Abstract
Measures shown to improve the adenoma detection during colonoscopy (excellent bowel preparation, cecal intubation, cap fitted colonoscope to examine behind folds, patient position change to optimize colon distention, trained endoscopy team focusing on detection of subtle flat lesions, and incorporation of optimum endoscopic examination with adequate withdrawal time) are applicable to clinical practice and, if incorporated are projected to facilitate comprehensive colonoscopy screening program for colon cancer prevention. To determine adenoma and serrated polyp detection rate under conditions designed to optimize quality parameters for comprehensive screening colonoscopy. Retrospective analysis of data obtained from a comprehensive colon cancer screening program designed to optimize quality parameters. Academic medical center. Three hundred and forty-three patients between the ages of 50 years and 75 years who underwent first screening colonoscopy between 2009 and 2011 among 535 consecutive patients undergoing colonoscopy. Comprehensive colonoscopy screening program was utilized to screen all patients. Cecal intubation was successful in 98.8% of patients. The Boston Bowel Preparation Scale for quality of colonoscopy was 8.97 (95% confidence interval [CI]; 8.94, 9.00). The rate of adenoma detection was 60% and serrated lesion (defined as serrated adenomas or hyperplastic polyps proximal to the splenic flexure) detection was 23%. The rate of precancerous lesion detection (adenomas and serrated lesions) was 66%. The mean number of adenomas per screening procedure was 1.4 (1.2, 1.6) and the mean number of precancerous lesions (adenomas or serrated lesions) per screening procedure was 1.6 (1.4, 1.8). Retrospective study and single endoscopist experience. A comprehensive colonoscopy screening program results in high-quality screening with high detection of adenomas, advanced adenomas, serrated adenomas, and multiple adenomas.
Keywords: Adenoma, colon, colonoscopy, detection, serrated adenoma
Introduction
Colonoscopic removal of adenomatous colon polyps has been shown to reduce the incidence of colorectal cancer and also to prevent death from colorectal cancer [1, 2]. Although colonoscopy is widely used for colorectal cancer screening, its miss rate for cancer and that for adenomas remains a concern [3–5]. The adenoma detection rate is an independent predictor of the risk of interval cancer and the ability of colonoscopy to reduce the incidence of colorectal cancer depends on the removal of adenomas [1, 6]. Recently, measurement of adenoma detection has been included in quality-improvement programs for colorectal cancer screening [7, 8]. The US Multi-Society Task Force on Colorectal Cancer has established target adenoma detection rates of >25% for men, and >15% for women undergoing screening colonoscopy [9]. Recently, the NHS Bowel Cancer Screening Program in the United Kingdom recommended target adenoma detection rate of ≥40% and mean adenoma detection per patient of 1.20 in patients [10].
The National Polyp Study predicted reduction in the incidence of colorectal cancer and colorectal cancer deaths in an adenoma-bearing cohort that had undergone clearing colonoscopy [1, 2]. However, recent dietary and chemoprevention trials suggest a lower level of protection [3, 11, 12]. Potential causes for colonoscopy failure to prevent colorectal cancer include the following: (a) inadequate bowel preparation [13–16], (b) failure to reach cecum [17, 18], (c) abbreviated withdrawal of colonoscope [19], (d) failure to examine polyps hidden behind folds [20–23], (e) inability to recognize subtle flat lesions such as sessile serrated adenomas [24–26], (f) incomplete polypectomy [5], (g) failure to provide interval surveillance colonoscopy program in patients with adenomas [27], and (g) biological differences in colorectal neoplasia growth [28].
From the standpoint of the practicing endoscopist who performs high-quality screening colonoscopy, a number of measures are applicable to clinical practice in order to (a) provide for comprehensive colonoscopy screening and (b) improve the quality of the procedure [29, 30]. These include achieving excellent quality of bowel preparation using a split dose bowel preparation [31–35], reaching the cecum to screen the entire length of the colon [18], taking adequate time to examine the colon [19], using a good technique to circumferentially examine a distended colon with a cap fitted colonoscope [36–39], using a high-definition endoscope with a trained eye to detect subtle flat lesions [24, 40], and reporting the colonoscopy findings using a quality structured reports [41].
