Abstract
The aim of this study was to determine the frequency of EGFR, KRAS, BRAF, and HER-2 mutations in brain metastases from non-small cell lung carcinomas (BM-NSCLC). A total of 77 samples of BM-NSCLC were included and 19 samples of BM from breast, kidney, and colorectal tumors were also studied as controls. These samples were collected from patients followed between 2008 and 2011 at Poitiers and Nice University Hospitals in France. The frequencies of EGFR, KRAS, BRAF, and HER-2 mutations in BM-NSCLC were 2.6, 38.5, 0, and 0% respectively. The incidence of KRAS mutation was significantly higher in female and younger patients (P < 0.05). No mutations of the four genes were found in BM from breast or kidney. However, among six BM from colorectal tumors, we identified KRAS mutations in three cases and BRAF mutations in two other cases. This study is the largest analysis on genetic alterations in BM-NSCLC performed to date. Our results suggest a low frequency of EGFR mutations in BM-NSCLC whereas KRAS mutations are as frequent in BM-NSCLC as in primitive NSCLC. These results raise the question of the variability of the brain metastatic potential of NSCLC cells in relation to the mutation pattern.
Keywords: BRAF, brain metastases, epidermal growth factor receptor, HER-2, KRAS, non-small cell lung cancer
Introduction
Among all malignant tumors, non-small cell lung cancer (NSCLC) is the main cause of brain metastases (BM). BM arise in approximately 20–40% of NSCLC patients [1]. They are mainly detected synchronously to NSCLC (50% of cases), but can also be prevalent (6% of cases) or metachronous [2]. They are symptomatic in 85% of patients. The prognosis is generally poor, with a median survival of 4–11 weeks in untreated patients, but it is improved in patients treated by whole-brain radiation therapy (WBRT), with a median survival of 3–6 months [3]. Surgery and stereotactic radiosurgery are therapeutic options for oligometastatic disease and must be considered when possible. Combined with WBRT, surgery or radiosurgery improves the overall survival rate, with a median of 6–11 months [4, 5]. WBRT remains the standard therapy for multiple brain metastases, but NSCLC is a radioresistant cancer and 30 Gy WBRT is not sufficient to sterilize the lesions. Treatment of BM by chemotherapy remains controversial, since uncertain penetration of anti-cancer drugs through the blood–brain barrier restrains their optimal use. Several targeted therapies have recently been developed in the treatment of NSCLC, the efficiency of which depends on predictive value of molecular biomarkers' mutational status. Indeed, approximately 60–80% of patients whose tumor samples contain somatic mutations in the kinase domain of epidermal growth factor receptor (EGFR) gene are responsive to EGFR tyrosine kinase inhibitors (TKI) gefitinib and erlotinib [6]. More than 80% of the detected mutations are located at amino acids 746–753 encoded by exon 19 and amino acid 858 encoded by exon 21 [7]. KRAS protein, other downstream effectors of EGFR such as serine-threonine kinase BRAF, and another member of the human epidermal growth factor receptor (HER) family, v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (HER-2), are also implicated in the tumorigenesis and progression of NSCLC [8, 9]. Approximately 97% of KRAS mutations in primary NSCLC involve codons 12 or 13. The most frequent BRAF and HER-2 mutations in NSCLC are amino acidic substitution of p.V600E in exon 15 and a 12-bp duplication coding for the amino acids YVMA at codon 776, respectively [10, 11]. Although the impact of these mutations has not been completely elucidated, recent publications have shown that they represent negative prognostic markers in NSCLC [12, 13]. While the predictive value of wild-type KRAS genotype for identifying patients who will benefit from anti-EGFR monoclonal antibodies treatment is now well established in metastatic colorectal cancer, the significance of KRAS and BRAF mutations in NSCLC is not yet comparably clear [14–17]. The potential effectiveness of BRAF, HER-2, MEK, and mTOR inhibitors in the presence of mutations is currently being investigated in clinical trials [18, 19]. While the molecular status of EGFR in primary NSCLC has been widely studied, data concerning the molecular status of BM from NSCLC are scarce [20–26]. However, it is known that BM from NSCLC responds to oral EGFR TKIs according to the presence of activating mutations [6, 27, 28]. Recently, studies about the molecular pathways that mediate brain colonization have shown that genetic factors play an important role and that the molecular status of oncogenes is part of the risk-stratification of patients and needs to be investigated [29].
In this article we present to the best of our knowledge, a molecular study with clinical data of the largest series of BM from NSCLC (BM-NSCLC). The aim was to investigate the frequencies of EGFR, KRAS, BRAF, and HER-2 mutations in BM-NSCLC samples from 77 patients operated in the neurosurgery departments of Nice and Poitiers University Hospitals (France). In addition, we established and compared the mutational status of eight pairs of primary NSCLC and matched BM-NSCLC and examined the frequencies of the same mutations in 19 BM from tumors other than NSCLC, such as breast, kidney, and colorectal cancers.
