Abstract
Information on the impact of hormone receptor status subtypes in breast cancer (BC) prognosis is still limited for Hispanics. We aimed to evaluate the association of BC molecular subtypes and other clinical factors with survival in a hospital-based female population of BC cases in Puerto Rico. We analyzed 663 cases of invasive BC diagnosed between 2002 and 2005. Information on HER-2/neu (HER-2) overexpression, estrogen (ER), and progesterone (PR) receptor status and clinical characteristics were retrieved from hospitals cancer registries and record review. Survival probabilities by covariates of interest were described using the Kaplan–Meier estimators. Cox proportional hazards models were employed to assess factors associated with risk of BC death. Overall, 17.3% of BC cases were triple-negative (TN), 61.8% were Luminal-A, 13.3% were Luminal-B, and 7.5% were HER-2 overexpressed. In the multivariate Cox model, among patients with localized stage, women with TN BC had higher risk of death (adjusted hazard ratio [HR]: 2.57, 95% confidence interval [CI]: 1.29–5.12) as compared to those with Luminal-A status, after adjusting for age at diagnosis. In addition, among women with regional/distant stage at diagnosis, those with TN BC (HR: 5.48, 95% CI: 2.63–11.47) and those HER-2+, including HER-2 overexpressed and Luminal-B, (HR: 2.73, 95% CI:1.30–5.75) had a higher mortality. This is the most comprehensive epidemiological study to date on the impact of hormone receptor expression subtypes in BC survival in Puerto Rico. Consistent to results in other populations, the TN subtype and HER-2+ tumors were associated with decreased survival.
Keywords: Breast cancer, Hispanic, Puerto Rico, subtypes, survival
Introduction
Breast cancer (BC) is the most common female malignancy in Puerto Rico and the United States (US) [1, 2]. Variations in BC occurrence and outcome exist by geographic regions and ethnic background [2–4]. The risk of developing BC is increasing faster in Puerto Rico than among non-Hispanic whites (NHW) in the US [5]. Despite lower incidence rates in Puerto Rico, these are increasing [1, 6], although they have remained stable for Hispanics and NHW [2, 6].
Breast cancer is a multifaceted disease comprising distinct biological subtypes with diverse etiology, therapeutic indications, and clinical outcomes [7, 8]. Human epidermal growth factor 2 (HER-2), estrogen (ER), and progesterone (PR) receptors are the three most common diagnostic markers that drive the clinical management of BC patients. Tumor-cell expression of these receptors has implications for disease progression as well as specific therapeutic interventions [9]. HER-2 overexpression predicts response to treatment, and is associated with more aggressive cancers and worse clinical outcomes, including survival [10]. Patients who are negative for ER and PR do not respond to established endocrine therapy and have poorer prognoses when compared with their ER and PR+ counterparts [11].
Biomarker phenotypes can be grouped into four tumor categories with different histological characteristics: Luminal-A is ER+ and/or PR+/HER-2−, Luminal-B is ER+ and/or PR+/HER-2+, HER-2 overexpressed is ER−/PR−/HER-2+, and triple-negative (TN) is ER−/PR−/HER-2− [10, 12]. The TN subtype is linked to aggressive cancers, metastasis [13], negative clinical outcomes, and is also frequently observed in BRCA1-related BC [14, 15].
Breast cancer development and mortality are also affected by demographic factors, including age [16, 17], race and ethnicity [4, 18, 19], and socio-economic status (SES) [20]. Although African Americans have lower incidence of BC, they have higher mortality from the disease than NHW [21, 22], a disparity that is accounted, in part, to the higher prevalence of TN BC in this group [23–25]. Several studies show that Hispanics have a lower incidence of BC but a higher BC-related mortality rate compared with NHW [6, 26], a finding also observed for Puerto Ricans [6]. Significant differences in the genetics and biology of BC in Hispanics, including a higher incidence of TN BCs [25–27] have been described as a significant contributor to their higher mortality [27]. Nonetheless, these results have not been consistent across studies [28]. A study in Puerto Rico suggests that the clinical outcome in Hispanic women with TN BC is more likely explained by SES and access to services rather than biological/genetic differences [29].
Despite availability of information on the impact of BC molecular subtypes in disease prognosis, these data are still limited for Hispanics. To further understand BC disparities in this group, we evaluated for the first time the association between BC molecular subtypes and other clinical factors with survival among Puerto Rico female BC cases.
Methods
The study population consisted of invasive BC cases diagnosed from 2002 to 2005 at the I. González Martínez Oncologic Hospital and the Auxilio Mutuo Hospital. During this period, 1072 incident cases of BC were identified. We reviewed medical records and pathology reports and extracted data from the cancer registries of both hospitals to collect information on HER-2 (corroborated by the presence of an immunohistochemistry [IHC] pathology report), ER and PR status of study participants, as well as data on age at diagnosis, disease grade, stage, death, and other clinical covariates. Patients with missing information on HER-2 (n = 359) or ER and/or PR status (n = 10), and those with inconclusive HER-2 results (n = 40) were excluded. In total, we analyzed data from 663 female patients at the I. González Martínez Hospital (n = 318) and the Auxilio Mutuo Hospital (n = 345). This study was approved by the Institutional Review Boards of the University of Puerto Rico Medical Sciences Campus and of both hospitals.
