Abstract
A major hallmark of chronic kidney injury is fibrosis, which is characterized by increased accumulation of extracellular matrix components that replace the damaged tissue. Normally, the synthesis and degradation of extracellular matrix components are finely regulated; however, when matrix replacement goes unchecked, there is unwanted and irreversible tissue scarring with consequent organ damage, organ failure, and, in certain cases, death. Many factors, including cell-matrix interactions, play a role in the development of renal fibrosis. Cell-matrix interactions are made possible by integrins, a family of transmembrane receptors that, upon binding to the extracellular matrix, activate intracellular signaling. Thus, they control various cell functions, including survival, proliferation, migration, and matrix homeostasis. Genetic mutations in humans and the development of animal models lacking integrins in selective parts of the kidney have improved our understanding of molecular mechanisms and pathways controlling matrix remodeling in kidney disease. Here we outline the major integrins involved in kidney disease and some of the major molecular mechanisms whereby integrins contribute to kidney fibrosis.
The kidneys are essential regulatory organs that play a critical role in the excretion of waste, fluids, electrolyte and acid-base homeostasis, BP control, and the production of various hormones. The adult human kidney consists of approximately 1 million filtering nephrons and a collecting system. Each nephron consists of a glomerulus, followed by a tubule that is divided into distinct segments. Nephrons are derived from the metanephric mesenchyme, and the collecting system derives from the ureteric bud. The glomerulus consists of a capillary network maintained in an open three-dimensional space by the mesangium and a glomerular filtration barrier composed of an inner fenestrated endothelial cell layer; an outer layer of visceral epithelial cells (podocytes); and the intervening glomerular basement membrane (GBM), composed of extracellular matrix (ECM) components. The Bowman capsule, which is also the beginning of the proximal tubule, surrounds the glomerulus. Cells that are both anatomically and functionally distinct form different segments of the renal tubule, from the proximal tubule to the distal collecting duct.
As with all organs, the interaction between cells and the surrounding ECM is required for normal kidney development and function. The principal cellular receptors that mediate cell-ECM interactions are integrins, heterodimeric transmembrane glycoproteins that consist of non–covalently associated α and β subunits. There are 18α and 8β subunits in mammals, which form 24 unique heterodimers with distinct specificities for different ECM components.1 Integrins are classified as collagen, laminin, and arginine-glycine-aspartic acid (RGD)–binding receptors. Each integrin subunit has a large extracellular domain that constitutes the ligand-binding domain, a single transmembrane domain, and a short cytoplasmic tail. A principal function of integrins is to anchor cells to the ECM, but they also signal bi-directionally across the plasma membrane. Thus, they control various cell functions, including cell proliferation, survival and migration, differentiation, and matrix homeostasis. Because the cytoplasmic tails of integrins lack enzymatic and actin-binding activity, they need to bind adaptor proteins for intracellular signal propagation.
Integrins play a critical role in kidney development, homeostasis, and renal disease. Because the role of integrins in kidney development was recently reviewed,2 we focus on their role in kidney disease. In particular, we highlight the contribution of various integrins in kidney disease based on their ligand specificity.
The Laminin Receptors
The principal laminin-binding receptors found in the kidney are integrins α3β1, α6β1, and α6β4. Integrin α3β1 is the most highly expressed integrin in the kidney and is found in both the glomerulus and the tubules. A definitive role for this integrin in normal kidney function comes from the finding that global integrin α3–null mice have abnormalities in both the collecting system and the glomerulus.3 Subsequent selective deletion of the integrin α3 subunit in the podocytes results in a severe developmental phenotype characterized by foot process effacement and severe proteinuria.4 Interestingly, this phenotype is similar to but slightly less severe than that seen when the integrin β1 subunit is selectively deleted in the same cell type,5,6 suggesting that integrin α3β1 is the major integrin involved in podocyte stability and glomerular development. By contrast, deletion of the α3 subunit exclusively in the developing collecting duct resulted in only a mild developmental phenotype,7 and constitutive deletion of the integrin α6 subunit does not cause any developmental renal phenotype.8 Thus, the role of specific laminin receptors in collecting system development is far less important than that seen in the glomerulus, and there may be redundancy with respect to their functions in this situation.