Materials and Methods
Samples
Formalin-fixed and paraffin-embedded BM tumor samples from fine needle aspiration or surgical resection were obtained from 96 patients, mainly of Caucasian origin, treated between 2008 and 2011 at Poitiers and Nice Hospitals in France. The histological types were as follows: Stage IV NSCLC n = 77, breast n = 7, kidney n = 6, and colorectal n = 6. None of the patients had previously been treated with EGFR inhibitors. The frequencies of EGFR, KRAS, BRAF, and HER-2 mutations in BM-NSCLC from our series were compared to frequencies of these mutations in a cohort of stage IV-primitive NSCLC samples evaluated for EGFR (n = 1235), KRAS (n = 1046), BRAF (n = 734), and HER-2 (n = 284) in our molecular diagnosis daily practice between 2009 and 2012, according to the recommendations of the French National Cancer Institute (INCa) (http://www.e-cancer.fr). The samples had been collected after informed consent of all patients according to the ethical rules of our institutions.
Genetic analysis
The presence of at least 50% tumor cells in samples was evaluated histologically. Genomic DNA was extracted using DNAeasy Blood & Tissue DNA isolation kit or QIAamp DNA Mini Kit (Qiagen, Hilden, Germany). Genotyping of EGFR exons 18, 19, 20, and 21 was performed by pyrosequencing method with PyroMark Q24 and CE-IVD-marked Therascreen EGFR Pyro associated Kit (Qiagen) or length analysis of fluorescently labelled PCR products for exons 19 and 21. To reveal the presence of p.L858R, PCR product of exon 21 was digested by Sau96I (New England Biolabs, Evry, France). Genotyping was carried out using 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA) and results were interpreted using Genemapper V4.1 software (Applied Biosystems).
Genotyping of KRAS exon 2 (codons 12 and 13) was performed by pyrosequencing method with the PyroMark Q24 and CE-IVD-marked Therascreen KRAS Pyro associated kit (Qiagen) or allelic discrimination using the 7500 Fast Real-Time PCR platform (Applied Biosystems). The design of sequences of the TaqMan probes was kindly provided by Pr Laurent-Puig [30].
Genotyping of BRAF exon 15 and HER-2 exon 20 was performed by pyrosequencing method with sequences designed using PyroMark Assay Design Software (Qiagen). Pyrosequencing was done according to the manufacturer's instructions (Qiagen). Results were interpreted using PyroMark Q24 2.0 software (Qiagen). The primers sequences and PCR conditions are available on request.
Statistical methods
Statistical analyses were performed to characterize the relationships between mutational status and clinical features by using chi square or Mann–Whitney tests. The statistical analyses were two-tailed ones and the level of significance was set at P = 0.05.
Results and Discussion
Clinical characteristics of patients
Seventy-seven consecutive cases of BM from NSCLC non-treated with TKI were studied (Table 1). The histological types were adenocarcinomas in 71 cases, squamous cell carcinomas in five cases, and large cell carcinoma in one case. All patients initially presented a stage IV disease. The mean age was 59.2 ± 10.1 years. A male predominance of 69.2% was observed (male-to-female ratio = 1:0.44). Sixty-six tumors were located in the supratentorial compartment while seven were cerebellar metastases. In addition, the clinical characteristics of 19 patients affected by BM from diverse primary tumors other than NSCLC are described in Table 2.
Table 1.
EGFR, KRAS, BRAF, HER-2, and ALK mutation status in brain metastases from NSCLC and patient characteristics
| Mutation status in brain metastases | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Sex | Age | EGFR | KRAS | BRAT | HER-2 | ALK | Metastases anatomical location | Smoking status |
| BM1 | F | 70 | wt | G12D | wt | wt | nc | Supratentorial | nc |
| BM2 | M | 40 | wt | G12C | wt | wt | nc | Supratentorial | Current smoker |
| BM3 | M | 60 | wt | wt | wt | wt | nc | Supratentorial | nc |
| BM4 | F | 47 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM5 | M | 54 | wt | wt | wt | wt | nc | nc | nc |
| BM6 | F | 50 | wt | G12C | wt | wt | nc | Supratentorial | nc |
| BM7 | M | 66 | wt | wt | wt | wt | nc | Infratentorial | nc |
| BM8 | M | 65 | wt | G12C | wt | wt | nc | Supratentorial | nc |
| BM9 | F | 75 | L858R | wt | wt | wt | nc | Supratentorial | nc |
| BM10 | M | 69 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM11 | F | 57 | wt | G12V | wt | nc | nc | Supratentorial | nc |
| BM12 | M | 80 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM13 | M | 56 | L858R | wt | wt | wt | nc | Supratentorial | Non smoker |