Information on tumor receptor expression of cases was obtained from record review of IHC pathological analyses. According to the staining intensity, the pathologist categorized HER-2 status as positive (IHC score = 3+), negative (IHC score = 0, 1+), or equivocal (inconclusive IHC score = 2+, inconclusive cases were excluded from the analyses); ER and PR status as positive or negative. Using this information, patients were categorized into four tumor subtypes, according to their tumor marker status as: (1) TN (HER-2−/ER−/PR−); (2) Luminal-A (HER-2−/ER and/or PR+); (3) Luminal-B (HER-2+/ER and/or PR+); and (4) HER-2 overexpressing (HER-2+, ER−, PR−) [12]. Data on age at diagnosis (<50 years, ≥50 years [cutoff used to differentiate early onset BC]) [30, 31], tumor histology (lobular, ductal, other), size (<2 cm, ≥2 cm), and grade (I, well differentiated; II, moderately well differentiated; III, poorly differentiated; and IV, undifferentiated) were also recorded. Information on stage at diagnosis (localized, regional, distant) was defined according to Surveillance Epidemiology and End Results (SEER) Summary Staging criteria. For cases diagnosed between 2002 and 2005, the SEER Summary Staging 2000 codes were used [32]. Lymph node metastasis (yes, no) at time of diagnosis was assessed, as well as information on vital status at last contact. Information on clinical characteristics of patients and of their date of last contact and vital status were corroborated with the Puerto Rico Central Cancer Registry.
We used descriptive statistics to characterize the study sample. We compared the characteristics associated with BC tumor subtypes among BC patients using chi-square distributions. Survival analysis was performed among patients diagnosed from 2002 to 2005, with the maximum follow-up date being 31 December 2009. Median follow-up time of patients was 24.3 months (minimum: 0.10 months, maximum: 83.2 months).
We described covariates' survival probabilities using the Kaplan–Meier estimators. The survival curves between categories of BC were compared using the Wilcoxon test in order to weight with the population at risk at the most recent time of follow-up; the number of population at risk with long time of follow-up was very small so the survival curves dramatically changed the slope. However, we also assessed the comparison of the survival curves with the log-rank test and the results were similar [33]. We used Cox proportional hazards models to estimate the magnitude of association between BC subtype and risk of death, after adjusting for stage and age at diagnosis through hazard ratio (HR) with 95% confidence interval (CI). We assessed interaction terms within the Cox model using the likelihood ratio test (LRT) and the proportional hazard with the Schoenfeld residual method [33]. Data analysis was performed using Stata 12.
Results
Study population
Median age of diagnosis of patients was 57.0 years (percentile 25: 48.1, percentile 75: 68.0). Overall, 17.3% of cases were TN, 61.8% were Luminal-A, 13.3% Luminal-B, and 7.5% were HER-2 overexpressed. Significant differences in the clinical characteristics studied were observed by BC subtypes (P < 0.05) (Table 1). Comparison of study individuals (n = 663), with those excluded because of missing information for molecular subtype (n = 409) showed that these groups did not differ in any of the clinical characteristics under study (P > 0.05, data not shown).
Table 1.