The importance of integrin α3β1 in glomerular homeostasis is emphasized by two recent studies showing that mutations of the integrin α3 subunit in humans is associated with severe renal abnormalities and premature death.9,10 Children carrying deletion or missense homozygote mutations have kidneys with atrophic glomeruli, FSGS, diffuse interstitial fibrosis, tubular atrophy, proteinuria, and loss and immaturity of the tubules. Interestingly, a A349S missense mutation results in no surface expression of integrin α3β1 because of the inability of the α3 subunit to heterodimerize with the β1 subunit.9 These children constitute the first demonstration that abnormalities in integrin structure or function lead to kidney disease in humans.
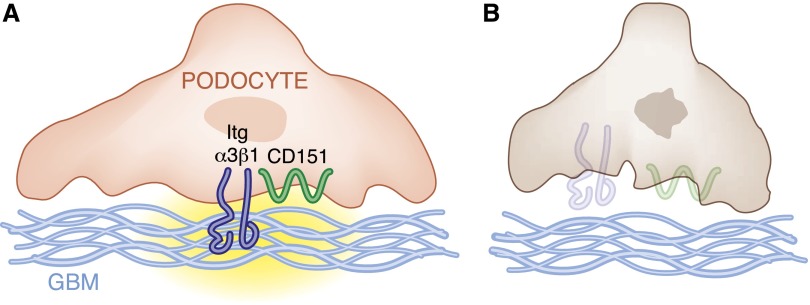
Although mutations in the integrin α3 subunit that cause human kidney disease were described only recently, patients with nonsense mutations in the tetraspanin protein CD151 (which binds to integrin α3β1 and regulates its function) were previously found to have glomerulosclerosis.11 The mechanisms for these glomerular abnormalities are well defined using laminin-binding integrin and CD151-null mice.4,12 CD151-null mice present with FSGS, similar to that observed in humans carrying nonsense mutations in CD151. The degree of glomerulosclerosis in these mice is similar to that of mice lacking the integrin α3 subunit in podocytes and is related to podocyte loss. Mechanistically, CD151 localizes to the podocyte-GBM interface, where it interacts with integrin α3β1. CD151 shifts integrin α3β1 from focal adhesions into tetraspanin webs, allowing for tight adhesion of podocytes to the GBM. In the absence of CD151, this shift does not occur, resulting in a weak adhesion of podocytes to the GBM and consequent podocyte detachment and loss. Thus, it is likely that mutations in the integrin α3 subunit or CD151 result in glomerulosclerosis in humans, primarily by leading to increased podocyte loss because integrin α3β1 is the principal integrin on the podocyte and CD151 plays a critical role in facilitating firm adhesion of podocytes to the GBM (Figure 1).
Figure 1.
Integrin α3β1 is required for podocyte adhesion to the glomerular basement membrane. (A) CD151/integrin α3β1 interactions play an important role in determining strong adhesion of podocytes to the GBM, thus protecting the glomerulus from stress-mediated injury. (B) Loss of CD151 or loss and/or mutations of the integrin α3 subunit leads to podocyte detachment and loss and consequent glomerular damage.
Because integrin α3β1 is a major laminin receptor, it is not surprising that patients carrying integrin α3 subunit mutations have many features of patients with Pierson syndrome. This autosomal recessive disease is caused by mutations in LAMB2, which encodes the laminin β2 chain, the principal laminin subunit in the GBM.13–15 Patients with Pierson syndrome and mice lacking laminin β216,17 present with glomerular injury characterized by diffuse mesangial sclerosis and nephrotic syndrome. Taken together, the diseases associated with mutations in integrin α3β1, CD151, and the laminin β2 suggest a key role for laminins and their principal receptors in normal glomerular function.