To maximize the effectiveness of colonoscopy as a prevention tool, an endoscopist (G. S. R.) incorporated multiple quality measures into clinical practice to develop a comprehensive colonoscopy screening program between 2001 and 2008 [42]. Although an expert panel recognized the importance of detection of serrated lesions, it is not clear whether this comprehensive colonoscopy screening program has an impact on serrated lesion detection [43]. Analysis of the data from this experience was undertaken to determine the adenoma detection and serrated lesion detection rate in a cohort of 343 consecutive patients between the ages of 50 years and 75 years who had undergone their first screening colonoscopy using comprehensive colonoscopy screening program by a single endoscopist (G. S. R.) between 2009 and 2011 at a single cancer center.
Material and Methods
Patients
All patients referred for their first screening colonoscopy between June 2009 and December 2011 who did not have a family or personal history of polyposis, inflammatory bowel disease, or a personal history of prior polypectomy or colorectal cancer were included in the retrospective study. The Institutional Review Board at the University of Texas MD Anderson Cancer Center approved the study. A single endoscopist (G. S. R.) performed all the procedures using a comprehensive colonoscopy screening program under conscious sedation (Table 1).
Table 1.
Comprehensive colonoscopy screening program
| Colon preparation | Split dose of polyethylene glycol electrolyte solution and bisacodyl in divided doses [32] (see Video S1) |
| Colonoscope | Cap fitted colonoscope [38] |
| Cleaning on insertion | Simethicone solution flush followed by suction to achieve BBPS of three in each segment before endoscope insertion into the cecum , [35] |
| Endoscope insertion | Colonoscope insertion to the cecum at 60–80 cm |
| Endoscopic screening | Cap fitted endoscope to examine in between folds [38] |
| Patient position change to optimize colon distension [37] | |
| Circumferential scanning for subtle flat lesions of the colon by focusing on disturbance to the mucosal innominate groove pattern, surface pit pattern, and vascular architecture [36] | |
| Training the eyes by reviewing the videos and photos of subtle lesions and the endoscopy team focus on finding subtle lesions [47, 48] | |
| Endoscopic report | Standardized colonoscopy reporting and data system for comprehensive recording of the procedure details [49]. |
| Follow-up | Counsel about pathology findings and surveillance colonoscopy intervals |
BBPS, Boston Bowel Preparation Scale.
Comprehensive colonoscopy screening program
The comprehensive colonoscopy screening program is summarized in Table 1.
Intensive bowel preparation regimen for colon preparation
All patients were instructed about colon preparation and were encouraged to view patient education videos developed of our institution on the YouTube (see Video S1). The protocol included six bisacodyl tablets in divided doses and 4 L of polyethylene glycol (PEG)-based electrolyte solution in a split dose. It consisted of a light breakfast and lunch without vegetables, salads, fruits and nuts, two bisacodyl tablets (5 mg) at noon, followed by another dose at 3.30 pm and 2 L of PEG solution on the day before the procedure. Those scheduled to have a procedure before noon were instructed to take two tablets of bisacodyl and 2 L of the PEG solution in the early morning 3 am, while those scheduled to have a procedure in the afternoon were instructed to take two tablets of bisacodyl and 2 L of the PEG solution at 7 am [32].
Colonoscopy screening
Setup of video monitor
The video monitor was setup about 4–5 feet from the endoscopist at or below the endoscopist's eye level [44]; usually this amounts to keeping the monitor above the bed rail on the other side of the patient.
Cap fitted colonoscopy
Either a cap fitted pediatric or adult variable stiffness colonoscope of the Olympus 160 series, with a fixed 140° angle of view was used for screening. The cap extended 2–3 mm from the tip of the endoscope [38]. Carbon dioxide was used only if the intubation through the sigmoid colon became technically challenging and if the patient required endoscopic mucosal resection. Conscious sedation was used for the procedures.
