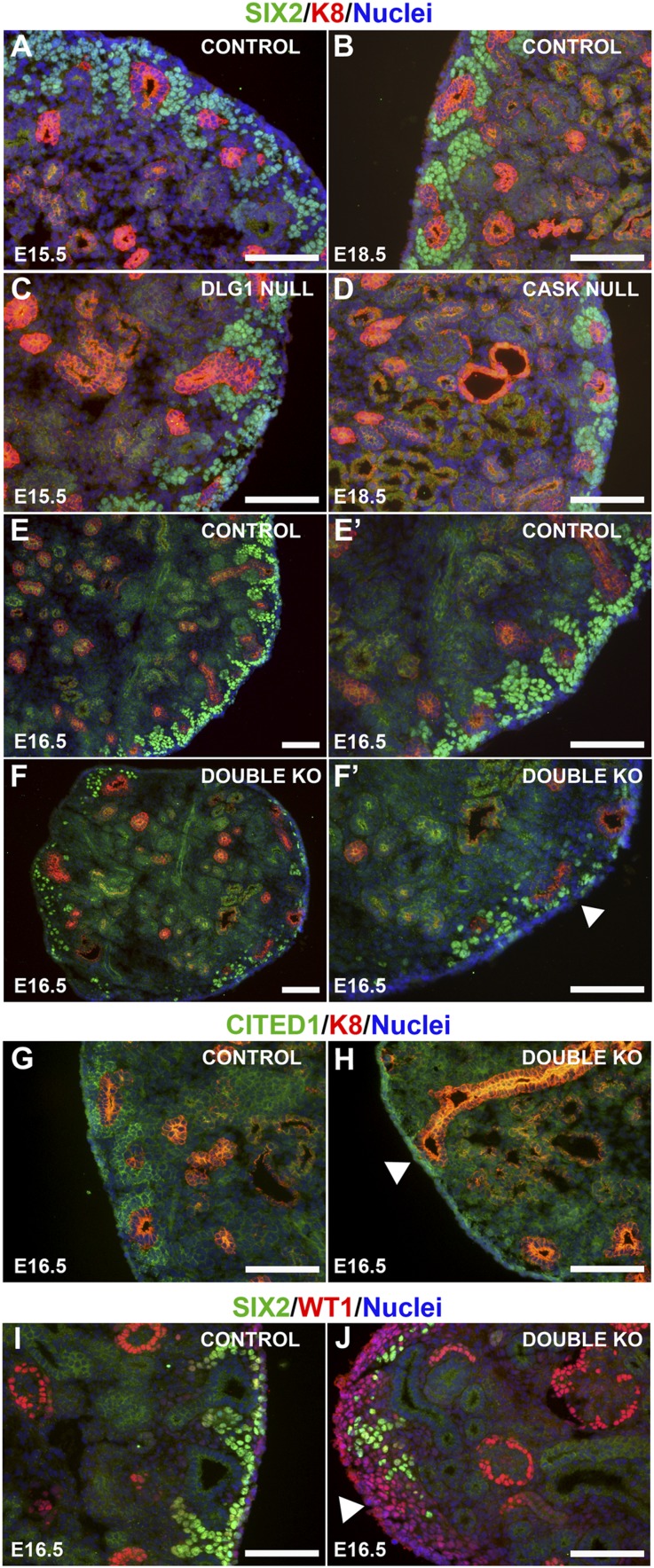
Figure 3.
Marker analyses in the nephrogenic zone. (A–F’) Immunostaining of kidneys of the indicated genotypes and ages for SIX2 (green; nephron progenitors) and K8 (red; UB tips and derivatives); nuclei are blue. SIX2+ cells aggregated around the UB tips in controls (A and B) and with loss of DLG1 (C; Dlg1fl/-;Pax-3Cre) or CASK (D; Cask−/−); however, DKO (Dlg1fl/-;Caskfl/fl;Pax-3Cre) kidneys (F and F’) showed a dramatic decrease of SIX2-positive cells in the nephrogenic zone compared with controls (E and E’). (G and H) Immunostaining of E16.5 kidneys for CITED1 (green) and K8 (red); nuclei are blue. DKO (Dlg1fl/fl;Caskfl/fl;Pax-3Cre) kidneys (H) showed fewer CITED1-expressing cells around the UB tips compared with controls (G). (I and J) Immunostaining of E16.5 kidneys for SIX2 (green) and WT1 (red; MM, renal vesicles, and podocytes); nuclei are blue. There were increased numbers of WT1-positive/SIX2-negative cells at the rim of the DKO (Dlg1fl/fl;Caskfl/fl;Pax-3Cre) kidneys. Scale bars, 100 µm.
To determine whether the depletion of cells in the nephrogenic zone involved decreased proliferation or increased apoptosis, we compared the expression of Ki67, a marker of cellular proliferation that is expressed during all active stages of the cell cycle, in control and DKO kidneys (Figure 2, C–F). At E12.5 we did not detect a significant reduction of Ki67 expression in the DKO kidneys compared with controls (Figure 2, D and I). However, at E16.5 we observed significantly decreased expression of Ki67 in the nephrogenic zone of DKO kidneys compared with controls (Figure 2, F and I). TUNEL assay at E12.5 revealed increased apoptosis in the nephrogenic zone of DKO kidneys (Figure 2, G, H, and J).