Clinical characteristics of the study population, overall and by tumor subtype (n = 663)
| Characteristics | Triple-negative HER-2−, ER−, PR− (n = 115, 17.3%) | Luminal-A HER-2−, ER and/or PR+ (n = 410, 61.8%) | Luminal-B HER-2+, ER and/or PR+ (n = 88, 13.3%) | Her-2 overexpressed HER-2+, ER−, PR− (n = 50, 7.5%) | P-value |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Age at diagnosis | |||||
| <50 years | 41 (35.6) | 108 (26.3) | 37 (42.0) | 26 (52.0) | <0.001 |
| ≥50 years | 74 (64.4) | 302 (73.7) | 51 (58.0) | 24 (48.0) | |
| Age at diagnosis (μ ± SD) | 55.8 ± 1.2 | 59.9 ± 0.7 | 56.3 ± 1.5 | 52.6 ± 2.2 | <0.001* |
| Tumor histology (n = 658) | |||||
| Invasive lobular | 4 (3.5) | 94 (23.1) | 13 (14.9) | 1 (2.0) | <0.001** |
| Invasive ductal | 101 (88.6) | 281 (69.0) | 70 (80.5) | 48 (96.0) | |
| Other | 9 (7.9) | 32 (7.9) | 4 (4.6) | 1 (2.0) | |
| Tumor grade (n = 566) | |||||
| I – Well differentiated | 5 (5.0) | 75 (21.9) | 5 (6.8) | 1 (2.2) | <0.001** |
| II – Moderately differentiated | 29 (28.7) | 180 (52.5) | 37 (48.7) | 18 (39.1) | |
| III – Poorly differentiated | 58 (57.4) | 79 (23.0) | 25 (32.9) | 25 (54.3) | |
| IV – Undifferentiated aggressive | 9 (8.9) | 9 (2.6) | 9 (11.8) | 2 (4.3) | |
| Tumor size (n = 544) | |||||
| <2 cm | 31 (30.4) | 189 (55.4) | 30 (44.1) | 10 (30.3) | <0.001 |
| ≥2 cm | 71 (69.6) | 152 (44.6) | 38 (55.9) | 13 (69.7) | |
| LN metastasis (n = 552) | |||||
| Negative | 51 (57.9) | 233 (66.9) | 40 (55.6) | 20 (45.5) | 0.014 |
| Positive | 37 (42.1) | 115 (33.1) | 32 (44.4) | 24 (54.5) | |
| Tumor staging (n = 612) | |||||
| Localized | 62 (59.6) | 248 (65.1) | 43 (55.1) | 24 (49.0) | 0.078 |
| Regional/distant | 42 (40.4) | 133 (34.9) | 35 (44.9) | 25 (51.0) | |
| Hospital | 0.335 | ||||
| Oncologic | 61 (53.0) | 187 (45.6) | 42 (47.7) | 28 (56.0) | 0.335 |
| Auxilio mutuo | 54 (47.0) | 223 (54.4) | 46 (52.3) | 22 (44.0) | |
Table 1 shows significant differences in the characteristics of the study population, by tumor subtypes.
Oneway Anova for comparing means.
Fishers exact test P-value.
Five-year survival
The overall 5-year survival for the entire sample was 71.2%. When stratified by tumor subtype, women with Luminal-A BC had the highest 5-year survival (80.2%) and those TN had the lowest (47.7%) (Table 2).
Table 2.
Five-year survival, overall, and by tumor subtypes
| Tumor subtype | 5-years survival | 95%CI |
|---|---|---|
| Triple-negative (HER-2−, ER−, PR−) | 47.7% | 32.2%–61.6% |
| Luminal-A (HER-2−, ER and/or PR +) | 80.2% | 72.4%–85.9% |
| Luminal-B (HER-2+, ER and/or PR+) | 63.0% | 42.2%–78.1% |
| HER-2 overexpressing (HER-2+, ER−, PR−) | 72.3% | 53.6%–84.5% |
| Overall survival | 71.2% | 64.9%–76.5% |
Table 2 shows that women with TN BC had the lowest 5-year survival.
Factors associated with risk of death
For Cox proportional hazards modeling, women with Luminal-B and HER-2 overexpressing disease were combined into a category called “HER-2+,” given reduced sample size of women with HER-2 overexpressing disease (n = 50) and given that survival curves significantly overlapped between these groups (data not shown). When using these categorizations, the proportional hazard assumption was satisfied (P = 0.80), and we proceeded with bivariate analysis. In Kaplan–Meier survival curves, significant differences in the survival functions of BC subtypes were observed (P < 0.0001). In Cox regression models, women with TN BC (HR: 3.02, 95% CI = 1.94–4.70) and those HER-2+ (HR: 1.79, 95% CI = 1.12–2.87) had higher risk of death as compared to those with Luminal-A disease. Other factors associated with risk of death in bivariate analysis (P < 0.05) included younger age at diagnosis and advanced stage; no differences were observed by tumor histology or by source hospital (P > 0.05) (Table 3).
Table 3.
Hazard ratio (HR) to assess the factors associated with risk of death (n = 663)
| Characteristics | HR crude (95% CI) |
|---|---|
| Breast cancer subtype | |
| Luminal-A (HER-2−, ER and/or PR+) | 1.00 |
| Triple-negative (HER-2−, ER−, PR−) | 3.02 (1.94–4.70) |
| HER-2+ (HER-2+, ER and/or PR±) | 1.79 (1.12–2.87) |
| Age at diagnosis | |
| <50 years | 1.57 (1.02–2.43) |
| ≥50 years | 1.00 |
| Tumor staging (n = 573) | |
| Localized | 1.00 |
| Regional/distant | 2.35 (1.58–3.48) |
| Tumor histology (n = 658) | |
| Invasive lobular | 1.00 |
| Invasive ductal | 1.68 (0.90–3.16) |
| Other | 2.46 (1.02–5.94) |
| Tumor grade (n = 566) | |
| I – Well differentiated | 1.00 |
| II – Moderately differentiated | 3.86 (1.20–12.46) |
| III – Poorly differentiated | 5.71 (1.77–18.46) |
| IV – Undifferentiated aggressive | 2.39 (0.40–14.33) |
| Tumor size (n = 544) | |
| <2 cm | 1.00 |
| ≥2 cm | 1.70 (1.06–2.70) |
| LN metastasis (n = 552) | |
| Negative | 1.00 |
| Positive | 2.12 (1.37–3.27) |
| Hospital | |
| Oncologic hospital | 1.00 |
| Auxilio mutuo | 1.18 (0.80–1.75) |
Table 3 presents the results from the crude Cox proportional hazards models showing that HER-2+ women and those with TN BC have increased risk of death as compared to those Luminal-A. Other factors associated to risk of death in bivariate analyses included age at diagnosis <50 years, regional/distant stage, and lymph node metastasis.