| BM14 | M | 54 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM15 | F | 54 | wt | G12C | wt | wt | nc | Supratentorial | nc |
| BM16 | M | 54 | wt | G12V | wt | wt | nc | Supratentorial | Current smoker |
| BM17 | M | 54 | wt | G12C | wt | wt | nc | nc | Current smoker |
| BM18 | M | 54 | wt | wt | wt | wt | No rearrangement | Supratentorial | nc |
| BM19 | M | 64 | wt | G12V | wt | wt | nc | nc | nc |
| BM20 | M | 67 | L858R | wt | wt | wt | nc | Infratentorial | nc |
| BM21 | M | 57 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM22 | M | 77 | wt | wt | wt | wt | No rearrangement | Supratentorial | Ex-smoker |
| BM23 | M | 66 | wt | G12C | wt | wt | nc | Supratentorial | nc |
| BM24 | M | 55 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM25 | F | 60 | wt | G12V | wt | wt | nc | Supratentorial | nc |
| BM26 | F | 59 | wt | G12D | wt | wt | nc | Supratentorial | Current smoker |
| BM27 | M | 79 | wt | G12V | wt | wt | nc | Supratentorial | Ex-smoker |
| BM28 | M | 57 | wt | wt | wt | wt | nc | Supratentorial | nc |
| BM29 | M | 59 | wt | wt | wt | wt | No rearrangement | nc | nc |
| BM30 | M | 61 | wt | wt | wt | wt | nc | Supratentorial | nc |
| BM31 | F | 52 | wt | G13D | wt | wt | nc | Supratentorial | nc |
| BM32 | M | 71 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM33 | M | 58 | wt | wt | wt | wt | nc | Infratentorial | Current smoker |
| BM34 | M | 83 | wt | wt | wt | wt | nc | Supratentorial | nc |
| BM35 | M | 61 | wt | G12C | wt | wt | nc | Supratentorial | Current smoker |
| BM36 | M | 66 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM37 | F | 54 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM38 | M | 60 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM39 | F | 42 | wt | G12C | wt | wt | nc | Supratentorial | nc |
| BM40 | F | 52 | wt | wt | wt | wt | No rearrangement | Supratentorial | Current smoker |
| BM41 | M | 54 | wt | G12F | wt | wt | nc | Supratentorial | Ex-smoker |
| BM42 | F | 68 | wt | G12A | wt | wt | nc | Supratentorial | Ex-smoker |
| BM43 | M | 52 | wt | G12V | wt | wt | nc | Supratentorial | Non smoker |
| BM44 | M | 52 | wt | G12V | wt | wt | nc | Supratentorial | Current smoker |
| BM45 | M | 52 | wt | G12C | wt | wt | nc | Supratentorial | Current smoker |
| BM46 | F | 58 | wt | G12C | wt | wt | nc | Supratentorial | Ex-smoker |
| BM47 | M | 61 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM48 | M | 67 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM49 | M | 58 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM50 | F | 57 | wt | wt | wt | wt | nc | Supratentorial | nc |
| BM51 | F | 65 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM52 | M | 59 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM53 | M | 81 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM54 | M | 70 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM55 | M | 65 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM56 | M | 66 | wt | wt | wt | wt | nc | Supratentorial | nc |
| BM57 | F | 49 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM58 | M | 69 | wt | wt | wt | wt | nc | Supratentorial | nc |
| BM59 | M | 67 | wt | wt | wt | wt | nc | Infratentorial | Current smoker |
| BM60 | F | 35 | wt | wt | wt | wt | nc | Infratentorial | nc |
| BM61 | M | 58 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM62 | M | 39 | wt | wt | wt | wt | nc | Supratentorial | nc |
| BM63 | F | 48 | wt | wt | wt | wt | nc | Supratentorial | Non smoker |
| BM64 | M | 53 | wt | G13D | wt | wt | nc | Supratentorial | Ex-smoker |
| BM65 | M | 80 | wt | G12V | wt | wt | nc | Supratentorial | Current smoker |
| BM66 | M | 52 | wt | wt | wt | wt | nc | Supratentorial | Ex-smoker |
| BM67 | M | 64 | wt | wt | wt | wt | nc | Supratentorial | Current smoker |
| BM68 | M | 55 | wt | wt | wt | wt | No rearrangement | Supratentorial | Ex-smoker |
| BM69 | F | 45 | wt | G12C | wt | wt | No rearrangement | Supratentorial | Current smoker |
| BM70 | F | 47 | wt | G12V | wt | wt | No rearrangement | Supratentorial | nc |
| BM71 | F | 53 | wt | G12C | wt | wt | No rearrangement | Supratentorial | nc |
| BM72 | F | 47 | wt | G12V | wt | wt | No rearrangement | Supratentorial | nc |
| BM73 | M | 57 | wt | G12A | wt | wt | No rearrangement | Supratentorial | nc |
| BM74 | M | 63 | wt | wt | wt | wt | No rearrangement | Supratentorial | nc |
| BM75 | M | 59 | wt | wt | wt | wt | No rearrangement | Supratentorial | nc |
| BM76 | M | 75 | wt | wt | wt | wt | No rearrangement | Supratentorial | nc |
| BM77 | F | 43 | wt | G12V | wt | wt | No rearrangement | Supratentorial | nc |
BM, brain metastase; wt, wild-type; ADC, adenocarcinoma; SCC, squamous cell carcinoma; LCC, large cell carcinoma; nc, noncommunicated.
Table 2.