Clean and dry the colon during colonoscope insertion
During the endoscope insertion, simethicone mixed water was routinely flushed in the left, transverse, and right colon and all the fluid was aspirated to create a dry colon before endoscope insertion to the cecum so that on withdrawal a clean dry colon (Boston Bowel Preparation Scale of 3 of 3 in each segment) was encountered for examination without the need for additional water flushes and suction [34, 35].
Cecal insertion without loops
Every attempt was made to reach the cecum, unless the endoscopist felt that it was risky and futile to pursue further. The colon loops were reduced constantly during the endoscope insertion so that the cecum could ideally be reached at 60–80 cm. A short straight endoscope allowed controlled withdrawal of the endoscope and circumferential scanning of the colon.
Screening – adequate distension of the colon
The patient's position was changed from left lateral to supine or right lateral position to allow adequate distension of the colon [37].
Screening – working the folds to pick up polyps behind folds
The colonoscopic screening involved working the folds with the cap to examine behind the ileo-cecal valve and in between the haustral folds. The cap was pushed against the colon wall to open up the mucosa if the folds were crowded [36].
Screening for subtle lesions – training the eye for subtle lesions
The endoscopist trained his eyes to detect subtle lesions initially by looking at the endoscopy atlases and videos on the subject [45–47], working closely with an expert in the field sharing and reviewing videos of subtle flat lesions on the web and at the American Society Gastrointestinal Endoscopy Courses on Colonoscopy. The endoscopist (G. S. R.) trained his endoscopy technicians by reviewing photos and videos of subtle lesions [47].
Screening for subtle lesions – technique to look for subtle lesions
The endoscope was withdrawn by keeping the endoscope close to the wall, as the endoscope worked the folds circumferentially and with to-and-fro movements, so that the subtle changes in the architecture of the innominate grooves, mucosal vasculature, and surface pit pattern could be closely observed with white light to identify subtle lesions [36]. Dye spray chromoendoscopy and narrow band imaging were not used to detect lesions. If any mucus was encountered, it was washed and the area was closely examined for serrated polyps. When a second pair of eyes (trained endoscopy technicians and nurses) spotted a lesion, that area was closely examined. Once all members of the team agreed about the presence or absence of a finding and it was appropriately dealt with, the endoscopist continued with examination of the rest of the colon. Video recording of all subtle lesions and all large lesion resections were routinely undertaken. Photos and videos were reviewed with the team to educate the team in finding subtle lesions. If polyps were found during insertion, they were removed at that time [48].
Screening time
The goal was to screen the colon and whatever time was required to screen the entire colon depending on the individual anatomy for any subtle lesion (see Video S2) was taken (a minimum of 6 min of screening time is required as part of the quality initiative of our endoscopy unit). The endoscopy unit at our institution allowed scheduling an hour for colonoscopies to deal with removal of all polyps, including large and flat lesion resections, and completion of a detailed procedure note and counseling of the patients and family about the findings of the procedure.
Polyp removal
Cold biopsy was used to remove smaller lesions (<5 mm) and endoscopic mucosal resection was used for lesions larger than 5 mm. All the lesions, including the large ones were removed during the same session. Each polyp was submitted in a separate jar with accurate labeling of the jar to correspond with the polyp removed and documented in the endoscopy report for pathological correlation and assessment of polyp burden.
Endoscopy report
Based on the recommendations of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable [49], we developed a standardized colonoscopy reporting system (Endoworks, Olympus Inc., Center Valley, PA) that provided for data entry and patient report generation. Two to three photos of the appendicular orifice, cecum, and ileocecal valve were taken to document cecal insertion. The Boston Bowel Preparation Scale was used to define the quality of colon preparation; photos were taken to document the quality of preparation [35]. Each polyp was submitted in a separate jar for pathology to help in the counting of the polyp burden.
Pathology of polyps
One of our dedicated gastrointestinal pathologists reviewed the pathology slides and reported the findings using standard terminology, including a comment on the completeness of resection of the large polyps.
Follow-up
The endoscopist contacted all the patients by phone 1–2 weeks after the procedure, followed by a letter that included counseling about biopsy findings and recommendations of surveillance examination based on the American Society of Gastrointestinal Endoscopy (ASGE) guidelines. Patients with no adenomas during the screening colonoscopy were recommended to reevaluate their risk for colon cancer at 5 years based on their family history and personal health and risk factors.