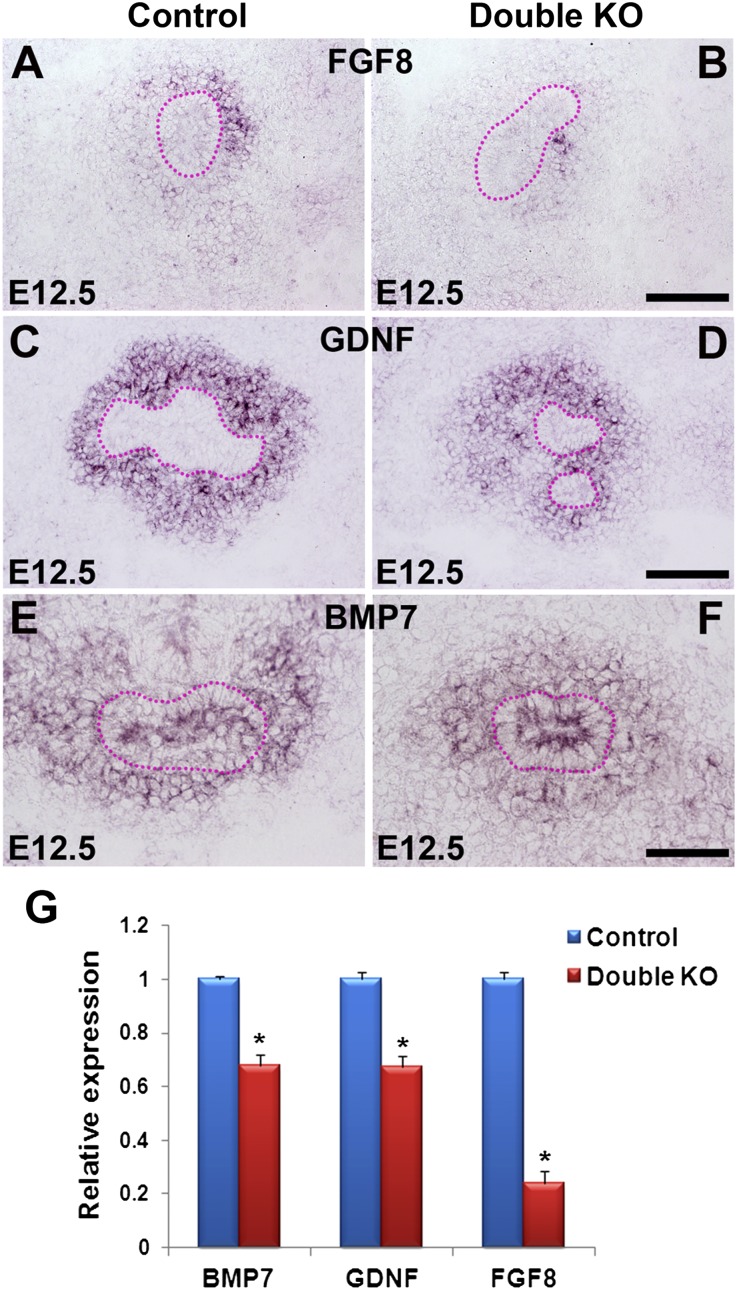
We next examined the expression of several molecular markers of nephron progenitors to determine the nature of the cells depleted in the nephrogenic zone. For this analysis we used kidneys at E15.5–16.5, an intermediate developmental stage when phenotypic changes were becoming obvious, but prior to a stage when the kidneys are severely dysplastic. We first confirmed through immunohistochemical analysis that deletion of either Dlg1 or Cask alone did not result in depletion of SIX2-positive cells in the nephrogenic zone (Figure 3, A–D). However, at E16.5 the numbers of SIX2- and CITED1-positive cells were dramatically reduced in the DKO kidneys compared with controls (Figure 3, E–H). SIX2, a homeobox transcription factor, is expressed in the cap mesenchyme.24 Six2-expressing cells are precursors of all the epithelial cells of the nephron, and Six2−/− mice have kidneys with a phenotype similar to that of our DKO kidneys, but they also exhibit ectopic nephron formation.4 CITED1, a transcription co-factor also expressed in the cap mesenchyme, becomes downregulated during the early stages of MET.25 Interestingly, we noted an expansion of SIX2-negative/WT1-positive cells in the cortex of DKO kidneys (Figure 3J); these cells seemed to replace the nephron progenitors as they became depleted. A similar phenomenon was observed in MM-specific Fgf8 mutant kidneys.23
Although we noted a drastic depletion of nephron progenitors at middle and late developmental stages, up to E12.5 the numbers of nephron progenitors were similar in DKOs and controls (Figure 4, A–D). Assembled confocal Z-stack images of immunostained whole kidneys at E11.5 and E12.5 demonstrated similar patterns of aggregation of SIX2-positive cells around UB tips in both controls and DKOs (Figure 4, A–D). In addition, UB formation and branching were similar between controls and DKOs up to E12.5. However, nephron progenitor depletion became apparent soon after E12.5 (Figure 4, E and F).
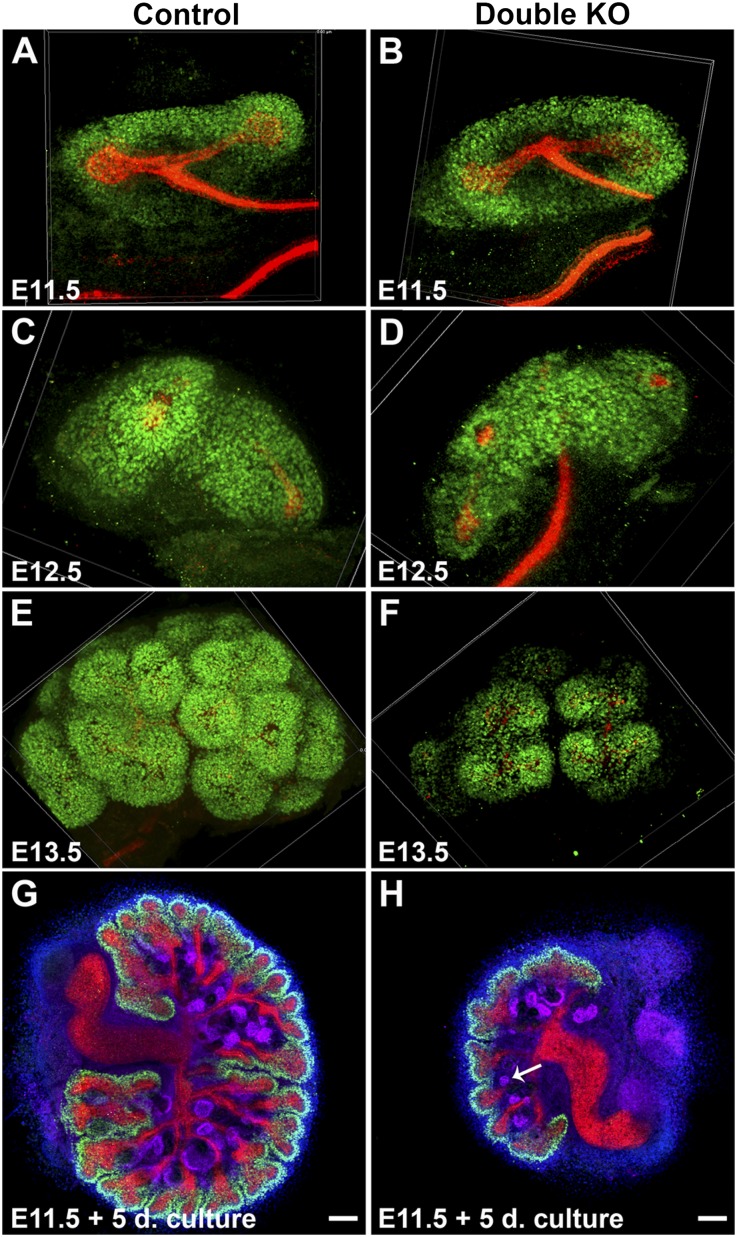
Figure 4.
Immunostained whole metanephroi at the indicated ages (A–F) or after organ culture (G and H). (A–F) Assembled confocal z-stack images of control and DKO kidneys immunostained for SIX2 (green; nephron progenitors) and K8 (red; UB). SIX2-positive cells were similar in abundance in E11.5 control and DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) kidneys and in E12.5 control and DKO (Dlg1fl/-;Caskfl/fl;Pax-3Cre) kidneys but were reduced in E13.5 DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) kidneys versus controls (E and F). (G and H) Whole kidneys removed at E11.5 were cultured for 5 days in serum-free medium and then immunostained for SIX2 (green), K8 (red), and WT-1 (purple; MM, renal vesicles and podocytes); nuclei are blue. Cultured DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) kidneys (H) were significantly smaller than controls (G), but they underwent MET (arrow in H points to a renal vesicle) and appeared to have more preserved SIX2-positive cells than their in vivo counterparts (e.g., Figure 3F). Scale bars, 100 µm.
To better characterize the development of the DKO kidneys, we cultured E11.5 metanephroi for 5 days (Figure 4, G and H). After culture, the DKO kidneys were 38% the size of controls, had 22±3 UB tips versus 60±4 in controls, and had 21±3 renal vesicles versus 55±4 in controls (mean ± SEM;P<0.05). The formation and location of renal vesicles appeared normal. Of note, SIX2-positive cells, although severely depleted in E16.5 DKO kidneys in vivo, were not as significantly reduced in the cultured DKO kidney. This may be due to the slower rate of kidney growth in culture than in vivo.