The initial Cox multivariate model considered the association between BC subtype and risk of death, after adjusting for stage and age at diagnosis. Given the presence of significant interaction terms in the Cox model (LRT P < 0.001), the survival analysis was performed separately in each stage, adjusting for age at diagnosis. In the Cox model among patients with localized stage, women with TN BC had higher risk of death (HR: 2.57, 95% CI = 1.29–5.12) as compared to those with Luminal-A status (Table 4). Meanwhile, among women with regional/distant staging, those with TN BC (HR: 5.48, 95% CI: 2.63–11.47) and HER-2+ (HR: 2.73, 95% CI = 1.30–5.75) had increased risk of death as compared to those with Luminal-A disease (Fig. 1A and B).
Table 4.
Hazard ratio (HR) to assess the factors associated with risk of death*
| Stage | ||
|---|---|---|
| Localized (n = 377) | Regional/distant (n = 196) | |
| Characteristics | HR adjusted (95% CI) | |
| Breast cancer subtype | ||
| Luminal-A (HER-2−, ER and/or PR +) | 1.00 | 1.00 |
| Triple-negative (HER-2−, ER−, PR−) | 2.57 (1.29–5.12) | 5.48 (11.47) |
| HER-2+ (HER-2+, ER and/or PR±) | 1.36 (0.60–3.09) | 2.73 (1.26–5.75) |
| Age at diagnosis | ||
| <50 years | 2.34 (1.06–5.13) | 1.85 (0.97–3.56) |
| ≥50 years | 1.00 | 1.00 |
Proportional hazards assumption was evaluated and found satisfied (staging localized: P = 0.6849; staging regional/distant: P = 0.8345) after stratification.
Table 4 shows that in both staging categories, women with TN BC had higher risk of death as compared to those with Luminal-A status, after adjusting for age at diagnosis.
Figure 1.
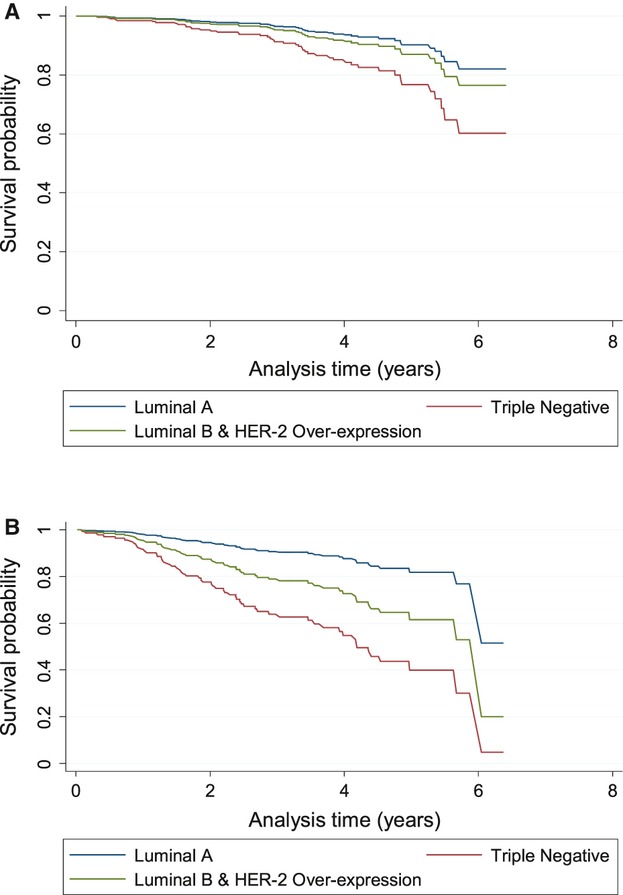
(A) Age-adjusted survival curves for women with localized stage at time of diagnosis, by breast cancer subtype. (B) Age-adjusted survival curves for women with regional/distant stage at time of diagnosis, by breast cancer subtype.