Data-collection and statistical analysis
The patients' demographics, medical, surgical, and cancer illnesses were retrieved from the hospital electronic medical record and the endoscopy data were retrieved from the colonoscopy report database and entered into an ACCESS Colonoscopy Database.
Nearly half of the patients in our cohort had a history of cancer (for example, breast cancer, prostate cancer, skin cancer, etc.). To evaluate the impact of cancer history on adenoma detection, the cohort was divided into those with history of any cancer “the cancer group” and those with no history of cancer, “the non-cancer group” and the results in both groups were compared.
Statistical analysis
Patients were selected from the overall screening colonoscopy database to meet the criteria of having their first screening colonoscopy with average risk between the ages of 50 years and 75 years. Patient characteristics and colonoscopy elements were tabulated overall and by cancer history. Colonoscopy experience was described by time in minutes and successful insertion and preparation. Among this defined population, each polyp found was classified as one of the following: cancer, precancerous (adenoma, serrated polyp, or both), or other (including non-neoplastic lesions and distal hyperplastic polyps). Specific definitions for detection rates and polyp burden are detailed in Table 2. Polyp counts for each type were summarized with counts and proportion for each patient and overall. Histograms of polyp counts were created overall and for men and women separately. The overall distribution of polyp types by cancer history was tested by the Jonkheere-Terpstra test as implemented in StatXact v8 (Cytel Inc., Cambridge, MA) to account for the natural orderings of polyp severity and cancer history. For individual comparisons of proportions, chi-square tests were implemented. For continuous measures, mean and 95% CI were reported. Comparisons between means were made with t-tests. All data analyses were performed in SAS 9.2 (SAS Institute, Inc., Cary, NC) unless otherwise noted. Plots were created in Stata/SE 12.1 (StataCorp LP, College Station, TX).
Table 2.
Definitions of adenoma detection rate and adenoma burden
| Adenoma detection rate | |
| Adenoma detection rate | Percentage of patients with tubular, tubullovillous, or villous adenomas |
| Advanced adenoma detection rate | Percentage of patients with advanced adenomas (>1 cm in size, villous histology, high-grade dysplasia) |
| Multiple adenoma detection rate | Percentage of patients with ≥3 adenomas |
| Serrated lesion detection rate | Percentage of patients with serrated adenomas or hyperplastic polyps proximal to splenic flexure |
| Precancerous lesion detection rate | |
| Precancerous lesion detection rate | Percentage of patients with adenomas or serrated lesions |
| Adenoma burden | |
| Mean number of adenomas per screening procedure | Total number of adenomas detected divided by the number of screening procedures |
| Mean number of adenomas per screening procedure positive for precancerous lesions | Total number of adenomas detected divided by the number of screening procedures positive for precancerous lesions |
Precancerous lesions include adenoma and serrated lesions.
Results
Three hundred forty-three patients who underwent screening colonoscopy between the ages of 50 years and 75 years by a single endoscopist, at the University of Texas, MD Anderson Cancer Center, between 2009 and 2011, were included in the study. In one patient, data on cancer history were missing. The remaining 342 patients were divided into two groups: (a) No cancer history group (n = 179) consisted of patients with no history of cancer, and (b). Cancer group (n = 163) consisted of patients with cancer history other than colorectal cancer.
Demographics
The patient characteristics are presented in Table 3 overall and by cancer history. The mean age of the 343 patients was 58.2 years, with 134 men and 209 women. The majority of the patients were Caucasians (71%), with a history of prior non-colon cancer in 163 (48%), comorbid medical conditions in 209 (61%), and a median body mass index (BMI) ranging from 15 to 59).
Table 3.