Laminins and their receptors are also highly expressed in the kidney tubules; however, their role in tubulointerstitial disease is poorly studied. On the basis of the observation that patients with integrin α3 mutations have generalized renal fibrosis, including the tubulointerstitum,9,10 this receptor probably plays a critical role in regulating tubulointerstitial fibrosis as well. This possibility can be investigated by selectively deleting the integrin α3 subunit in specific nephron segments. It is also highly likely that α6-containing integrins are associated with fibrotic kidney disease because integrin α6β4 plays a key role in regulating tight adhesion of cells to basement membranes. In this regard, a patient presenting with FSGS and a mutation in the integrin β4 subunit has been described.18 Studies in mice null for the integrin α6 and/or β4 subunit in the kidney need to be conducted to define the contribution of integrins α6β1 and α6β4 in kidney repair after injury.
The Collagen Receptors
The two major collagen receptors, integrins α1β1 and α2β1, are widely expressed in the kidney as well as in leukocytes, which play a role in inflammatory forms of renal injury. Integrin α1β1 primarily binds collagen type IV, a major constituent of basement membranes, whereas integrin α2β1 binds collagen type I, whose expression is upregulated in renal disease.19 Neither of these integrins affects renal development because integrin α1- and α2-null mice do not show any obvious kidney phenotypes. No human diseases are directly attributed to mutations in these integrins; however, a large body of evidence suggests that their expression is altered in the course of human kidney disease and that they modulate renal fibrosis in rodents.20
The first evidence that collagen-binding receptors regulate renal fibrosis comes from studies demonstrating that anti–integrin α1 antibodies reduce scarring in rat models of glomerular injury by inhibiting leukocyte function and the immune response.21 It was subsequently shown that integrin α1–null mice have decreased glomerular fibrosis in a mouse model of Alport syndrome.22 In contrast to these studies, there is overwhelming evidence that integrin α1–null mice have an exacerbation of glomerulosclerosis in response to multiple forms of glomerular injury, including models of diabetic nephropathy and adriamycin-induced glomerular disease.23,24 The more severe glomerular injury in the integrin α1–null mice is characterized by increased mesangial accumulation of collagen type IV and increased production of profibrotic reactive oxygen species. In vitro studies demonstrate that integrin α1β1 negatively regulates collagen synthesis by multiple mechanisms. In this regard, this receptor controls collagen synthesis at the transcriptional level.25 In addition, integrin α1β1 negatively regulates the production of reactive oxygen species by downregulating epidermal growth factor receptor activation.26 Epidermal growth factor receptor is inactivated by integrin α1β1–mediated activation of the T cell protein tyrosine phosphatase as well as regulation of caveolin-1 levels and phosphorylation.27,28 In addition, a study recently demonstrated that the affinity of integrin α1β1 to collagen type IV regulates integrin-mediated intracellular signaling and ultimately collagen type IV production.29 In particular, the tighter the binding to collagen by the integrin, the less collagen is produced. Taken together, overwhelming evidence indicates that deletion or inhibition of integrin α1β1 exacerbates glomerulosclerosis, suggesting that maneuvers to increase ligand/integrin binding that promote integrin α1β1 activation are beneficial in the setting of renal injury.