Signaling Pathways Contributing to Nephron Progenitor Maintenance
To identify the signaling pathways and molecules required for nephron progenitor maintenance that were affected by the absence of DLG1 and CASK, we profiled gene expression by microarray analysis. Because nephron progenitor depletion occurred after E12.5 in the DKOs, all analyses described here were performed at E12.5. We found significant downregulation of Fgf8 mRNA (−1.7-fold change, P<0.005), which was confirmed by in situ hybridization and RT-PCR analyses (Figure 5, A, B, and G, and Supplemental Figure 2). Fgf8 is expressed in the MM immediately surrounding the UB at early stages of kidney development and is later expressed at a higher level in pretubular aggregates and renal vesicles. MM-specific Fgf8−/− kidneys show progressive depletion of nephron progenitors, similar to our phenotype, and demonstrate halted morphogenesis from the renal vesicle to the S-shaped stage.23 Other mRNAs found to be reduced by microarray included Cited1 (−1.8-fold change, P<0.005); Lhx1, a transcription factor expressed in the UB, vesicles, comma- and S-shaped bodies, collecting ducts, and podocytes of immature glomeruli26,27 (−2.4-fold change, P<0.005); and Osr2 (−5.0-fold change, P<0.005), which is expressed in the mesenchyme surrounding the tubular epithelium in the mesonephros and metanephros.28 But on the basis of the phenotypes of the Cited1, Lhx1, and Osr2 mutants, which were not similar to our DKO phenotype, they were not pursued further.
Figure 5.
Analysis of secreted growth factor expression in E12.5 kidneys. (A–F) In situ hybridization shows Fgf8 expression in the MM of the control (A) but greatly reduced expression in the DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) (B); strong Gdnf expression in the MM of the control (C) and moderately reduced expression in the DKO (Dlg1fl/-;Caskfl/fl;Six2-EGFP/Cre) (D); and slightly reduced Bmp7 expression in the DKO (Dlg1fl/-;Caskfl/fl;Six2-EGFP/Cre) (F) compared with control (E). UBs are outlined by dotted lines in A–F. (G) Quantitative RT-PCR analysis of E12.5 kidney RNA demonstrates reduced expression of Bmp7, Gdnf, and Fgf8 in the double mutants compared with control. Data represent mean values; bars indicate SEM. *P<0.05. Scale bars in A–F, 100 µm.
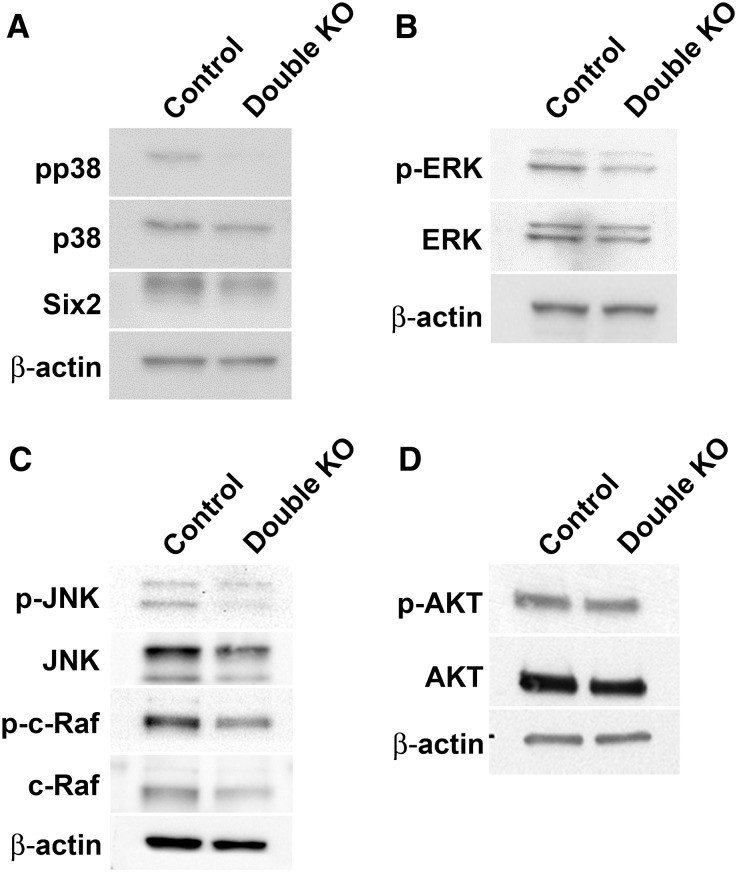
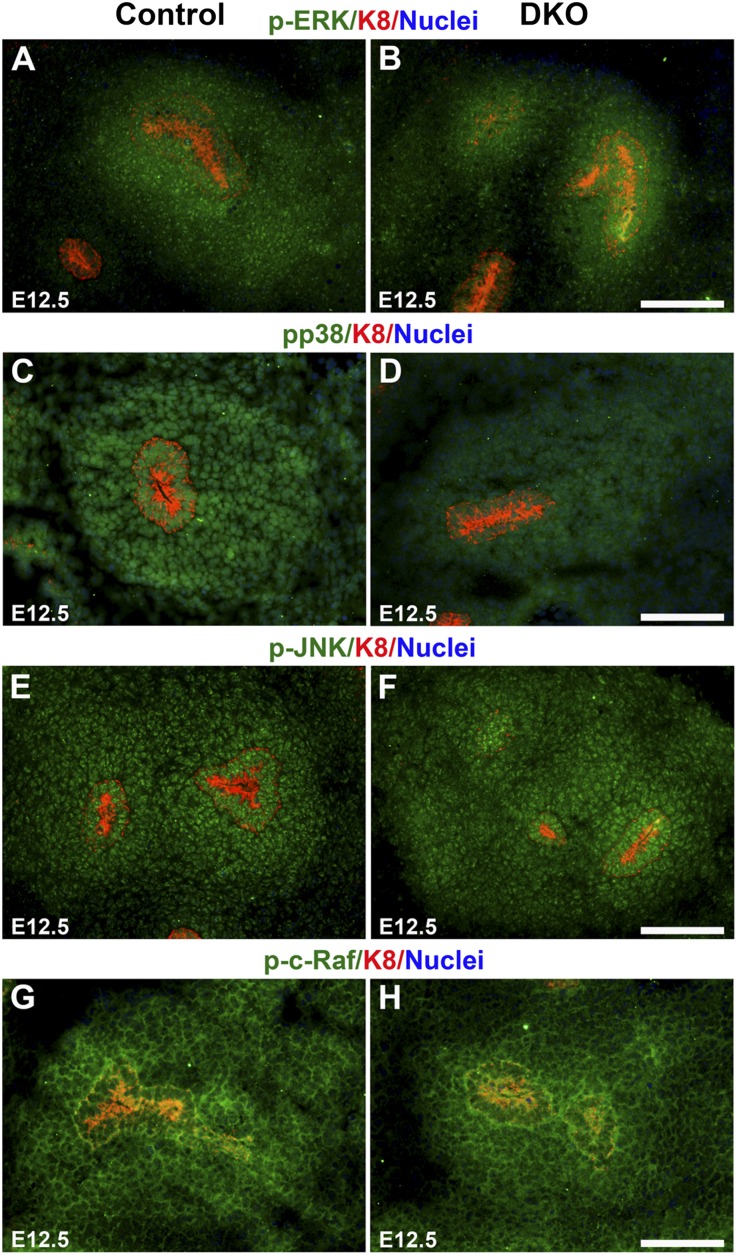
Because the MM-specific Fgf8 null phenotype is similar to our DKO phenotype, we explored intracellular components of the FGF signaling pathway, which includes the PLCγ/PKC, Ras/ERK, and PI3K pathways.29 We found that components of the Ras pathway were reduced in the DKO kidneys. By Western blot, levels of phospho-p38 MAPK (pp38; 62% of control), ERK and phospho-ERK (p-ERK; 66% and 57% of control, respectively), c-Raf and phospho-c-Raf (p-c-Raf; 55% and 66% of control, respectively), and JNK and phospho-JNK (p-JNK; 76% and 66% of control, respectively) were lower in the DKOs (Figure 6, A–C). However, levels of AKT and phospho-AKT (p-AKT), components of the PI3K signaling pathway, were similar to controls (86% and 90% of control, respectively; Figure 6D), as was the level of p38MAPK (98% of control). Previous studies have established the presence of ERK, JNK, and p38 MAPK in the cap mesenchyme.30–32 By immunostaining E12.5 metanephroi, we found that the levels of p-ERK and pp38 MAPK expression were reduced in the MM of the DKO kidneys compared with controls (Figure 7, A–D), whereas levels of p-JNK and p-c-Raf were not (Figure 7, E–H).