Patient characteristics overall and by cancer history
| All patients | Patients without cancer history | Patients with cancer history | |
|---|---|---|---|
| Characteristic | N (%) | N (%) | N (%) |
| All patients | 343 (100) | 179 (100) | 163 (100) |
| Age in years (median 57) | |||
| 50–59 | 213 (62) | 128 (72) | 85 (52) |
| 60–69 | 108 (31) | 48 (27) | 59 (36) |
| 70–75 | 22 (6) | 3 (2) | 19 (12) |
| Gender | |||
| Female | 209 (61) | 107 (60) | 102 (63) |
| Male | 134 (39) | 72 (40) | 61 (37) |
| Race | |||
| Caucasian | 242 (71) | 124 (69) | 117 (72) |
| African American | 40 (12) | 21 (12) | 19 (12) |
| Asian | 39 (11) | 26 (15) | 13 (8) |
| Hispanic | 20 (6) | 6 (3) | 14 (9) |
| Other | 2 (1) | 2 (1) | 0 (0) |
| Body mass index | |||
| Underweight | 7 (2) | 3 (2) | 4 (2) |
| Normal weight | 89 (26) | 43 (24) | 46 (28) |
| Overweight | 135 (39) | 76 (42) | 59 (36) |
| Obese | 96 (28) | 44 (25) | 51 (31) |
| Missing | 16 (5) | 13 (7) | 3 (2) |
| Smoking status | |||
| Yes | 23 (7) | 10 (6) | 13 (8) |
| Former | 65 (19) | 29 (16) | 36 (22) |
| No | 255 (74) | 140 (78) | 114 (70) |
| Comorbid conditions | |||
| Yes | 209 (61) | 97 (54) | 112 (69) |
| No | 124 (36) | 79 (44) | 45 (28) |
| Missing | 10 (3) | 3 (2) | 6 (4) |
| Cancer history | |||
| Yes | 163 (48) | 0 (0) | 163 (100) |
| No | 179 (52) | 179 (100) | 0 (0) |
| Missing | 1 (0) | – | – |
There was no difference between the cancer (163) and no cancer (179) groups except for the older age of patients with means of 60.0 (58.9, 61.0) and 56.5 (55.8, 57.2) years, respectively (P < 0.001), and comorbid conditions (69% vs. 54%, P = 0.01) in the cancer group compared to the no cancer group.
Colonoscopy
Patients primarily received fentanyl and midozolam (83%) with the pediatric size colonoscope (96%). Procedure success and time measures are presented in Table 4. Cecal intubation was successful 98.8% of patients. The Boston Bowel Preparation Scale for quality of colonoscopy was 8.97 (95% CI 8.94, 9.00). Average cecal insertion time and total procedure time were 12.0 and 41.0, respectively. There was no difference between the cancer and no cancer groups in cecal insertion, quality of colonoscopy preparation, cecal insertion time, and total procedure time.
Table 4.
Colonoscopy findings in 343 consecutive patients between the ages of 50 years and 75 years undergoing first screening colonoscopy
| Characteristic | All patients | No cancer | Cancer |
|---|---|---|---|
| All patients N* | 343 | 179 | 163 |
| Cecal insertion – successful N (%) | 339 (98.8%) | 179 (100%) | 159 (97.6%) |
| Cecal insertion time in minutes mean (95% confidence interval) | 12.0 (11.2, 12.7) N = 315 | 11.8 (10.7, 12.9) N = 166 | 12.2 (11.1, 13.3) N = 148 |
| Total procedure time in minutes mean (95% confidence interval) | 41.0 (39.7, 42.4) N = 322 | 41.2 (39.4, 43.0) N = 167 | 40.8 (38.7, 42.8) N = 154 |
| Boston Bowel Preparation Scale (0–9) mean (95% confidence interval) | 8.97 (8.94, 9.00) | 8.98 (8.95, 9.00) | 8.96 (8.91, 9.00) |
Measures that have N specified were not available for all patients, otherwise all patients were included in the calculation.
Average cecal insertion and total colonoscopy procedure times in patients with no polyps were 12.8 (10.2, 15.5) min and 36.0 (32.1, 39.8) min, respectively, while the average cecal insertion and total colonoscopy procedure times in patients with polyps was 11.8 (11.0, 12.7) min and 42.0 (40.3, 43.2) min, respectively. The overall procedure was on average 5.8 (1.7, 9.9) min longer among patients with polyps (P = 0.01).