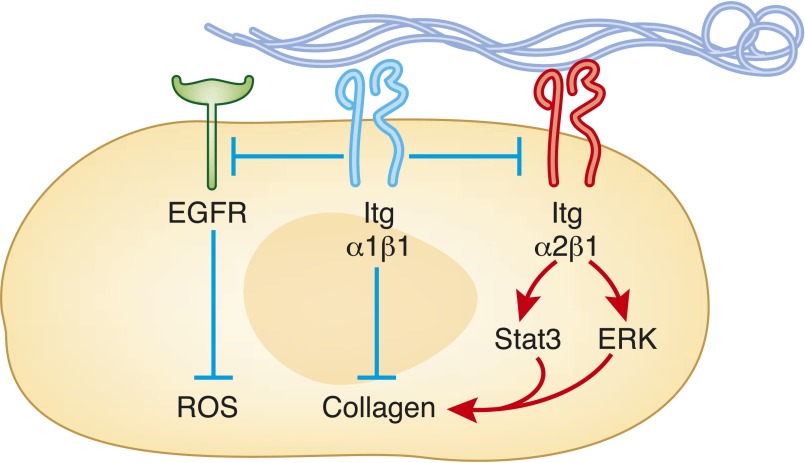
In contrast to integrin α1β1, integrin α2β1 is a positive regulator of collagen synthesis and reactive oxygen species production. This is consistent with recent evidence that genetic deletion of the integrin α2 subunit or selective chemical antagonism of integrin α2β1 protects mice from the development of glomerular fibrosis after adriamycin administration or partial renal ablation.30 A possible mechanism whereby integrin α2β1 promotes collagen synthesis and subsequent glomerular injury is by activation of Stat3 in mesangial cells. Another novel mechanism whereby integrin α2β1 has been shown to promote ERK-mediated collagen synthesis is its ability to act as a receptor for IgA1 in mesangial cells,31 suggesting that various pathways control integrin α2β1–mediated collagen production. Although these studies propose that integrin α2β1 induces glomerular fibrosis, integrin α2–null mice seem to develop mild proteinuria and glomerular damage at 6 months of age because of increased expression of the profibrotic TGF-β and connective tissue growth factor.32 This effect appears to be strain specific because mild glomerular disease is observed only in 6-month-old C57/Black632 but not BALB/C or 129Sv integrin α2–null mice (Pozzi, unpublished observation). Despite these contradictory results, it appears that targeting integrin α2β1 with specific small molecule inhibitors might be beneficial for the treatment of renal fibrosis. To determine whether and at what stage of renal injury this receptor should be targeted, it is important to define how integrin α2β1 controls collagen synthesis. In this regard, integrin α1β1 negatively regulates integrin α2β1–mediated signaling, suggesting that concomitant activation of integrin α1β1 and inhibition of integrin α2β1 might be the optimal manner in which to modulate signaling by these very important regulators of collagen synthesis (Figure 2).
Figure 2.
Collagen binding integrins exert opposing effects in the regulation of glomerular homeostasis. In mesangial cells, integrin α2β1 binding to collagen leads to activation of intracellular signaling (i.e., ERK or Stat3) resulting in increased collagen production. In contrast, activation of integrin α1β1, directly or via negative regulation of integrin α2β1 or epidermal growth factor receptor signaling, leads to reduced production of profibrotic signaling. ROS, reactive oxygen species.
The RGD-Binding Integrins
Two β1 integrins (α5β1, α8β1), five αV integrins (αvβ1, αvβ3, αvβ5, αvβ6, and αvβ8), and αIIbβ3 (found only on platelets) bind to ligands containing an RGD-active site primarily found in fibronectin and vitronectin.
The fibronectin receptor integrin α5β1 does not play a major role in kidney disease; however, to the best of our knowledge, mice selectively lacking the integrin α5 subunit in the kidneys have not been generated. By contrast, constitutive deletion of the integrin α8 subunit leads to renal agenesis or dysgenesis due to loss of binding to nephronectin, a major integrin α8β1 ligand33–35 (reviewed in detail by Mathew et al.2). However, when crossed onto certain backgrounds, integrin α8–null mice develop kidneys, thus allowing us to determine the role of integrin α8β1 in renal disease. In this context, integrin α8–null mice present with abnormalities of the mesangium characterized by hypercellularity and altered glomerular capillaries, most likely due to impaired ability to protect the glomerulus from mechanical stress, including hypertension.36–38 In addition, there is evidence that a polymorphism within the promoter of the integrin α8 subunit regulates the progression of polycystic kidney disease.39 Altogether these findings suggest that mutations or loss of function of the α8 subunit could render humans more susceptible to stress-induced glomerular damage or polycystic kidney disease.