EGFR, KRAS, BRAF, and HER-2 mutation status in brain metastases from breast, kidney, and colorectal primitive tumors and patient characteristics
| Mutation status in brain metastases | |||||||
|---|---|---|---|---|---|---|---|
| Primitive anatomical location | No | Sex | Age | EGFR | KRAS | BRAF | HER-2 |
| Breast | BM70 | F | 79 | wt | wt | wt | wt |
| BM71 | F | 63 | wt | wt | wt | wt | |
| BM72 | F | 63 | wt | wt | wt | wt | |
| BM73 | F | 71 | wt | wt | wt | wt | |
| BM74 | F | 40 | wt | wt | wt | wt | |
| BM75 | F | 37 | wt | wt | wt | wt | |
| BM76 | F | 60 | wt | wt | wt | wt | |
| Kidney | BM77 | M | 50 | wt | wt | wt | wt |
| BM78 | M | 67 | wt | wt | wt | wt | |
| BM79 | F | 69 | wt | wt | wt | wt | |
| BM80 | F | 57 | wt | wt | wt | wt | |
| BM81 | M | 64 | wt | wt | wt | wt | |
| BM82 | F | 57 | wt | wt | wt | wt | |
| Colorectal | BM83 | F | 77 | wt | wt | wt | wt |
| BM84 | M | 67 | wt | wt | V600E | wt | |
| BM85 | F | 80 | wt | G13D | wt | wt | |
| BM86 | M | 58 | wt | wt | V600E | wt | |
| BM87 | M | 59 | wt | G12A | wt | wt | |
| BM88 | M | 76 | wt | G12V | wt | wt | |
EGFR mutations in brain metastases from NSCLC and in matched primitive NSCLC
We detected p.L858R mutation of EGFR in three out of 77 cases of BM-NSCLC (3.9%). One patient was a non-smoker male while the smoking status of the other two patients (one male and one female) was not known. No mutation of KRAS, BRAF, and HER-2 was detected in these three EGFR-mutated samples (Table 1). Recent studies have shown that the EGFR TKI are active in patients with BM-NSCLC but unfortunately, none of the three patients in our series had received this treatment [27, 28]. The frequency of 3.9% of EGFR mutations in our series of BM-NSCLC is lower than rates in grouped series of primary tumors and all anatomically located metastases. Indeed, the frequency of EGFR mutation in published series of NSCLC is approximately 10–16% in patients of non-Asian origin [31, 32]. In France, according to the INCa register, frequency of EGFR-activating mutation was 10% in 20,750 NSCLC patients tested in 2011 (http://www.e-cancer.fr). In our own experience of daily practice in Nice and Poitiers between 2009 and 2012, while using the same experimental methods as those described here, we observed a mutation frequency of 9.5% (1235 NSCLC patients). The ethnic origins of patients are determining factors since frequencies of EGFR mutation are much higher (40%) in patients of Asian origin [33]. The difference between frequency of 3.9% in BM-NSCLC versus 9.5 or 10% in NSCLC is statistically significant (P < 0.05) and this low frequency of EGFR mutations in BM is consistent with results from other studies in Caucasian patients. Sun et al. [34], Daniele et al. [21], and Cortot et al. [20] reported frequencies of 1/42, 0/28, and 0/13 of EGFR mutations in BM-NSCLC, respectively. Several hypotheses can be raised in explanation of this low frequency in BM-NSCLC. First, the low frequency of EGFR mutations could be explained by the male gender, smoker and non-Asian predominance in our BM-NSCLC series since it has been shown that EGFR mutations in NSCLC are statistically associated with female gender, non-smoking status, and Asian origin [35, 36]. Male and Caucasian predominance is also present in the three studies mentioned above. Smoking status was not mentioned. As a second hypothesis, it should be considered that tumor heterogeneity at the molecular level might be responsible for the differences in frequency between primitive-NSCLC and BM-NSCLC. We could study only eight matched cases and found no discrepancy of EGFR status in primitive tumors and matched BM (Table 3). Other studies have reported heterogeneity of EGFR status, but a recent article in a large Asian series explains these discordances by technical artefacts due to heterogeneity in the amplification of EGFR mutated [22, 23, 26, 37]. In the studies of Sun et al. and Cortot et al. [20, 34], all cases showed concordant results between NSCLC primary tumors and BM. The report by Daniele et al. [21] only covers mutation status in BM. Finally, as a third hypothesis, one may think that EGFR mutational status impacts the capacity to metastasize. Doebele et al. [10] have shown that the mutation profile (EGFR/KRAS/ALK) did impact the metastatic spread pattern and one can hypothesize that wild-type EGFR clones have enhanced brain metastatic potential and that patients with EGFR-mutated tumors may develop fewer BM than patients with wild-type EGFR tumors. Consistent with this hypothesis, two recent studies have reported a longer median time to progression for patients with BM harboring EGFR mutations in their primitive NSCLC compared to patients whose EGFR mutational status was not known or wild type [27, 38]. Another study has suggested a lower risk of CNS invasion in patients with advanced EGFR-mutated NSCLC treated with initial systemic therapy by gefitinib or erlotinib than the risk reported in historical series (19% vs. 40%) [39]. However, these results may be dependent on EGFR-TKI treatment rather than EGFR status by itself. On the other hand, the study of Li et al. [40] has suggested that BM would be more frequent in patients with tumors bearing EGFR mutations. In this retrospective study including 110 patients with NSCLC, EGFR status was determined in primitive tumors and compared with the development of BM. The frequencies of EGFR mutation were 64% and 31% in the patients with and without BM, respectively. Thus, current published data are far too limited to draw any firm conclusion and it is necessary to study large case series.
Table 3.