Discussion
This study describes the influence of BC molecular subtypes on risk of death in a Hispanic population. Although the distribution of molecular subtypes in our clinic-based study may not be representative of Puerto Rico, our estimates have similarities and contrasts with those from other US populations. Our TN status (17.3%) results are similar to those for NHW (16.7%) and lower than African Americans estimates (24.6%) [34], although higher than those described for Hispanics (10.7%) in another study [12]. Our estimates for HER-2 positivity were similar to previous data for Hispanics (Luminal-B: 13.3% vs. 14.2% and HER-2 overexpressing: 7.5% vs. 6.6%) [12, 35]. Meanwhile, our results for TN status are somewhat similar to those overall (13.1%) and for Hispanics (17.6%) in a big US cohort [36], while our estimates of HER-2 positivity are similar to theirs (Luminal-B: 15.5% and HER-2 overexpressed: 7.2%) and specifically to their Hispanic population (Luminal-B: 16.4% and HER-2 overexpressed: 9.7%) [36].
Our study reveals differences in the clinical characteristics of patients with different molecular subtypes, with a higher proportion of women with TN status and HER-2+ having larger tumor size, advanced stage at diagnosis and lymph node metastasis. Also, the highest proportion of cases <50 years was observed among those HER-2+ and those with TN BC, as compared to those with Luminal-A BC. This is consistent with previous studies that have documented younger age at diagnosis, larger tumor size, lymphovascular invasion and higher grade at diagnosis among TN BC cases [37–40] and those HER-2+ [37, 41, 42] as compared to those with Luminal-A disease.
Our overall 5-year survival estimates (71.2%) are lower than those of US [28, 36] women and other populations [43, 44]. Meanwhile, similar to previous studies, women with Luminal-A BC (80.2%) had the highest 5-year survival [36] and those with TN disease had the lowest 5-year survival (47.7%) [43, 45]. Nonetheless, some studies [28, 36] have also observed lowest survival among patients with HER-2 overexpression, similar to that of TN cases, a pattern that was not observed in our study and that could not be assessed in multivariate analyses given the small proportion of HER-2 overexpressed tumors.
The clinical correlates of BC survival in this population are also similar to those described previously in the US. In stratified analysis, our results showed that among women with regional/distant stage at diagnosis, those with HER-2+ had more than twofold higher risk of death, when compared to those in the Luminal-A group. Nonetheless, among those with localized disease, this excess risk of death was not observed for women HER-2+. HER-2 receptor overexpression has been clearly linked to a greater risk of tumor recurrence after initial remission [32, 45], as well as diminished survival [34, 46]. The inclusion of trastuzumab in the initial treatment of BC patients with HER-2+ tumors has changed the recurrence rate and survival of these patients. In Puerto Rico, Herceptin had been available since its initial launch in 1998. It was approved for treatment of metastatic HER-2+ BC. This intervention most probably affected the survival of some of these patients. However, in 2006, trastuzumab was also approved by the Food and Drug Administration for adjuvant treatment of BC patients. As a result, the patients included in this study were not treated in the adjuvant setting. Future studies evaluating the outcome of treatment of HER-2+ patients will determine whether adjuvant trastuzumab affects patient survival. While we do not have complete data on initial treatment regimen, it is possible that the known risk of adverse outcomes of HER-2 overexpressing tumors, coupled with the availability of effective therapeutic agents to treat these HER-2+ tumors [47] may be causing physicians to treat these tumors more quickly and aggressively than others. This may lead to improved outcomes for these patients, including greater survival, and may partly explain why HER-2 overexpression showed no association with mortality in our sample of women with localized disease.
Meanwhile, consistent with previous studies [28, 48, 49] among women with both localized and regional/distant stage at diagnosis, those with TN BC had 3–5 times increased risk of death as compared to those with Luminal-A disease. Poor prognosis of patients with TN BC was also seen in another study in Puerto Rico [29]. Also, as in previous studies [16, 20, 28, 34, 50], patients with age at diagnosis <50 years had increased risk of death.
Similar with results in other populations, the TN subtype was associated with decreased survival time [24, 25] and the Luminal-A subtype was associated with the greatest survival [28]. Given that studies suggest that the TN subtype is more common among Hispanics than among NHW [26], these results highlight the importance of HER-2 and hormone receptor screening in this population.
Future studies should aim to further elucidate other socio-economic and cultural factors that may impact BC survival in Puerto Rico. This is important given that 41.5% of Puerto Ricans live at or below poverty line [51] and given the significant financial burden of a BC diagnosis [3]. It is also pertinent to evaluate the effect of lifestyles and other clinical factors on BC survival in this population, and their potential interactions with BC molecular subtypes.
Our study may be limited by selection bias, as 38.2% of BC cases were excluded because of missing information on HER-2 or ER/PR receptors. However, study individuals did not differ from those excluded in any of the clinical characteristics studied. The absence of this information in medical records is of concern given that routine HER-2 and ER/PR receptor screening recommendations for treatment decision were established prior to the study period [52, 53]. Also, our hospital-based data may not be representative of all BC cases in Puerto Rico.