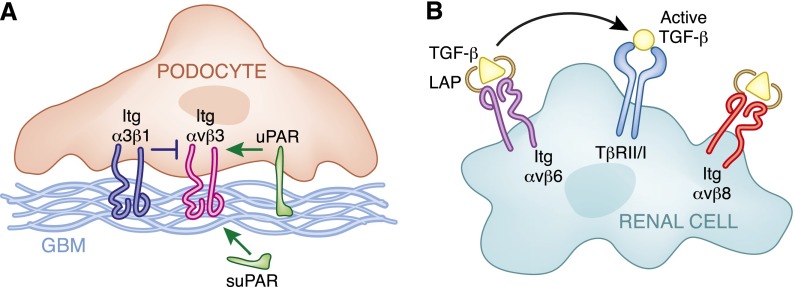
The αv subunit is ubiquitously expressed in the adult kidney; however, the αvβ heterodimers present in the different nephron segments are unclear. There are two distinct forms of αv integrins: those that primarily interact with the RGD present in fibronectin and vitronectin (αvβ1, αvβ3, and αvβ5) and those that bind to the RGD motif of the TGF-β–binding latency–associated peptide (LAP) (αvβ6 and αvβ8). Although the integrin αv subunit has never been selectively deleted in any cell types within the kidney, some indirect evidence suggests that integrin αvβ3 plays a role in regulating the glomerular filtration barrier (reviewed in detail by Reiser et al.40,41). In this context, activation of integrin αvβ3 by the urokinase plasminogen receptor (uPAR) promotes podocyte injury in a lipopolysaccharide-mediated renal injury model in mice.42 More recently, research showed that a soluble form of uPAR, namely suPAR, interacts with and activates integrin αvβ3 in podocytes, leading to FSGS in humans.43 Although these studies suggest a highly novel mechanism whereby integrin αvβ3 might contribute to FSGS, the conclusion that activation of this integrin in podocytes is deleterious was primarily made by using conformational antibodies able to detect activated integrin αvβ3 and by blocking integrin function with blocking antibodies or cyclic RGD. Thus, to conclusively determine the role of this integrin in podocyte homeostasis, it is important to define how the glomerulus responds to injury in the absence of integrin αvβ3. It is also critical to clarify how this integrin alters the response of the podocytes to the signals from uPAR. Although activation of small GTPases is a plausible explanation,42 the mechanism whereby this occurs has not been defined. The picture is also complicated by the fact that integrin αvβ3 function or affinity can be modulated by multiple factors, including integrin α3β1, which is the principal receptor whereby podocytes interact with the GBM.44 Thus, more mechanistic studies are required to investigate how integrin αvβ3 regulates the glomerular filtration barrier and whether it does in fact play a direct role in glomerular diseases in humans. Some of the factors involved in activation and inhibition of integrin αvβ3 in podocytes are illustrated in Figure 3A.
Figure 3.
αv integrins modulates glomerular ECM homeostasis by multiple mechanisms. (A) Schematic representations of mechanisms of activation (i.e., uPAR and suPAR) and inhibition (i.e., integrin α3β1) of integrin αvβ3 in podocytes that contribute to alterations in podocyte cell function. (B) In renal cells (i.e., tubular or mesangial cells), binding of the LAP/TGF-β complex to integrin αvβ6 contributes to release of active TGF-β and increased TGF-β receptor–mediated signaling. In contrast, binding of the LAP/TGF-β complex to integrin αvβ8 sequesters TGF-β, thereby decreasing TGF-β–mediated signaling.
Integrins αvβ6 and αvβ8 regulate the levels of free and active TGF-β in tissues by binding to the LAP/TGF-β complex. Although neither of these integrins has been shown to play a role in human disease, they alter kidney function in rodents. Integrin αvβ6 contributes to unilateral ureter obstruction–mediated renal fibrosis by facilitating TGF-β release and activation, and loss of the integrin β6 subunit protects mice from this form of injury.45 Similarly, integrin β6–null mice are protected from glomerulosclerosis in an Alports model.46 In contrast to the integrin β6–null mice, which have no developmental phenotype, the integrin β8–null mice demonstrate subtle renal abnormalities characterized by azotemia, albuminuria, and mild podocyte foot process effacement.47 The proposed mechanism for these findings is that in the glomerulus, integrin αvβ8 binds the LAP/TGF-β complex, keeping the TGF-β in an inactive state, and in the absence of integrin αvβ8 increased levels of bioactive TGF-β lead to glomerular damage. Thus, integrins αvβ6 and αvβ8 appear to have opposite effects in kidney injury, with αvβ6 increasing and αvβ8 decreasing the amount of active TGF-β (Figure 3B).