EGFR, KRAS, BRAF, and HER-2 mutation status in paired primitive NSCLC and BM samples
| Mutation status in primitive NSCLC | Mutation status in brain metastases | |||||||
|---|---|---|---|---|---|---|---|---|
| EGFR | KRAS | BRAF | HER-2 | EGFR | KRAS | BRAF | HER-2 | |
| BM3 | wt | wt | wt | wt | wt | wt | wt | wt |
| BM9 | L858R | wt | wt | wt | L858R | wt | wt | wt |
| BM13 | L858R | wt | wt | wt | L858R | wt | wt | wt |
| BM20 | L858R | wt | wt | wt | L858R | wt | wt | wt |
| BM22 | wt | wt | wt | wt | wt | wt | wt | wt |
| BM26 | wt | G13D | wt | wt | wt | G13D | wt | wt |
| BM35 | wt | G12C | wt | wt | wt | G12C | wt | wt |
| BM43 | wt | G12V | wt | wt | wt | G12V | wt | wt |
KRAS mutations in brain metastases from NSCLC and in matched primitive NSCLC
We have detected mutations of KRAS in 30 out of 77 (39.0%) BM-NSCLC cases (Table 1). Among them, mutations in codon 12 were observed in 26 cases and in codon 13 in four cases (Table 4). Little information concerning the brain localization is available in the few reports on KRAS status in lung cancer metastases [20, 25, 26, 41, 42]. To note, the frequency of 39.0% is not statistically different from the frequency of 31.3% that we observed in 1046 primary NSCLC between 2009 and 2012 using the same technology as for the BM-NSCLC. According to the INCa register, the frequency of KRAS mutation was 25.4% in 17,153 patients (primary and all metastatic sites) tested in 2011 (http://www.e-cancer.fr). This frequency is lower, but the use of various techniques with lower sensitivities than pyrosequencing and allelic discrimination can explain this difference. In our cohort of patients with BM-NSCLC, the incidence of KRAS mutation was significantly higher in female than in male patients and in younger ones (P < 0.05) (Table 5). These points have already been reported in several studies about NSCLC and the sex-linked factors that are related to lung cancer risk deserve consideration [43, 44]. No association of frequency of KRAS mutations with tumor differentiation was found. Correlation of KRAS mutation with smoking history has previously been reported [45]. In our study, smoking status was known in 44 cases, and only three patients were nonsmokers, including one case with KRAS mutation. No correlation was found between KRAS mutations and smoking status. Interestingly, we have found no KRAS mutation when the metastases were located in the cerebellum (Table 5). As for EGFR mutations, we did not observe discordant results between primitive and metastatic tumors for KRAS mutations, indicating that they are generally acquired prior to metastatic spread, but our matched series and studies on this subject are still too few to draw firm conclusions (Table 3). As we found comparable frequencies of KRAS mutations in primitive NSCLC and in BM, our data are consistent with the idea that KRAS mutational status does not influence the capacity of cells from NSCLC to metastasize. In NSCLC, several studies showed that KRAS mutations were associated with decreased time to progression and shorter survival [12, 16]. mTOR and MEK inhibitors are currently being evaluated in clinical trials in patients with KRAS mutations [18, 19]. In BM-NSCLC, further investigations are needed to evaluate the efficacy of these inhibitors.
Table 4.
Summary of KRAS mutations in brain metastases from NSCLC (n = 30)
| KRAS | Nucleotide change | Codon change | Cases |
|---|---|---|---|
| Codon 12 | GGT>GAT | G12D | 1 (3.3%) |
| GGT>TGT | G12C | 11 (36.7%) | |
| GGT>GTT | G12V | 11 (36.7%) | |
| GGT>GCT | G12A | 2 (6.6%) | |
| GGT>TTT | G12F | 1 (3.3%) | |
| Codon 13 | GGOGAC | G13D | 3 (9.9%) |
| GGOTGC | G13C | 1 (3.3%) |
Table 5.
Correlations between KRAS status and clinicopathologic factors in BM from NSCLC patients
| Gender | Histopathology | Anatomical location | Smoking status | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KRAS mutation status | Age (mean) | Male | Female | Poorly differentiated | Moderately differentiated | Well differentiated | Infratentorial | Supratentorial | Non-smoker | Ex-smoker | Current smoker |
| Wild-type | 61.1 ± 10.0 | 38 | 9 | 10 | 11 | 3 | 7 | 24 | 1 | 10 | 16 |
| Mutated | 56.3 ± 9.8 | 15 | 15 | 7 | 6 | 1 | 0 | 24 | 1 | 4 | 9 |
| Statistical significativity | P < 0.05 | χ2 = 8.125 P < 0.05 | χ2 = 0.396 P > 0.05 | χ2 = 6.21 P < 0.05 | χ2 = 0. 454 P > 0.05 | ||||||
BRAF and HER-2 mutations in brain metastases from NSCLC
No mutations of BRAF or HER-2 were found, respectively, in 77 and 76 analyzed cases of BM-NSCLC (Table 1). To our knowledge, this is the first study to have investigated BRAF and HER-2 mutational status in BM-NSCLC. Concerning primary-NSCLC and all metastatic sites analyzed in 2011, the INCa register indicates BRAF and HER-2 mutation frequencies of 1.8% in 10,017 patients and 0.9% in 7731 patients, respectively (http://www.e-cancer.fr). Our daily practice between 2009 and 2012, with the same technology shows BRAF and HER-2 mutation frequencies of 2.5% (734 patients) and 1.4% (284 patients), respectively. Genetic rearrangement between echinoderm microtubule-associated protein-like 4 and anaplastic lymphoma kinase (EML4-ALK) was examined in 14 BM-NSCLC and one was positive. Although this genetic alteration is usually described as exclusive with EGFR or KRAS mutations, in our series, this patient also harbors KRAS mutation (BM73). The spectrum of the five driver mutations in our series of BM-NSCLC is illustrated in Figure 1.
Figure 1.