Figure 6.
Western blot analysis of the levels of various signaling pathway intermediates relevant to cell proliferation and apoptosis in E12.5 kidneys. (A) Phospho(p)-p38 MAPK levels were significantly reduced in the double mutants compared with controls. SIX2 levels were similar or slightly reduced in double mutants compared with controls after normalization to actin. (B and C) Levels of p-ERK, ERK, p-JNK, JNK, p-c-Raf, and c-Raf were reduced in the double mutants compared with control, with p-JNK and p-ERK being more reduced than total JNK and ERK. (D) Levels of p-AKT and AKT were similar or slightly reduced in the double mutants versus controls.
Figure 7.
Expression of FGF signaling pathway components in the MM. Immunostaining of E12.5 kidneys of the indicated genotype for FGF signaling components (green), K8 (red; UB tips and derivatives), and nuclei (blue). Levels of p-ERK (A and B) and pp38 MAPK (C and D) expression were reduced in the MM of the DKO (Dlg1fl/fl;Caskfl/fl;Six2-EGFP/Cre) kidneys compared with controls, whereas levels of p-JNK (E and F) and p-c-Raf (G and H) were similar in the MM of the DKO kidneys and controls. Scale bars, 100 µm.
That Dlg1fl/fl;Caskfl/fl;Hoxb7-Cre/EGFP kidneys were normal (Figure 1I) indicates that the reduced UB branching in the constitutive DKOs cannot be attributed to intrinsic UB defects. We therefore hypothesized that the reduced UB branching was due to reduced expression of Gdnf, which is secreted by the MM to promote UB outgrowth and branching.33 By in situ hybridization and RT-PCR, we confirmed that Gdnf mRNA levels were modestly lower in the E12.5 DKO kidneys compared with controls (Figure 5, C, D, and G). Moreover, as nephron progenitors become depleted, GDNF levels should decline further, leading to further attenuation of UB branching. Levels of mRNA for Fgf10, which can also promote UB branching,34 were similar by in situ hybridization in control and DKO kidney at E12.5 (Supplemental Figure 3, A and B).
We also analyzed the expression of another regulator of nephron progenitor proliferation, BMP7.5,6,35 Through in situ hybridization and RT-PCR analyses, we found slightly reduced levels of BMP7 in the E12.5 DKOs compared with controls (Figure 5, E–G). JNKs and p38 MAPK can be activated by BMP signaling,5,36 so the reduction in pp38 MAPK and p-JNK (Figure 6, A and C) may stem partly from the reduced BMP7.
On the basis of a recent report of inhibition of Fgf8 and Wnt4 expression in progenitors by SIX2/TCF/LEF and their activation in WNT9B-induced cells by βcat/TCF/LEF,37 we also examined Wnt4 expression but found no difference between the controls and DKOs, either in progenitors or in renal vesicles (Supplemental Figure 3, C and D). Fgf8 was also unchanged in the vesicles (Supplemental Figure 2). Finally, there was no difference in β-catenin staining between controls and DKOs (Supplemental Figure 3, E and F).
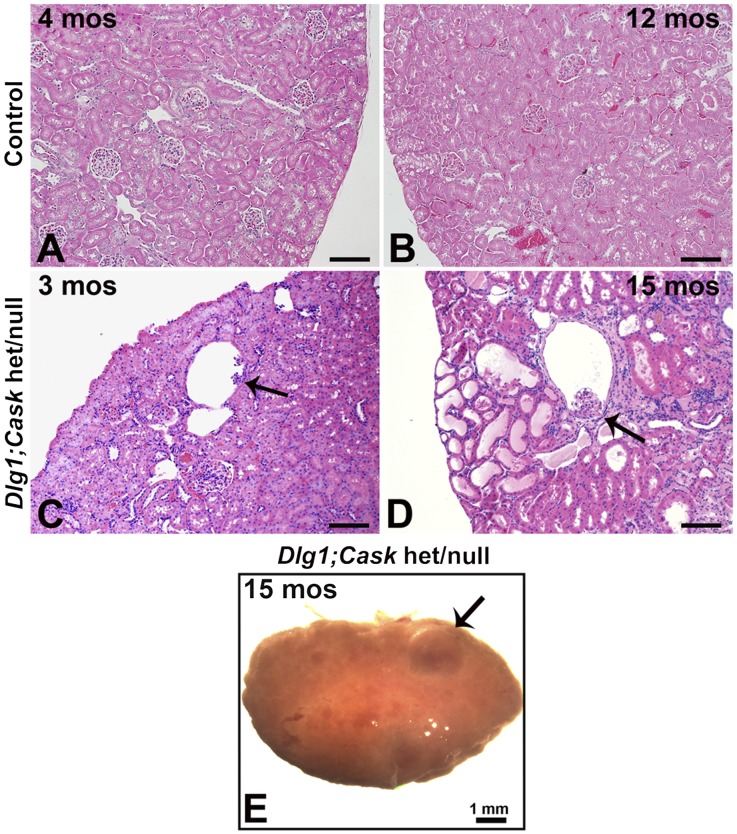
Dlg1;Cask het/null Kidneys Are Small and Cystic
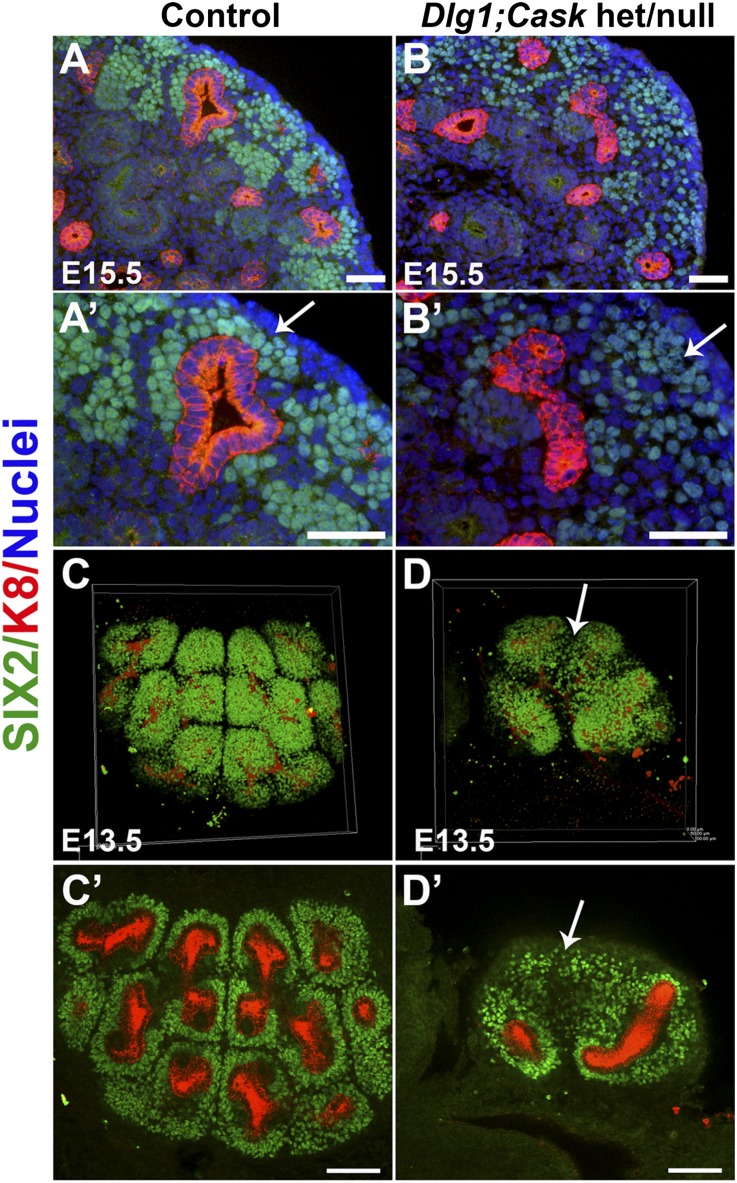
Although Cask mutant kidneys showed no growth defects (Figure 3D, and data not shown), Dlg1+/fl;Caskfl/fl;Six2-EGFP/Cre (het/null) kidneys were smaller than controls, but not as small or dysplastic as the DKO kidneys (Figure 1, E–G). In the E15.5 het/null nephrogenic zone, SIX2-positive cells failed to aggregate tightly around the UB tips (Figure 8, B and B’), in contrast to controls (Figure 8, A and A’). This failure to properly aggregate was detectable as early as E13.5; three-dimensional confocal image reconstruction of immunostained embryonic kidneys at E13.5 showed many SIX2-positive cells to be dispersed (Figure 8, D and D’), in contrast to controls (Figure 8, C and C’).
Figure 8.