Polyp characteristics and detection
Tables 5 and 6 present the polyps detected by their characteristics. There were 882 polyps detected among 297 (87%) of the 343 screened patients. According to their worst polyps classification, there was one patient with one cancerous polyp, 226 patients with precancerous lesions, 70 patients with polyps that were either distal hyperplastic or non-neoplastic, and 46 patients with no polyps detected. There was no overall difference between patients with a cancer history versus none (P = 0.98). Figure 1 presents the distribution of polyp counts overall (Fig. 1A) as well as for adenomas (Fig. 1B) and serrated lesions (Fig. 1C) per person. Overall, most patients had 1, 2, or 3 polyps and the highest number in one patient was 11 polyps. Figure 2 presents the same information separated by men and women, where the higher numbers of polyps detected in men is visible with the small proportion of men with 0 polyps and more men with two or more polyps compared to women (Fig. 2A).
Table 5.
Polyp characteristics overall and by cancer history
| All patients | No cancer history | Cancer history | ||||
|---|---|---|---|---|---|---|
| Classification | Patients N (%) | Polyps N | Patients N (%) | Polyps N | Patients N (%) | Polyps N |
| All patients | 343 (100) | 179 (100) | 163 (100) | |||
| All polyps | 297 (87) | 882 | 157 (88) | 454 | 139 (85) | 416 |
| Cancer | 1 (0) | 1 | 0 (0) | 0 | 1 (0) | 1 |
| Precancerous polyps | 226 (66) | 479 | 118 (66) | 236 | 107 (66) | 233 |
| Adenoma | 207 (60) | 422 | 110 (61) | 212 | 96 (59) | 201 |
| Multiple (≥3) | 47 (14) | – | 22 (12) | – | 24 (15) | – |
| Tubular | 190 (55) | 376 | 103 (58) | 187 | 86 (53) | 180 |
| Tubulovillous | 3 (1) | 3 | 3 (2) | 3 | 0 (0) | 0 |
| Advanced | 10 (3) | 13 | 7 (4) | 9 | 3 (2) | 4 |
| Serrated polyps | 79 (23) | 100 | 38 (21) | 46 | 40 (25) | 53 |
| Serrated adenoma* | 36 (11) | 43 | 21 (12) | 22 | 15 (9) | 21 |
| Proximal hyperplastic | 49 (14) | 57 | 21 (12) | 24 | 27 (17) | 32 |
| Advanced | 4 (1) | 5 | 3 (2) | 3 | 1 (1) | 2 |
| Distal hyperplastic/Non-neoplastic** | 70 (20) | 402 | 39 (22) | 218 | 31 (19) | 182 |
| No polyps found | 46 (13) | – | 22 (12) | – | 24 (15) | – |
Patients with serrated adenomas count both in the adenoma and serrated categories.
Patient counts include patients who had polyps, but all identified polyps were free from cancer or precancerous features. The polyp count includes all such polyps even if the patient also had precancerous or cancerous polyps.
Table 6.