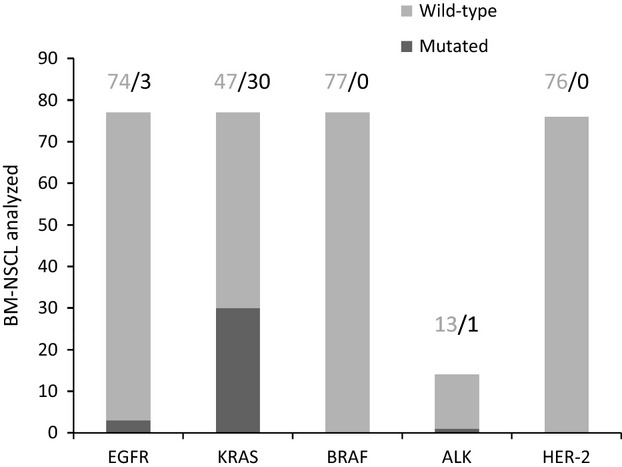
Spectrum of analyzed mutations in our series of BM-NSCLC.
EGFR, KRAS, BRAF, and HER-2 mutations in BM from primitive tumors other than lung adenocarcinomas
Frequencies of EGFR, KRAS, BRAF, and HER-2 mutations were compared between BM from lung, breast, kidney, and colorectal tumors (Table 2). All metastases studied were wild type for EGFR. No KRAS mutations were found in metastases from breast and kidney whereas three of six metastases from colorectal tumors were mutated. Despite the small size of the series, the results suggest that BM from lung and colorectal tumors are more frequently KRAS-mutated than those from breast and kidney. Indeed, we have noticed statistical differences in frequency of KRAS mutations between BM from NSCLC and from breast and kidney (P < 0.05), but no difference between NSCLC and colorectal tumor (P > 0.05) (Table 6). A high frequency of mutations in BM from colorectal cancers has been reported by Tie et al. [46]. Two cases of BM from colorectal tumors were BRAF mutated (p.V600E). A similar observation was made in BM from melanoma by El-Osta et al. [47] that showed more BM in melanoma patients with mutated BRAF versus wild type. No mutation of HER-2 was found in all cases of metastases.
Table 6.
Correlations between frequency of KRAS mutations in BM from breast, kidney, or colorectal tumors and BM from NSCLC
| BM from breast tumors | BM from kidney tumors | BM from colorectal tumors | |
|---|---|---|---|
| BM-NSCLC | χ2 = 4.988 P < 0.05 | χ2 = 4.331 P < 0.05 | χ2 = 0.072 P> 0.05 |
In summary, we report the first large series analyzing EGFR, KRAS, BRAF, and HER-2 mutations in brain metastases of NSCLC. While the frequencies of KRAS and BRAF mutations were similar to frequencies usually described in primitive or other metastatic locations of NSCLC, the frequency of EGFR mutations was low. Mechanistic studies are needed to evaluate the association of these mutations with the metastatic spread. We can assume that they are not necessary to trigger the metastatic process since the majority of patients with metastases do not have these mutations. BM-NSCLC have been shown to respond to oral EGFR TKIs and these data highlight the potential value of detecting mutations for choosing the most appropriate targeted treatment and for surveillance strategies.
Acknowledgments
The authors thank Anabelle Cortes-Maurel, Estelle Lemarie, Anaïs Balbous, and Thibault Fabas for expert technical assistance.
Conflict of Interest
None declared.
References
- 1.Mamon HJ, Yeap BY, Jänne PA, Reblando J, Shrager S, Jaklitsch MT, et al. High risk of brain metastases in surgically staged IIIA non-small-cell lung cancer patients treated with surgery, chemotherapy, and radiation. J. Clin. Oncol. 2005;23:1530–1537. doi: 10.1200/JCO.2005.04.123. [DOI] [PubMed] [Google Scholar]
- 2.Komaki R, Cox JD, Stark R. Frequency of brain metastases in adenocarcinoma with large cell carcinoma of the lung: correlation with survival. Int. J. Radiat. Oncol. Biol. Phys. 1983;9:1467–1470. doi: 10.1016/0360-3016(83)90319-x. [DOI] [PubMed] [Google Scholar]
- 3.Sundström JT, Minn H, Lertola KK, Nordman E. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann. Med. 1998;30:296–299. doi: 10.3109/07853899809005858. [DOI] [PubMed] [Google Scholar]
- 4.Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 5.Bailon O, Kallel A, Chouahnia K, Billot S, Ferrari D, Carpentier AF. Management of brain metastases from non-small cell lung carcinoma. Rev. Neurol. 2011;167:579–591. doi: 10.1016/j.neurol.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 7.Pao W, Miller VA. Epidermal growth factor receptor mutations, small-molecule kinase inhibitors, and non-small-cell lung cancer: current knowledge and future directions. J. Clin. Oncol. 2005;23:2556–2568. doi: 10.1200/JCO.2005.07.799. [DOI] [PubMed] [Google Scholar]
- 8.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 9.Pao W, Girard N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 10.Doebele RC, Lu X, Sumey C, Maxson DA, Weickhardt AJ, Oton AB, et al. Oncogene status predicts patterns of metastatic spread in treatment-naive nonsmall cell lung cancer. Cancer. 2012;118:4502–4511. doi: 10.1002/cncr.27409. doi: 10.1002/cncr.27409. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shigematsu H, Takahashi T, Nomura M, Majmudar K, Suzuki M, Lee H, et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65:1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 12.Brugger W, Triller N, Blasinska-Morawiec M, Curescu S, Sakalauskas R, Manikhas GM, et al. Prospective molecular marker analyses of EGFR and KRAS from a randomized, placebo-controlled study of erlotinib maintenance therapy in advanced non-small-cell lung cancer. J. Clin. Oncol. 2011;29:4113–4120. doi: 10.1200/JCO.2010.31.8162. Erratum in: J. Clin. Oncol. 2011;29:4725. [DOI] [PubMed] [Google Scholar]