Analysis of Dlg1/Cask het/null kidneys. (A and B) Immunostaining at E15.5 for SIX2 (green; nephron progenitors) and K8 (red; UB); nuclei are blue. (A and A’) SIX2-positive cells were closely aggregated around the UB tips in controls (arrow in A′), whereas they were much less well aggregated around the UB tips in het/null (Dlg1+/fl;Cask−/−;Pax-3Cre) kidneys (B and B’, arrow in B′). Scale bars, 50 µm. (C and D) Three-dimensional reconstructions and confocal z-stack sections of immunostained kidneys at E13.5. SIX2-positive cells (green) were closely apposed to UB tips in controls (C and C’), whereas some SIX2-positive cells were dispersed from the UB tips in het/null (Dlg1+/fl;Caskfl/fl;Pax-3Cre) kidneys (arrows in D and D’). Scale bars, 100 µm.
Unlike the DKOs, het/null mice were viable and fertile. However, compared with controls, by 90 days of age these mice had developed glomerular cysts (Figure 9, A and C). At 15 months the het/null kidneys had numerous glomerular cysts, dilated tubules, protein casts, and diffuse areas of inflammation, in contrast to controls, and appeared grossly cystic (Figure 9, B, D, and E). Analysis of plasma from a het/null mouse at 15 months showed an elevated BUN and creatinine of 97.3 mg/dl and 0.4 mg/dl, respectively.
Figure 9.
Analysis of adult Dlg1/Cask het/null kidneys. (A–D) Hematoxylin-eosin staining at 90 days and 15 months shows the presence of normal glomeruli in control kidneys (A and B) and glomerulocystic structures (arrows) in Dlg1/Cask het/null (Dlg1+/fl;Caskfl/fl;Six2-EGFP/Cre) kidneys (C and D). At 15 months, multiple tubules were dilated and fibrosis was evident (D), and the kidneys appeared grossly cystic (E). Scale bars in A–D, 100 µm.
Discussion
Our findings demonstrate a novel genetic interaction between Dlg1 and Cask in the developing kidney. Mice deficient in both Dlg1 and Cask were born with severely hypoplastic and dysplastic kidneys, and this phenotype could be recapitulated by combined Dlg1 and Cask deletion solely in nephron progenitors. This indicates cell autonomous roles for Dlg1 and Cask in these stem-like cells. The rapid loss of nephron progenitors in DKOs suggests a crucial role for scaffolding proteins in maintaining this stem cell population.
Previous reports of DLG1-deficient mice described the presence of occasional renal hypoplasia.14–16 Although the small kidney size was speculated to be a result of defective cell proliferation,15 the mechanism for the renal hypoplasia has been unclear. Our studies here show that proliferation in the MM was markedly reduced when both DLG1 and CASK were deleted, and that apoptosis was also increased in the nephrogenic zone. A tumor suppressor function for DLG1 has been demonstrated in several studies in which reduced DLG1 levels were observed in human colon, breast, and cervical cancers.38 On the other hand, DLG1 functions in an opposite manner in a different cellular context by promoting oncogenesis via interaction with the adenovirus type 9 E4-ORF1 oncogene in mouse embryo fibroblasts to activate Ras-mediated PI3K signaling.39 Thus, DLG1, depending on its protein binding partners, can promote proliferation in certain cell types. Moreover, loss-of-function mutations in CASK have been associated with microcephaly and cerebellar hypoplasia in humans.40 Consistent with these concepts, our results suggest that Dlg1 and Cask cooperatively maintain the proliferating nephron progenitor population to ensure nephron endowment. In investigating the mechanism by which combined Dlg1 and Cask deletion led to depletion of nephron progenitors, we found that Fgf8 expression in the DKO MM was significantly reduced. Similarly, MM deletion of Fgf8 by Pax3-Cre causes increased cell death in the nephrogenic zone and progressive loss of nephron progenitors.23 In direct support of the involvement of FGF signaling in the DKO phenotype, the levels of several components of the Ras pathway were reduced in the DKOs, including ERK, p-ERK, c-Raf, p-c-Raf, JNK, p-JNK, and pp38 MAPK by Western blotting and p-ERK and pp38 MAPK by immunostaining. The reduced levels of total ERK, c-Raf, and JNK may be due to autoregulatory loops in which reduced activation of these proteins leads to their reduced expression.