Conclusions
It is clear that multiple different integrins play a role in renal disease, and it is not surprising that there is no common theme as to how integrins regulate kidney disease— they are differentially expressed in different cell types in the kidney, bind to a diverse set of ligands, and regulate multiple different signaling pathways. It has only been in the last year that strong direct evidence has shown that mutations in integrins cause kidney disease in humans. Nevertheless, a large body of in vivo work in mice and in vitro studies in isolated kidney cells emphasizes the potential role of integrins in kidney disease in humans, in particular kidney fibrosis. Thus, several integrin-specific topics related to kidney disease should be explored further. Although it is clear that integrin α3β1 is vital for the normal function of the glomerular filtration barrier, the role of the other laminin-binding integrins, such as α6β1 and α6β4, in the injured glomerulus is unknown. In addition, the roles of the laminin receptors in tubulointerstitial disease are unclear.
There is overwhelming evidence in mouse models that the collagen-binding receptors integrins α1β1 and α2β1 regulate renal fibrosis. Thus, it would be helpful to determine whether mutations in these integrins are indeed associated with fibrosis in different human kidney diseases. In addition, further effort should be placed on targeting these collagen receptors to prevent fibrosis; modulating their function will probably not cause major adverse effects because they are not required for normal development. Similarly, targeting integrin αvβ6 with small molecule inhibitors might be an effective way of dampening TGF-β signaling, which has been implicated in numerous fibrotic kidney diseases. Finally, more work is needed to define how activation of integrin αvβ3 is required for the development of uPAR- and suPAR-dependent FSGS because this exciting and provocative finding has major implications for human disease.
Acknowledgments
This work was in part supported by the Veterans Affairs Merit Reviews 1I01BX002025-01 (A.P.) and 1I01BX002196-01 (R.Z.); the National Institutes of Health grants DK095761 (A.P.), >DK075594> (R.Z.), DK069221 (R.Z.), DK65123 (R.Z. and A.P.), and DK083187 (R.Z.); the O’Brien Center DK79341-01 (A.P., R.Z.); and an American Heart Association Established Investigator Award (R.Z.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Hynes RO: Integrins: Bidirectional, allosteric signaling machines. Cell 110: 673–687, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Mathew S, Chen X, Pozzi A, Zent R: Integrins in renal development. Pediatr Nephrol 27: 891–900, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R: Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122: 3537–3547, 1996 [DOI] [PubMed] [Google Scholar]
- 4.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A: Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175: 33–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R: Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanasaki K, Kanda Y, Palmsten K, Tanjore H, Lee SB, Lebleu VS, Gattone VH, Jr, Kalluri R: Integrin beta1-mediated matrix assembly and signaling are critical for the normal development and function of the kidney glomerulus. Dev Biol 313: 584–593, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Chattopadhyay N, Qin S, Szekeres C, Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH, Kreidberg JA: Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development 136: 843–853, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M: Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet 13: 370–373, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Nicolaou N, Margadant C, Kevelam SH, Lilien MR, Oosterveld MJ, Kreft M, van Eerde AM, Pfundt R, Terhal PA, van der Zwaag B, Nikkels PG, Sachs N, Goldschmeding R, Knoers NV, Renkema KY, Sonnenberg A: Gain of glycosylation in integrin α3 causes lung disease and nephrotic syndrome. J Clin Invest 122: 4375–4387, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Has C, Spartà G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, Waters A, He Y, Anikster Y, Esser P, Straub BK, Hausser I, Bockenhauer D, Dekel B, Hildebrandt F, Bruckner-Tuderman L, Laube GF: Integrin α3 mutations with kidney, lung, and skin disease. N Engl J Med 366: 1508–1514, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamatic Crew V, Burton N, Kagan A, Green CA, Levene C, Flinter F, Brady RL, Daniels G, Anstee DJ: CD151, the first member of the tetraspanin (TM4) superfamily detected on erythrocytes, is essential for the correct assembly of human basement membranes in kidney and skin. Blood 104: 2217–2223, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, Zuurbier CJ, Janssen H, Sonnenberg A: Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest 122: 348–358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Zenker M, Pierson M, Jonveaux P, Reis A: Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am J Med Genet A 138: 73–74, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Zenker M, Tralau T, Lennert T, Pitz S, Mark K, Madlon H, Dötsch J, Reis A, Müntefering H, Neumann LM: Congenital nephrosis, mesangial sclerosis, and distinct eye abnormalities with microcoria: an autosomal recessive syndrome. Am J Med Genet A 130A: 138–145, 2004 [DOI] [PubMed] [Google Scholar]
- 16.Jarad G, Cunningham J, Shaw AS, Miner JH: Proteinuria precedes podocyte abnormalities inLamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest 116: 2272–2279, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miner JH, Go G, Cunningham J, Patton BL, Jarad G: Transgenic isolation of skeletal muscle and kidney defects in laminin beta2 mutant mice: Implications for Pierson syndrome. Development 133: 967–975, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kambham N, Tanji N, Seigle RL, Markowitz GS, Pulkkinen L, Uitto J, D’Agati VD: Congenital focal segmental glomerulosclerosis associated with beta4 integrin mutation and epidermolysis bullosa. Am J Kidney Dis 36: 190–196, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Heino J: The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol 19: 319–323, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Borza CM, Pozzi A: The role of cell-extracellular matrix interactions in glomerular injury. Exp Cell Res 318: 1001–1010, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook HT, Khan SB, Allen A, Bhangal G, Smith J, Lobb RR, Pusey CD: Treatment with an antibody to VLA-1 integrin reduces glomerular and tubulointerstitial scarring in a rat model of crescentic glomerulonephritis. Am J Pathol 161: 1265–1272, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cosgrove D, Rodgers K, Meehan D, Miller C, Bovard K, Gilroy A, Gardner H, Kotelianski V, Gotwals P, Amatucci A, Kalluri R: Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol 157: 1649–1659, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Moeckel G, Morrow JD, Cosgrove D, Harris RC, Fogo AB, Zent R, Pozzi A: Lack of integrin alpha1beta1 leads to severe glomerulosclerosis after glomerular injury. Am J Pathol 165: 617–630, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu L, Su Y, Paueksakon P, Cheng H, Chen X, Wang H, Harris RC, Zent R, Pozzi A: Integrin α1/Akita double-knockout mice on a Balb/c background develop advanced features of human diabetic nephropathy. Kidney Int 81: 1086–1097, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner H, Broberg A, Pozzi A, Laato M, Heino J: Absence of integrin alpha1beta1 in the mouse causes loss of feedback regulation of collagen synthesis in normal and wounded dermis. J Cell Sci 112: 263–272, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Chen X, Abair TD, Ibanez MR, Su Y, Frey MR, Dise RS, Polk DB, Singh AB, Harris RC, Zent R, Pozzi A: Integrin alpha1beta1 controls reactive oxygen species synthesis by negatively regulating epidermal growth factor receptor-mediated Rac activation. Mol Cell Biol 27: 3313–3326, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen X, Whiting C, Borza C, Hu W, Mont S, Bulus N, Zhang MZ, Harris RC, Zent R, Pozzi A: Integrin alpha1beta1 regulates epidermal growth factor receptor activation by controlling peroxisome proliferator-activated receptor gamma-dependent caveolin-1 expression. Mol Cell Biol 30: 3048–3058, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borza CM, Chen X, Mathew S, Mont S, Sanders CR, Zent R, Pozzi A: Integrin alpha1beta1 promotes caveolin-1 dephosphorylation by activating T cell protein-tyrosine phosphatase. J Biol Chem 285: 40114–40124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi M, Pedchenko V, Greer BH, Van Horn WD, Santoro SA, Sanders CR, Hudson BG, Eichman BF, Zent R, Pozzi A: Enhancing integrin α1 inserted (I) domain affinity to