- 13.Marchetti A, Felicioni L, Malatesta S, Grazia Sciarrotta M, Guetti L, Chella A, et al. Clinical features and outcome of patients with non-small-cell lung cancer harboring BRAF mutations. J. Clin. Oncol. 2011;29:3574–3579. doi: 10.1200/JCO.2011.35.9638. [DOI] [PubMed] [Google Scholar]
- 14.Bonanno L, Schiavon M, Nardo G, Bertorelle R, Bonaldi L, Galligioni A, et al. Prognostic and predictive implications of EGFR mutations, EGFR copy number and KRAS mutations in advanced stage lung adenocarcinoma. Anticancer Res. 2010;30:5121–5128. [PubMed] [Google Scholar]
- 15.Lièvre A, Blons H, Laurent-Puig P. Oncogenic mutations as predictive factors in colorectal cancer. Oncogene. 2010;29:3033–3043. doi: 10.1038/onc.2010.89. [DOI] [PubMed] [Google Scholar]
- 16.Massarelli E, Varella-Garcia M, Tang X, Xavier AC, Ozburn NC, Liu DD, et al. KRAS mutation is an important predictor of resistance to therapy with epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. Clin. Cancer Res. 2007;13:2890–2896. doi: 10.1158/1078-0432.CCR-06-3043. [DOI] [PubMed] [Google Scholar]
- 17.Roberts PJ, Stinchcombe TE, Der CJ, Socinski MA. Personalized medicine in non-small-cell lung cancer: is KRAS a useful marker in selecting patients for epidermal growth factor receptor-targeted therapy? J. Clin. Oncol. 2010;28:4769–4777. doi: 10.1200/JCO.2009.27.4365. [DOI] [PubMed] [Google Scholar]
- 18.Johnson BE, Jackman D, Jänne PA. Rationale for a phase I trial of erlotinib and the mammalian target of rapamycin inhibitor everolimus (RAD001) for patients with relapsed non small cell lung cancer. Clin. Cancer Res. 2007;13:s4628–4631. doi: 10.1158/1078-0432.CCR-07-0717. [DOI] [PubMed] [Google Scholar]
- 19.Mahoney CL, Choudhury B, Davies H, Edkins S, Greenman C, Haaften G, et al. LKB1/KRAS mutant lung cancers constitute a genetic subset of NSCLC with increased sensitivity to MAPK and mTOR signalling inhibition. Br. J. Cancer. 2009;100:370–375. doi: 10.1038/sj.bjc.6604886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortot AB, Italiano A, Burel-Vandenbos F, Martel-Planche G, Hainaut P. KRAS mutation status in primary nonsmall cell lung cancer and matched metastases. Cancer. 2010;116:2682–2687. doi: 10.1002/cncr.25014. [DOI] [PubMed] [Google Scholar]
- 21.Daniele L, Cassoni P, Bacillo E, Cappia S, Righi L, Volante M, et al. Epidermal growth factor receptor gene in primary tumor and metastatic sites from non-small cell lung cancer. J. Thorac. Oncol. 2009;4:684–688. doi: 10.1097/JTO.0b013e3181a52359. [DOI] [PubMed] [Google Scholar]
- 22.Gow CH, Chang YL, Hsu YC, Tsai MF, Wu CT, Yu CJ, et al. Comparison of epidermal growth factor receptor mutations between primary and corresponding metastatic tumors in tyrosine kinase inhibitor-naive non-small-cell lung cancer. Ann. Oncol. 2009;20:696–702. doi: 10.1093/annonc/mdn679. [DOI] [PubMed] [Google Scholar]
- 23.Han C, Zou H, Ma J, Zhou Y, Zhao J. Comparison of EGFR and KRAS status between primary non-small cell lung cancer and corresponding metastases: a systematic review and meta-analysis. Zhongguo Fei Ai Za Zhi. 2010;13:882–891. doi: 10.3779/j.issn.1009-3419.2010.09.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsumoto S, Takahashi K, Iwakawa R, Matsuno Y, Nakanishi Y, Kohno T, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int. J. Cancer. 2006;119:1491–1494. doi: 10.1002/ijc.21940. [DOI] [PubMed] [Google Scholar]
- 25.Monaco SE, Nikiforova MN, Cieply K, Teot LA, Khalbuss WE, Dacic S. A comparison of EGFR and KRAS status in primary lung carcinoma and matched metastases. Hum. Pathol. 2010;41:94–102. doi: 10.1016/j.humpath.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 26.Munfus-McCray D, Harada S, Adams C, Askin F, Clark D, Gabrielson E, et al. EGFR and KRAS mutations in metastatic lung adenocarcinomas. Hum. Pathol. 2011;42:1447–1453. doi: 10.1016/j.humpath.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Porta R, Sánchez-Torres JM, Paz-Ares L, Massutí B, Reguart N, Mayo C, et al. Brain metastases from lung cancer responding to erlotinib: the importance of EGFR mutation. Eur. Respir. J. 2011;37:624–631. doi: 10.1183/09031936.00195609. [DOI] [PubMed] [Google Scholar]
- 28.Preusser M, Capper D, Ilhan-Mutlu A, Berghoff AS, Birner P, Bartsch R, et al. Brain metastses: pathobiology and emerging targeted therapies. Acta Neuropathol. 2012;123:205–222. doi: 10.1007/s00401-011-0933-9. [DOI] [PubMed] [Google Scholar]