Numerous in vivo and in vitro studies have established essential roles for FGF signaling in kidney development. Deletion of both Fgfr1 and Fgfr2 in the MM results in increased apoptosis and decreased proliferation of mutant mesenchyme dorsal to the UB at E10.5, leading to renal agenesis.9 In addition, FGF2, a ligand for FGF receptor 1 and 2, prevents apoptosis of the MM and promotes its condensation.35,41,42 Most recently, FGF-9 and -20 have been shown to maintain the stemness of nephron progenitors, and absence of both these factors in mice results in renal agenesis.43 Of note, FGF and epidermal growth factor signaling through the Ras and PI3K pathways, but not the ERK pathway, is required for survival and renewal of nephron progenitors.8 Although ERK signaling was reduced in our DKOs (Figures 6 and 7), this may not be the main signaling pathway responsible for the DKO phenotype. Another explanation for the difference regarding ERK may be that the previously mentioned study was performed on cultures of primary nephron progenitors, whereas our studies were performed in vivo. We did find that another signaling pathway downstream of Ras, the p38 MAPK pathway, was reduced in the DKOs; the relevance of this is suggested by the fact that FGF2-induced proliferation has been shown to require the activation of p38 MAPK in fibroblasts.44
We also observed slightly reduced expression of Bmp7, which is required for maintenance of nephron progenitors, and of Gdnf, which is secreted from the MM and promotes UB budding and branching. The defective UB branching that we observed is therefore probably secondary to reduced GDNF secretion from the MM. The slight reduction in Bmp7 expression may synergize with the reduced Fgf8 expression to further dampen the Ras signaling pathway; indeed, BMP7 has been shown to promote nephron progenitor proliferation through the JNK pathway, which is downstream of Ras.5
The potential involvement of CASK and DLG1 in the FGF signaling pathway can be explained by the direct interactions between CASK and syndecan and between DLG1 and MEK2.45–48 Syndecans function as co-receptors for fibroblast growth factors (FGFs).47,49 They appear to modulate the activation of FGF receptors by FGFs, and their expression is highly regulated and cell-type specific.50 In vitro studies have demonstrated a specific interaction between the PDZ domain of CASK and the C-terminal tail of syndecan-2.45,47 In mammalian brain, syndecan-2 has been shown to co-localize with CASK at synapses.47 More recently, human DLG1 was found to interact directly with MEK2, a component of the ERK pathway.46,48 Altogether, these findings support the possibility that DLG1 and CASK may regulate Ras signaling. Further studies will be needed to clarify this potentially important mechanism for maintaining nephron progenitors.
Another mechanism by which DLG1 and CASK may affect kidney development is by mediating migration of nephron progenitor cells toward the UB. Dlg1;Cask het/null kidneys, which were small but functional, showed a defect in tight aggregation of nephron progenitor cells around the UB tips. Recent studies have demonstrated an important role for DLG1 in cell migration.51,52 Scratch-induced migration assays using monolayer Schwann cell cultures demonstrated that the Dlg1-silenced cells failed to migrate and also exhibited reduced expression of the polarity protein Par3, which regulates directional cell migration.51 Another study revealed that DLG1 was absent in the basal plasma membrane of nonmigrating astrocytes, whereas it was present at the front border of migrating astrocytes.52 Furthermore, DLG1 interacts with the tumor suppressor protein adenomatous polyposis coli to polarize the microtubule cytoskeleton, a process required for migration.52
A recent study reported renal/urologic anomalies, including fusion of kidneys, unilateral kidney ectasia, and malrotated kidney, in patients with CASK loss-of-function mutations.53 Although these defects did not include our DKO phenotype of renal hypoplasia, they do suggest that scaffolding proteins may play an important role in congenital anomalies of the kidney and urinary tract (CAKUT). Because deletion of Cask alone in mouse nephron progenitors did not result in a kidney phenotype, we speculate that the renal anomaly observed in humans is the result of a combined effect with a variation in a protein that interacts with CASK, such as DLG1, Scribble, or the Lethal Giant Larvae homolog. Given these findings, a potential role for DLG1 and CASK variants in human CAKUT merits further investigation.
Concise Methods
Genetically Altered Mice
All animal studies were approved by the Washington University Animal Studies Committee. Genetically altered mice have been previously described, as follows, with Mouse Genome Informatics nomenclature in parentheses: Dlg1− (null; Dlg1tm1Jhm) and Dlg1fl (conditional; Dlg1tm2.1Jhm),14,54 generated at Washington University; Caskfl (conditional; Casktm1Sud),55 from The Jackson Laboratory; Pax3-Cre transgene (Tg(Pax3-cre)1),21,56 from Dr. Jonathan Epstein (University of Pennsylvania, Philadelphia, PA); Hoxb7-Cre-EGFP transgene (Tg[Hoxb7-cre]5526Cmb)57 from Dr. Carlton Bates (University of Pittsburgh, Pittsburgh, PA, USA); Six2-EGFP-Cre transgene (Tg[Six2-EGFP/cre]1Amc)58 from Dr. Andrew McMahon (University of Southern California, Los Angeles, CA); and Sox2-Cre transgene (Tg[Sox2-cre]1Amc)59 from The Jackson Laboratory. Cask is X-linked; Caskfl/fl and Cask−/− refer to homozygous females or hemizygous males. The germline Cask null allele was generated by mating a Caskfl mouse to a Sox2-Cre mouse. Western blot analysis has shown that DLG1 is absent from Dlg1−/− mice and significantly reduced in Dlg1+/− mice.14
Histology and Antibodies
Immunostaining on frozen or paraffin sections were performed as described.14 Antibodies used were as follows: rabbit anti-SIX2 (Proteintech, Chicago, IL), rat anti–keratin 8 (K8; TROMA-1, Developmental Studies Hybridoma Bank), mouse anti- WT1 (Thermo Scientific Pierce, Rockford, IL), rabbit anti-CITED1 (Thermo Scientific Pierce), anti–p-ERK, anti-pp38 MAPK, anti–p-c-Raf, anti–p-JNK (rabbit, Cell Signaling, Danvers, MA), rabbit anti-CASK (Invitrogen, Carlsbad, CA), mouse anti-DLG1 (BD Biosciences, San Jose, CA), rabbit anti-Ki67 (Novocastra, Buffalo Grove, IL), mouse anti–β-catenin (BD Biosciences), and FITC- and Cy3-conjugated secondary antibodies (Molecular Probes, Eugene, OR). Hoechst 33342 (Sigma) was used to label nuclei. For whole organ staining, embryos were fixed overnight in 4% paraformaldehyde at 4°C and washed in PBS containing 0.3% Triton X-100 three times, 20 minutes each, at room temperature. The kidneys were then dissected and blocked in 5% normal goat serum with PBS containing 0.3% Triton X-100 overnight at 4°C, incubated overnight in primary antibody at 4°C, and washed three times with PBS-Triton X-100 0.3% for 20 minutes each. They were then incubated overnight in secondary antibody at 4°C and washed three times with PBS-Triton X-100 0.3% for 20 minutes each and visualized with confocal microscopy (Nikon C-1 confocal system). TUNEL analysis was performed with the In Situ Cell Death Detection Kit, Fluorescein (Ver16.0, Roche, Mannheim, Germany). Light microscopy was performed as described.60
In situ Hybridization
In situ hybridization was performed on 10-µM frozen sections as described.14 Gdnf, Fgf8, Bmp7, Fgf10, and Wnt4 plasmids were gifts from Drs. Masato Hoshi (Washington University School of Medicine), Gail Martin (University of California, San Francisco), Jordan Kreidberg (Children’s Hospital Boston), Bridgid Hogan (Duke University), and Andrew McMahon (University of Southern California), respectively.
Organ Culture
E11.5 kidneys were dissected in ice-cold PBS and placed on 0.4-µM transwell filters. The kidneys were cultured 5 days at 37°C, 5% CO2 in serum-free defined media.61 Staining was performed in the same manner as the whole organ immunostaining described above.