This is the most comprehensive study to date on the impact of BC molecular subtypes on BC survival in Puerto Rico. Consistent with other populations, the TN subtype was associated with decreased survival, even when stratified by tumor staging. Furthermore, HER-2+ cases with localized disease also had higher risk of death as compared to those with Luminal-A BC. Given the observed biological differences in BC between racial/ethnic groups [17] and the recognized need for additional studies on BC subtypes [54], our study contributes to this area by revealing the survival disadvantage of women with these molecular subtypes in a population of Hispanic origin. Considering that targeted treatments exist for these tumor subtypes, our results highlight the need for more effective management of TN and HER-2+ cancers in Puerto Rico, including phenotype specific BC control strategies.
Acknowledgments
We acknowledge the contribution of Yarí Valle, Paula Ortiz-Bachier, Joanne Díaz-Rodríguez, Anjanet Pérez, Arelis Santana, Adriana Padilla, and Daphne Díaz-Vázquez in data collection.
Conflict of Interest
None declared.
References
- 1.Figueroa-Vallés NR, Ortiz-Ortiz KJ, Pérez-Ríos N, Villanueva-Rosa E, Traverso-Ortiz M, Torres-Cintrón CR, et al., editors. Cancer in Puerto Rico, 2004–2009. San Juan, Puerto Rico: Puerto Rico Central Cancer Registry; 2012. [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al. Bethesda, MD: National Cancer Institute; 2012. SEER cancer statistics review, 1975–2009 (vintage 2009 populations) Available at http://seer.cancer.gov/csr/1975_2009_pops09/ (accessed on November 17, 2011) [Google Scholar]
- 3.Torres-Cintron M, Ortiz AP, Perez-Irizarry J, Soto-Salgado M, Figueroa-Valles NR, Dela Torre-Feliciano T, et al. Incidence and mortality of the leading cancer types in Puerto Rico: 1987–2004. P. R. Health Sci. J. 2010;29:317–329. [PubMed] [Google Scholar]
- 4.Jack RH, Davies EA, Renshaw C, Tutt A, Grocock MJ, Coupland MH, et al. Differences in breast cancer hormone receptor status in ethnic groups: a London population. Eur. J. Cancer. 2012;49:696–702. doi: 10.1016/j.ejca.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Nazario CM, Figueroa-Valles N, Rosario RV. Breast cancer patterns and lifetime risk of developing breast cancer among Puerto Rican females. P. R. Health Sci. J. 2000;19:7–13. [PubMed] [Google Scholar]
- 6.Ortiz AP, Soto-Salgado M, Calo W, Nogueras G, Figueroa-Valles N, Suarez E, et al. Disparities in breast cancer in Puerto Rico and among Hispanics, non-Hispanic whites, and non-Hispanics blacks in the United States, 1992-2004. Breast J. 2010;16:666–668. doi: 10.1111/j.1524-4741.2010.00990.x. doi: 10.1111/j.1524-4741.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg S, Stopeck A, Rugo HS. Systemic treatment of early breast cancer? A biological perspective. J. Surg. Oncol. 2011;103:619–626. doi: 10.1002/jso.21842. doi: 10.1002/jso.21842. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann M, Pusztai L the Biedenkopf Expert Panel Members. Use of standard markers and incorporation of molecular markers into breast cancer therapy. Cancer. 2011;117:1575–1582. doi: 10.1002/cncr.25660. doi: 10.1002/cncr.25660. [DOI] [PubMed] [Google Scholar]
- 9.Liedtke C, Kiesel L. Breast cancer molecular subtypes – modern therapeutic concepts for targeted therapy of a heterogeneous entity. Maturitas. 2012;73:288–294. doi: 10.1016/j.maturitas.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 10.Brown M, Tsodikov A, Bauer KR, Parise CA, Caggiano V. The role of human epidermal growth factor receptor 2 in the survival of women with estrogen and progesterone receptor-negative, invasive breast cancer: the California cancer registry, 1999-2004. Cancer. 2008;112:737–747. doi: 10.1002/cncr.23243. doi: 10.1002/cncr.23243. [DOI] [PubMed] [Google Scholar]
- 11.Brady-West DC, McGrowder DA. Triple negative breast cancer: therapeutic and prognostic implications. Asian Pac. J. Cancer Prev. 2011;12:2139–2143. [PubMed] [Google Scholar]
- 12.Kwan ML, Kushi LH, Weltzien E, Maring B, Cutner SE, Fulton RS, et al. Epidemiology of breast cancer subtypes in two prospective cohort studies of breast cancer survivors. Breast Cancer Res. 2009;11:R31. doi: 10.1186/bcr2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishihara A, Tsuda H, Kitagawa K, Yoneda M, Shiraishi T. Morphological characteristics of basal-like subtype of breast carcinoma with special reference to cytopathological features. Breast Cancer. 2009;16:179–185. doi: 10.1007/s12282-009-0108-x. [DOI] [PubMed] [Google Scholar]