- 29.Eichler AF, Chung E, Kodack DP, Loeffler JS, Fukumara D, Jain RK. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 2011;8:344–356. doi: 10.1038/nrclinonc.2011.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lièvre A, Bachet JB, Boige V, Cayre A, Buc D, Le Corre E, et al. KRAS mutations as an independent prognostic factor in patients with advanced colorectal cancer treated with cetuximab. J. Clin. Oncol. 2008;26:374–379. doi: 10.1200/JCO.2007.12.5906. [DOI] [PubMed] [Google Scholar]
- 31.Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N. Engl. J. Med. 2009;361:958–967. doi: 10.1056/NEJMoa0904554. [DOI] [PubMed] [Google Scholar]
- 32.Marchetti A, Martella C, Felicioni L, Barassi F, Salvatore S, Chella A, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J. Clin. Oncol. 2005;23:857–865. doi: 10.1200/JCO.2005.08.043. [DOI] [PubMed] [Google Scholar]
- 33.Wu YL, Zhong WZ, Li LY, Zhang XT, Zhang L, Zhou CC, et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J. Thorac. Oncol. 2007;2:430–439. doi: 10.1097/01.JTO.0000268677.87496.4c. [DOI] [PubMed] [Google Scholar]
- 34.Sun M, Behrens C, Feng L, Ozburn N, Tang X, Yin G, et al. HER family receptor abnormalities in lung cancer brain metastases and corresponding primary tumors. Clin. Cancer Res. 2009;15:4829–4837. doi: 10.1158/1078-0432.CCR-08-2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Calvo E, Baselga J. Ethnic differences in response to epidermal growth factor receptor tyrosine kinase inhibitors. J. Clin. Oncol. 2006;24:2158–2163. doi: 10.1200/JCO.2006.06.5961. [DOI] [PubMed] [Google Scholar]
- 36.D'Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, et al. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J. Clin. Oncol. 2011;29:2066–2070. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yatabe Y, Matsuo K, Mitsudomi T. Heterogeneous distribution of EGFR mutations is extremely rare in lung adenocarcinoma. J. Clin. Oncol. 2011;29:2972–2977. doi: 10.1200/JCO.2010.33.3906. [DOI] [PubMed] [Google Scholar]
- 38.Eichler AF, Kahle KT, Wang DL, Joshi VA, Willers H, Engelman JA, et al. EGFR mutation status and survival after diagnosis of brain metastases in nonsmall cell lung cancer. Neuro Oncol. 2010;12:1193–1199. doi: 10.1093/neuonc/noq076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heon S, Yeap BY, Britt GJ, Costa DB, Rabin MS, Jackman DM, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin. Cancer Res. 2010;16:5873–5882. doi: 10.1158/1078-0432.CCR-10-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Z, Lu J, Zhao Y, Guo H. 2011. The retrospective analysis of the frequency of EGFR mutations and the efficacy of gefitinib in NSCLC patients with brain metastases. ASCO Annual Meeting. Abstract no. e18065.
- 41.Da Silva L, Simpson PT, Smart CE, Cocciardi S, Waddell N, Lane A, et al. HER3 and downstream pathways are involved in colonization of brain metastases from breast cancer. Breast Cancer Res. 2010;12:R46. doi: 10.1186/bcr2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalikaki A, Koutsopoulos A, Trypaki M, Souglakos J, Stathopoulos E, Georgoulias V, et al. Comparison of EGFR and K-RAS gene status between primary tumors and corresponding metastases in NSCLC. Br. J. Cancer. 2008;99:923–929. doi: 10.1038/sj.bjc.6604629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blons H, Côté JF, Riquet D, Le Corre M, Fabre-Guilevin E, Laurent-Puig P, et al. Epidermal growth factor receptor mutation in lung cancer are linked to bronchiolalveolar differentiation. Am. J. Surg. Pathol. 2006;30:1309–1315. doi: 10.1097/01.pas.0000213285.65907.31. [DOI] [PubMed] [Google Scholar]
- 44.Planchard D, Loriot Y, Goubar A, Commo F, Soria JC. Differential expression of biomarkers in men and women. Semin. Oncol. 2009;36:553–565. doi: 10.1053/j.seminoncol.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Ahrendt SA, Decker PA, Alawi EA, Zhu Yr YR, Sanchez-Cespedes M, Yang SC, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 46.Tie J, Lipton L, Desai J, Gibbs P, Jorissen RN, Christie M, et al. KRAS mutation is associated with lung metastases in patients with curatively resected colorectal cancer. Clin. Cancer Res. 2011;17:1122–1130. doi: 10.1158/1078-0432.CCR-10-1720. [DOI] [PubMed] [Google Scholar]
- 47.El-Osta H, Falchook G, Tsimberidou A, Hong D, Naing A, Kim K, et al. BRAF mutations in advanced cancers: clinical characteristics and outcomes. PLoS ONE. 2011;6:e25806. doi: 10.1371/journal.pone.0025806. [DOI] [PMC free article] [PubMed] [Google Scholar]