ligand potentiates integrin α1β1-mediated down-regulation of collagen synthesis. J Biol Chem 287: 35139–35152, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borza CM, Su Y, Chen X, Yu L, Mont S, Chetyrkin S, Voziyan P, Hudson BG, Billings PC, Jo H, Bennett JS, Degrado WF, Eckes B, Zent R, Pozzi A: Inhibition of integrin α2β1 ameliorates glomerular injury. J Am Soc Nephrol 23: 1027–1038, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaneko Y, Otsuka T, Tsuchida Y, Gejyo F, Narita I: Integrin α1/β1 and α2/β1 as a receptor for IgA1 in human glomerular mesangial cells in IgA nephropathy. Int Immunol 24: 219–232, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, Müller GA, Gross O: Integrin α2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenesis Tissue Repair 3: 19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Müller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF: Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell 88: 603–613, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Müller U, Reichardt LF: Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J Cell Biol 154: 447–458, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Linton JM, Martin GR, Reichardt LF: The ECM protein nephronectin promotes kidney development via integrin alpha8beta1-mediated stimulation of Gdnf expression. Development 134: 2501–2509, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bieritz B, Spessotto P, Colombatti A, Jahn A, Prols F, Hartner A: Role of alpha8 integrin in mesangial cell adhesion, migration, and proliferation. Kidney Int 64: 119–127, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Hartner A, Cordasic N, Klanke B, Müller U, Sterzel RB, Hilgers KF: The alpha8 integrin chain affords mechanical stability to the glomerular capillary tuft in hypertensive glomerular disease. Am J Pathol 160: 861–867, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartner A, Cordasic N, Menendez-Castro C, Volkert G, Yabu JM, Kupraszewicz-Hutzler M, Rascher W, Hilgers KF: Lack of alpha8-integrin aggravates podocyte injury in experimental diabetic nephropathy. Am J Physiol Renal Physiol 299: F1151–F1157, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Zeltner R, Hilgers KF, Schmieder RE, Porst M, Schulze BD, Hartner A: A promoter polymorphism of the alpha 8 integrin gene and the progression of autosomal-dominant polycystic kidney disease. Nephron Clin Pract 108: c169–c175, 2008 [DOI] [PubMed] [Google Scholar]
- 40.Reiser J, Sever S: Podocyte biology and pathogenesis of kidney disease. Annu Rev Med 64: 357–366, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reiser J, Wei C, Tumlin J: Soluble urokinase receptor and focal segmental glomerulosclerosis. Curr Opin Nephrol Hypertens 21: 428–432, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Wei C, Möller CC, Altintas MM, Li J, Schwarz K, Zacchigna S, Xie L, Henger A, Schmid H, Rastaldi MP, Cowan P, Kretzler M, Parrilla R, Bendayan M, Gupta V, Nikolic B, Kalluri R, Carmeliet P, Mundel P, Reiser J: Modification of kidney barrier function by the urokinase receptor. Nat Med 14: 55–63, 2008 [DOI] [PubMed] [Google Scholar]
- 43.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borza CM, Borza DB, Pedchenko V, Saleem MA, Mathieson PW, Sado Y, Hudson HM, Pozzi A, Saus J, Abrahamson DR, Zent R, Hudson BG: Human podocytes adhere to the KRGDS motif of the alpha3alpha4alpha5 collagen IV network. J Am Soc Nephrol 19: 677–684, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma LJ, Yang H, Gaspert A, Carlesso G, Barty MM, Davidson JM, Sheppard D, Fogo AB: Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta6(-/-) mice. Am J Pathol 163: 1261–1273, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahm K, Lukashev ME, Luo Y, Yang WJ, Dolinski BM, Weinreb PH, Simon KJ, Chun Wang L, Leone DR, Lobb RR, McCrann DJ, Allaire NE, Horan GS, Fogo A, Kalluri R, Shield CF, 3rd, Sheppard D, Gardner HA, Violette SM: Alphav beta6 integrin regulates renal fibrosis and inflammation in Alport mouse. Am J Pathol 170: 110–125, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khan S, Lakhe-Reddy S, McCarty JH, Sorenson CM, Sheibani N, Reichardt LF, Kim JH, Wang B, Sedor JR, Schelling JR: Mesangial cell integrin αvβ8 provides glomerular endothelial cell cytoprotection by sequestering TGF-β and regulating PECAM-1. Am J Pathol 178: 609–620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]