- 14.Livasy CA, Karaca G, Nanda R, Tretiakova MS, Moore DT, Perou CM, et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod. Pathol. 2006;19:264–271. doi: 10.1038/modpathol.3800528. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee S, Reis-Filho JS, Ashley S, Steele D, Ashworth A, Lakhani SR, et al. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J. Clin. Pathol. 2006;59:729–735. doi: 10.1136/jcp.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K, Chie EK, Han W, Noh DY, Oh DY, Im SA, et al. Age 40 years is an independent prognostic factor predicting inferior overall survival in patients treated with breast conservative therapy. Breast J. 2011;17:75–78. doi: 10.1111/j.1524-4741.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- 17.Jatoi I, Anderson WF. Qualitative age interactions in breast cancer studies: a mini-review. Future Oncol. 2010;6:1781–1788. doi: 10.2217/fon.10.139. [DOI] [PubMed] [Google Scholar]
- 18.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Lane D, Dolan NC, et al. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. J. Natl. Cancer Inst. 2005;97:439–448. doi: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 19.Haque R, Ahmed S, Schottinger J, Kwan M, Shi J, Chung J, et al. PS1-05: disparities in breast cancer survival by molecular subtype and race/ethnicity. Clin. Med. Res. 2012;10:145. [Google Scholar]
- 20.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. J. Womens Health (Larchmt) 2009;18:883–893. doi: 10.1089/jwh.2008.1127. [DOI] [PubMed] [Google Scholar]
- 21.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J. Natl. Cancer Inst. 2009;101:984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lepeak L, Tevaarwerk A, Jones N, Williamson A, Cetnar J, LoConte N. Persistence in breast cancer disparities between African Americans and whites in Wisconsin. WMJ. 2011;110:21–25. [PMC free article] [PubMed] [Google Scholar]
- 23.Morris GJ, Naidu S, Topham AK, Guiles F, Xu Y, McCue P, et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: a single-institution compilation compared with the National Cancer Institute's Surveillance, Epidemiology, and End Results database. Cancer. 2007;110:876–884. doi: 10.1002/cncr.22836. [DOI] [PubMed] [Google Scholar]
- 24.Amirikia KC, Mills P, Bush J, Newman LA. Higher population-based incidence rates of triple-negative breast cancer among young African-American women: implications for breast cancer screening recommendations. Cancer. 2011;117:2747–2753. doi: 10.1002/cncr.25862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 26.Patel TA, Colon-Otero G, Bueno Hume C, Copland JA, III, Perez EA. Breast cancer in Latinas: gene expression, differential response to treatments, and differential toxicities in Latinas compared with other population groups. Oncologist. 2010;15:466–475. doi: 10.1634/theoncologist.2010-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lara-Medina F, Perez-Sanchez V, Saavedra-Perez D, Blake-Cerda M, Arce C, Motola-Kuba D, et al. Triple-negative breast cancer in hispanic patients: high prevalence, poor prognosis, and association with menopausal status, body mass index, and parity. Cancer. 2011;117:3658–3669. doi: 10.1002/cncr.25961. [DOI] [PubMed] [Google Scholar]
- 28.Carey LA, Perou CM, Livasy CA, Dressier LG, Cowan D, Conway K, et al. Race, breast cancer subtypes, and survival in the carolina breast cancer study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 29.Giraldo-Jimenez MY, Cabanillas F, Negron V, Echenique M, Mojica P, Santiago K, et al. Triple negative breast cancer: a retrospective study of Hispanics residing in Puerto Rico. P. R. Health Sci. J. 2012;31:45–51. [PubMed] [Google Scholar]
- 30.Pal T, Bonner D, Kim J, Monteiro AN, Kessler N, Royer R, et al. Early onset breast cancer in a registry-based sample of african-american women: BRCA mutation prevalence, and other personal and system-level clinical characteristics. Breast J. 2013;19:189–192. doi: 10.1111/tbj.12083. [DOI] [PubMed] [Google Scholar]
- 31.Ademuyiwa FO, Groman A, Hong C-C, Kumar S, Levine EG, Miller A, et al. Time-trends in survival in young women with breast cancer in a SEER population-based study. Cancer Res. 2012;72(Suppl. 24) doi: 10.1007/s10549-013-2425-1. P3-07-02. [DOI] [PubMed] [Google Scholar]
- 32.Young JL, Jr, Roffers SD, Reis LAG, Fritz AG, Hurlbut AA. SEER summary staging manual – 2000 codes and coding instructions (SSS2000) Bethesda, MD: National Cancer Institute; 2001. NIH Publication No. 01-4969. [Google Scholar]
- 33.Hosmer D, Lemeshow S, May S. Applied survival analysis: regression modeling of time-to-event data. 2nd ed. New Jersey: John Wiley & Sons; 2008. [Google Scholar]
- 34.Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12:R99. doi: 10.1186/bcr2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortiz AP, Frıas O, Gonzalez-Keelan C, Suarez E, Capo D, Perez J, et al. Clinicopathological factors associated to her-2 status in a hospital-based sample of breast cancer patients in Puerto Rico. P. R. Health Sci. J. 2010;3:265–271. [PMC free article] [PubMed] [Google Scholar]