Western Blot Analysis
E12.5 kidneys were harvested in radio-immunoprecipitation assay cell lysis buffer (50 mM Tris-HCl at pH 8.0, 100 mM NaCl, 1% NP-40, and 5 mM EDTA at pH 8.0), containing phosphatase and protease inhibitors (Roche, Mannheim, Germany). Protein concentration was measured by the BCA protein assay kit (Thermo Scientific Pierce, Rockford, IL). Total protein (25 μg) was separated by 10% SDS-PAGE and transferred to a polyvinylidene fluoride membrane (Millipore Corp., Bedford, MA). Western blot analyses were performed with the aforementioned antibodies and with anti-ERK, anti-AKT, anti-pAKT, anti–β-Actin, anti-p38 MAPK, anti–c-Raf, and anti-JNK (Cell Signaling). The signals were detected with an enhanced chemiluminescence kit (Amersham Pharmacia, Buckinghamshire, United Kingdom).
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis
Total RNA was isolated from E12.5 kidneys by RNeasy Micro Kit (Qiagen, Valencia, CA). cDNA was synthesized from 1 µg RNA with SuperScript3 Reverse transcription (Invitrogen, Carlsbad, CA). Quantitative real-time PCR amplification was performed using the fast mode (annealing and extending at 60°C) of the 7900HT Fast Real-Time PCR System (Applied Biosystems, Forrest City, CA). 20 μl reaction mixtures contained 10 nM primers, 1 μl cDNA, and 10 μl Fast SYBR Green Master Mix (Applied Biosystems). The primer sequences used were: GDNF-F: 5′- TCTCCTTCGTGCCCTCAGAA -3′; GDNF-R: 5′- CCTATGAGAATGCTGCCGAAA -3′ (NM_010275); BMP7-F: 5′- GATGAGCGCCCTTTCCTTCT -3′; BMP7-R: 5′- AGGCGTTTGCTGTGGTAGCT- 3′ (NM_007557); FGF8-F: 5′-TCTCCAGCACGATCTCTGTGAA-3′; FGF8-R: 5′-GGAAGCTAATTGCCAAGAGCAA-3′.23 Primers were designed by Primer Express Software Version 3.0 (Applied Biosystems). The mRNA transcript levels were measured and normalized to glyceraldehyde-3-phosphate dehydrogenase.
RNA Isolation and Microarray Analysis
Total RNA was isolated as described above and submitted to the WU Genome Technology Access Center for microarray hybridization. Briefly, RNA quality was assessed by Agilent 2100 Bioanalyzer (Agilent, Palo Alto, CA). The RNA was then amplified and labeled with biotin by T7 linear amplification (reverse transcription, followed by in vitro transcription) using the MessageAmp TotalPrep amplification kit (Life Technologies, Carlsbad, CA). The labeled RNA was then hybridized to Illumina Mouse6v2 Expression BeadChips (Illumina Inc., San Diego, CA). These were then washed and stained with streptavidin-Cy3 per the standard Illumina protocol and scanned on an Illumina BeadArray Reader (n=4 per group). Statistical analysis of microarray data was performed using the Partek Genomics Suite, version 6.6 (Partek Inc., St. Louis, MO).
Statistical Analyses
The statistical significance of differences between control and DKO samples were determined by the unpaired t test, and differences were considered significant when P<0.05.
Supplementary Material
Acknowledgments
We thank Jennifer Richardson for mouse genotyping; Gloriosa Go for technical assistance; the WU Mouse Genetics Core for care of mice; the Pulmonary Morphology Core for histology; Drs. Masato Hoshi and Sung-Ho Huh for valuable discussions; and Drs. Bates, Epstein, and McMahon for providing the Cre mice that made this study possible.
This work was supported by National Institutes of Health grants R01DK081156 (J.H.M. and W.S.) and R01DK078314 (J.H.M.) and in part by the Children’s Discovery Institute. Microscopy and Mouse Genetics Core services were supported by the WU O’Brien Center for Kidney Disease Research (National Institutes of Health (NIH) P30DK079333). We thank the WU Genome Technology Access Center for help with microarray analysis. The Center is partially supported by Cancer Center Support Grant P30CA091842 and ICTS/CTSA Grant UL1RR024992 from the NIH.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012111074/-/DCSupplemental.
References
- 1.Saxén L, Sariola H: Early organogenesis of the kidney. Pediatr Nephrol 1: 385–392, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Dressler GR: The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Vainio S, Lin Y: Coordinating early kidney development: Lessons from gene targeting. Nat Rev Genet 3: 533–543, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Self M, Lagutin OV, Bowling B, Hendrix J, Cai Y, Dressler GR, Oliver G: Six2 is required for suppression of nephrogenesis and progenitor renewal in the developing kidney. EMBO J 25: 5214–5228, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blank U, Brown A, Adams DC, Karolak MJ, Oxburgh L: BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development 136: 3557–3566, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley AT, Lyons KM, Robertson EJ: A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9: 2795–2807, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Bates CM: Role of fibroblast growth factor receptor signaling in kidney development. Pediatr Nephrol 22: 343–349, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Brown AC, Adams D, de Caestecker M, Yang X, Friesel R, Oxburgh L: FGF/EGF signaling regulates the renewal of early nephron progenitors during embryonic development. Development 138: 5099–5112, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poladia DP, Kish K, Kutay B, Hains D, Kegg H, Zhao H, Bates CM: Role of fibroblast growth factor receptors 1 and 2 in the metanephric mesenchyme. Dev Biol 291: 325–339, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Boyle SC, Kim M, Valerius MT, McMahon AP, Kopan R: Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development 138: 4245–4254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods DF, Hough C, Peel D, Callaini G, Bryant PJ: Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol 134: 1469–1482, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim E, Sheng M: PDZ domain proteins of synapses. Nat Rev Neurosci 5: 771–781, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Caruana G, Bernstein A: Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol 21: 1475–1483, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahoney ZX, Sammut B, Xavier RJ, Cunningham J, Go G, Brim KL, Stappenbeck TS, Miner JH, Swat W: Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proc Natl Acad Sci U S A 103: 19872–19877, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iizuka-Kogo A, Ishidao T, Akiyama T, Senda T: Abnormal development of urogenital organs in Dlgh1-deficient mice. Development 134: 1799–1807, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Naim E, Bernstein A, Bertram JF, Caruana G: Mutagenesis of the epithelial polarity gene, discs large 1, perturbs nephrogenesis in the developing mouse kidney. Kidney Int 68: 955–965, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Fanning AS, Anderson JM: Protein modules as organizers of membrane structure. Curr Opin Cell Biol 11: 432–439, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Caruana G: Genetic studies define MAGUK proteins as regulators of epithelial cell polarity. Int J Dev Biol 46: 511–518, 2002 [PubMed] [Google Scholar]
- 19.Lee S, Fan S, Makarova O, Straight S, Margolis B: A novel and conserved protein-protein interaction domain of mammalian Lin-2/CASK binds and recruits SAP97 to the lateral surface of epithelia. Mol Cell Biol 22: 1778–1791, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard MA, Elias GM, Elias LA, Swat W, Nicoll RA: The role of SAP97 in synaptic glutamate receptor dynamics. Proc Natl Acad Sci U S A 107: 3805–3810, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Chen F, Epstein JA: Neural crest expression of Cre recombinase directed by the proximal Pax3 promoter in transgenic mice. Genesis 26: 162–164, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Chang CP, McDill BW, Neilson JR, Joist HE, Epstein JA, Crabtree GR, Chen F: Calcineurin is required in urinary tract mesenchyme for the development of the pyeloureteral peristaltic machinery. J Clin Invest 113: 1051–1058, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grieshammer U, Cebrián C, Ilagan R, Meyers E, Herzlinger D, Martin GR: FGF8 is required for cell survival at distinct stages of nephrogenesis and for regulation of gene expression in nascent nephrons. Development 132: 3847–3857, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Costantini F, Kopan R: Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18: 698–712, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyle S, Shioda T, Perantoni AO, de Caestecker M: Cited1 and Cited2 are differentially expressed in the developing kidney but are not required for nephrogenesis. Dev Dyn 236: 2321–2330, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Fujii T, Pichel JG, Taira M, Toyama R, Dawid IB, Westphal H: Expression patterns of the murine LIM class homeobox gene lim1 in the developing brain and excretory system. Dev Dyn 199: 73–83, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Karavanov AA, Karavanova I, Perantoni A, Dawid IB: Expression pattern of the rat Lim-1 homeobox gene suggests a dual role during kidney development. Int J Dev Biol 42: 61–66, 1998 [PubMed] [Google Scholar]