- 36.Parise CA, Bauer KR, Caggiano V. Variation in breast cancer subtypes with age and race/ethnicity. Crit. Rev. Oncol. Hematol. 2010;76:44–52. doi: 10.1016/j.critrevonc.2009.09.002. doi: 10.1016/j.critrevonc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Ibrahim E, Al-Gahmi AM, Zeenelin AA, Zeckri JM, Elkhodary TR, Gaballa HE, et al. Basal vs. luminal A breast cancer subtypes: a matched case-control study using estrogen receptor, progesterone receptor, and HER-2 as surrogate markers. Med. Oncol. 2009;26:372–378. doi: 10.1007/s12032-008-9131-6. doi: 10.1007/s12032-008-9131-6. [DOI] [PubMed] [Google Scholar]
- 38.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13(15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen PL, Taghian AG, Katz MS, Niemierko A, Abi Raad RF, Boon WL, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J. Clin. Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 40.Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008;52:108–118. doi: 10.1111/j.1365-2559.2007.02889.x. doi: 10.1111/j.1365-2559.2007.02889.x. [DOI] [PubMed] [Google Scholar]
- 41.Menard S, Balsari A, Casalini P, Tagliabue E, Campiglio M, Bufalino R, et al. HER-2-positive breast carcinomas as a particular subset with peculiar clinical behaviors. Clin. Cancer Res. 2002;8:520–525. [PubMed] [Google Scholar]
- 42.Pal SK, Childs BH, Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res. Treat. 2011;125:627–636. doi: 10.1007/s10549-010-1293-1. doi: 10.1007/s10549-010-1293-1; 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue C, Wang X, Peng R, Shi Y, Qin T, Liu D, et al. Distribution, clinicopathologic features and survival of breast cancer subtypes in Southern China. Cancer Sci. 2012;103:1679–1687. doi: 10.1111/j.1349-7006.2012.02339.x. doi: 10.1111/j.1349-7006.2012.02339.x; 10.1111/j.1349-7006.2012.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaha DC, Lazar E, Lazureanu C. Clinicopathologic features and five years survival analysis in molecular subtypes of breast cancer. Rom. J. Morphol. Embryol. 2010;51:85–89. [PubMed] [Google Scholar]
- 45.Park YH, Lee S, Cho EY, Choi YL, Lee JE, Nam SJ, et al. Patterns of relapse and metastatic spread in HER2-overexpressing breast cancer according to estrogen receptor status. Cancer Chemother. Pharmacol. 2010;66:507–516. doi: 10.1007/s00280-009-1190-7. [DOI] [PubMed] [Google Scholar]
- 46.Brouckaert O, Wildiers H, Floris G, Neven P. Update on triple-negative breast cancer: prognosis and management strategies. Int. J. Womens Health. 2012;4:511–520. doi: 10.2147/IJWH.S18541. doi: 10.2147/IJWH.S18541; 10.2147/IJWH.S18541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burstein HJ, Keshaviah A, Baron AD, Hart RD, Markom PK, Gelman R, et al. Trastuzumab plus vinorelbine or taxane chemotherapy for HER2-overexpressing metastatic breast cancer: the trastuzumab and vinorelbine or taxane study. Cancer. 2007;110:965–972. doi: 10.1002/cncr.22885. [DOI] [PubMed] [Google Scholar]
- 48.Ohzawa H, Sakatani T, Niki T, Yasuda Y, Hozumi Y. Pathological responses and survival of patients with human epidermal growth factor receptor 2-positive breast cancer who received neoadjuvant chemotherapy including trastuzumab. Breast Cancer. 2012 doi: 10.1007/s12282-012-0424-4. doi: 10.1007/s12282-012-0424-4. [DOI] [PubMed] [Google Scholar]
- 49.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin. Med. Res. 2009;7:4–13. doi: 10.3121/cmr.2009.825. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vona-Davis L, Rose DP, Hazard H, Howard-McNatt M, Adkins F, Partin J, et al. Triple-negative breast cancer and obesity in a rural appalachian population. Cancer Epidemiol. Biomarkers Prev. 2008;17:3319–3324. doi: 10.1158/1055-9965.EPI-08-0544. doi: 10.1158/1055-9965.EPI-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Negociado del Censo Federal, Censo de Población y Vivienda de. 2010. Puerto Rico; Perfil Demográfico 3, Perfil de Características Económicas Seleccionadas (DP3); y Junta de Planificación. Programa de Planificación Económica y Social, Oficina del Censo.
- 52.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007;131:18–43. doi: 10.5858/2007-131-18-ASOCCO. doi: 2. [DOI] [PubMed] [Google Scholar]
- 53.Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Oncol. Pract. 2010;6:195–197. doi: 10.1200/JOP.777003. doi: 10.1200/JOP.777003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Ann. Oncol. 2012;23(Suppl. 6):vi7–vi12. doi: 10.1093/annonc/mds187. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]