- 28.Lan Y, Kingsley PD, Cho ES, Jiang R: Osr2, a new mouse gene related to Drosophila odd-skipped, exhibits dynamic expression patterns during craniofacial, limb, and kidney development. Mech Dev 107: 175–179, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Dorey K, Amaya E: FGF signalling: Diverse roles during early vertebrate embryogenesis. Development 137: 3731–3742, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Giovanni V, Alday A, Chi L, Mishina Y, Rosenblum ND: Alk3 controls nephron number and androgen production via lineage-specific effects in intermediate mesoderm. Development 138: 2717–2727, 2011 [DOI] [PubMed] [Google Scholar]
- 31.Hoshi M, Batourina E, Mendelsohn C, Jain S: Novel mechanisms of early upper and lower urinary tract patterning regulated by RetY1015 docking tyrosine in mice. Development 139: 2405–2415, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oxburgh L, Brown AC, Fetting J, Hill B: BMP signaling in the nephron progenitor niche. Pediatr Nephrol 26: 1491–1497, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costantini F, Shakya R: GDNF/Ret signaling and the development of the kidney. Bioessays 28: 117–127, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Michos O, Cebrian C, Hyink D, Grieshammer U, Williams L, D’Agati V, Licht JD, Martin GR, Costantini F: Kidney development in the absence of Gdnf and Spry1 requires Fgf10. PLoS Genet 6: e1000809, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dudley AT, Godin RE, Robertson EJ: Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13: 1601–1613, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu C, Goswami M, Talley J, Chesser-Martinez PL, Lou CH, Sater AK: TAK1 promotes BMP4/Smad1 signaling via inhibition of erk MAPK: A new link in the FGF/BMP regulatory network. Differentiation 83: 210–219, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Park JS, Ma W, O’Brien LL, Chung E, Guo JJ, Cheng JG, Valerius MT, McMahon JA, Wong WH, McMahon AP: Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell 23: 637–651, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts S, Delury C, Marsh E: The PDZ protein discs-large (DLG): the ‘Jekyll and Hyde’ of the epithelial polarity proteins. FEBS J 279: 3549–3558, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Frese KK, Latorre IJ, Chung SH, Caruana G, Bernstein A, Jones SN, Donehower LA, Justice MJ, Garner CC, Javier RT: Oncogenic function for the Dlg1 mammalian homolog of the Drosophila discs-large tumor suppressor. EMBO J 25: 1406–1417, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Najm J, Horn D, Wimplinger I, Golden JA, Chizhikov VV, Sudi J, Christian SL, Ullmann R, Kuechler A, Haas CA, Flubacher A, Charnas LR, Uyanik G, Frank U, Klopocki E, Dobyns WB, Kutsche K: Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat Genet 40: 1065–1067, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D: Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol 273: F757–F767, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Perantoni AO, Dove LF, Karavanova I: Basic fibroblast growth factor can mediate the early inductive events in renal development. Proc Natl Acad Sci U S A 92: 4696–4700, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschké P, Salomon R, Antignac C, Ornitz DM, Kopan R: FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell 22: 1191–1207, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maher P: p38 mitogen-activated protein kinase activation is required for fibroblast growth factor-2-stimulated cell proliferation but not differentiation. J Biol Chem 274: 17491–17498, 1999 [DOI] [PubMed] [Google Scholar]
- 45.Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM: Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol 142: 129–138, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gaudet S, Langlois MJ, Lue RA, Rivard N, Viel A: The MEK2-binding tumor suppressor hDlg is recruited by E-cadherin to the midbody ring. BMC Cell Biol 12: 55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M: Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol 142: 139–151, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maïga O, Philippe M, Kotelevets L, Chastre E, Benadda S, Pidard D, Vranckx R, Walch L: Identification of mitogen-activated protein/extracellular signal-responsive kinase kinase 2 as a novel partner of the scaffolding protein human homolog of disc-large. FEBS J 278: 2655–2665, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Masola V, Gambaro G, Tibaldi E, Brunati AM, Gastaldello A, D’Angelo A, Onisto M, Lupo A: Heparanase and syndecan-1 interplay orchestrates fibroblast growth factor-2-induced epithelial-mesenchymal transition in renal tubular cells. J Biol Chem 287: 1478–1488, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carey DJ: Syndecans: multifunctional cell-surface co-receptors. Biochem J 327: 1–16, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cotter L, Ozçelik M, Jacob C, Pereira JA, Locher V, Baumann R, Relvas JB, Suter U, Tricaud N: Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science 328: 1415–1418, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A: Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol 170: 895–901, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moog U, Kutsche K, Kortüm F, Chilian B, Bierhals T, Apeshiotis N, Balg S, Chassaing N, Coubes C, Das S, Engels H, Van Esch H, Grasshoff U, Heise M, Isidor B, Jarvis J, Koehler U, Martin T, Oehl-Jaschkowitz B, Ortibus E, Pilz DT, Prabhakar P, Rappold G, Rau I, Rettenberger G, Schlüter G, Scott RH, Shoukier M, Wohlleber E, Zirn B, Dobyns WB, Uyanik G: Phenotypic spectrum associated with CASK loss-of-function mutations. J Med Genet 48: 741–751, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Stephenson LM, Sammut B, Graham DB, Chan-Wang J, Brim KL, Huett AS, Miletic AV, Kloeppel T, Landry A, Xavier R, Swat W: DLGH1 is a negative regulator of T-lymphocyte proliferation. Mol Cell Biol 27: 7574–7581, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M, Kavalali ET, Südhof TC: Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A 104: 2525–2530, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jarad G, Miner JH: The Pax3-Cre transgene exhibits a rostrocaudal gradient of expression in the skeletal muscle lineage. Genesis 47: 1–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao H, Kegg H, Grady S, Truong HT, Robinson ML, Baum M, Bates CM: Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev Biol 276: 403–415, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP: Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hayashi S, Lewis P, Pevny L, McMahon AP: Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev 119[Suppl 1]: S97–S101, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Jarad G, Cunningham J, Shaw AS, Miner JH: Proteinuria precedes podocyte abnormalities inLamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rogers SA, Ryan G, Hammerman MR: Insulin-like growth factors I and II are produced in the metanephros and are required for growth and development in vitro. J Cell Biol 113: 1447–1453, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.