Polyp characteristics by shape
| All | 0–1p N (%) | 0–1s N (%) | 0–IIa N (%) | 0–IIb N (%) | 0–IIc N (%) | |
|---|---|---|---|---|---|---|
| All polyps | 670 | 8 (100) | 158 (100) | 465 (100) | 37 (100) | 2 (100) |
| Location | ||||||
| Right | ||||||
| Appendiceal orifice | 1 (0) | – | – | 1 (0) | – | – |
| Ascending colon | 119 (18) | 1 (13) | 33 (21) | 79 (17) | 6 (16) | – |
| Cecum | 73 (11) | 1 (13) | 16 (10) | 48 (10) | 6 (16) | 2 (100) |
| Distal transverse colon | 2 (0) | – | – | – | 2 (5) | – |
| Hepatic flexure | 35 (5) | – | 11 (7) | 20 (4) | 4 (11) | – |
| Ileocecal valve | 8 (1) | – | – | 8 (2) | – | – |
| Transverse colon | 81 (12) | – | 17 (11) | 59 (13) | 5 (14) | – |
| Left | ||||||
| Descending colon | 95 (14) | – | 17 (11) | 73 (16) | 5 (14) | – |
| Rectosigmoid junction | 14 (2) | – | 5 (3) | 9 (2) | – | – |
| Rectum | 111 (17) | 1 (13) | 34 (22) | 74 (16) | 2 (5) | – |
| Sigmoid colon | 129 (19) | 5 (63) | 25 (16) | 92 (20) | 7 (19) | – |
| Unspecified location | 2 (0) | – | – | 2 (0) | – | – |
| Diagnosis | ||||||
| Adenoma | 326 (49) | 7 (88) | 93 (59) | 199 (43) | 25 (68) | 2 (100) |
| Serrated adenoma | 32 (5) | 1 (13) | 3 (2) | 23 (5) | 5 (14) | – |
| Hyperplastic polyps* | 45 (7) | – | 5 (3) | 39 (8) | 1 (3) | – |
558 polyps were collected prior to including Paris Classification of polyps in our endoscopy reports and are not included here. Additionally, there was 1 polyp that was 0–III in the sigmoid colon that was diagnosed as cancer. (*Hyperplastic polyps located proximal to the splenic flexure.)
Figure 1.
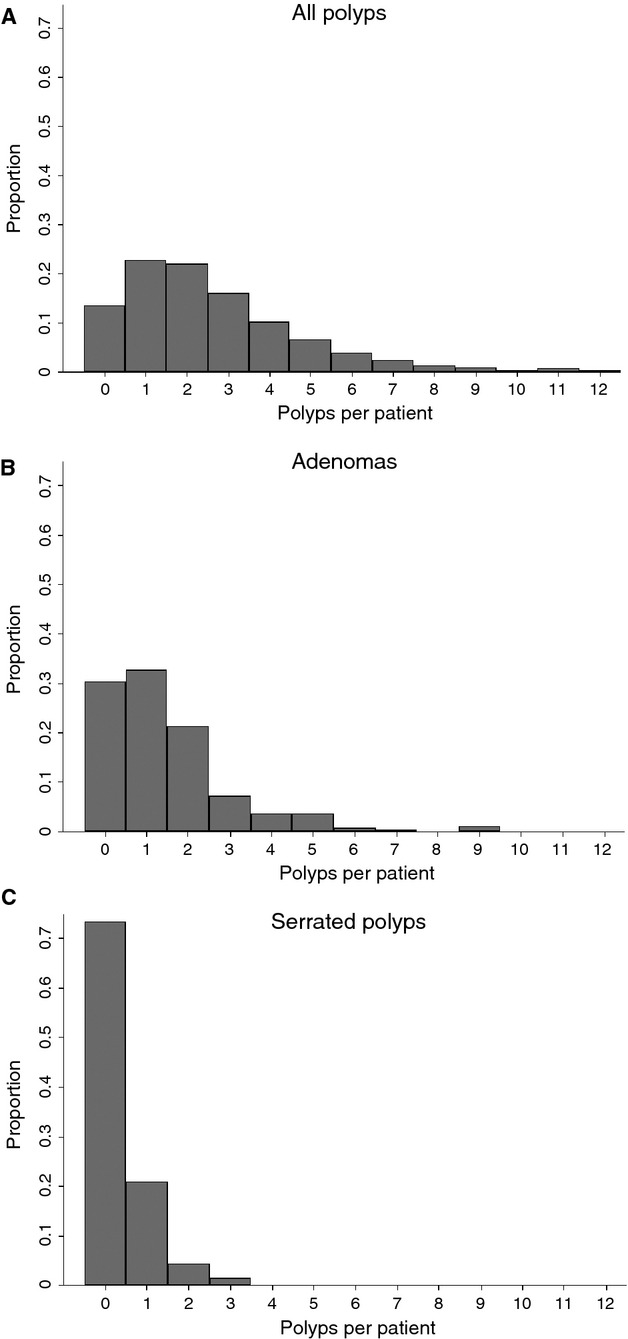
Distribution of polyps per patient for all polyps (A), adenomas (B), and serrated polyps (C).
Figure 2.
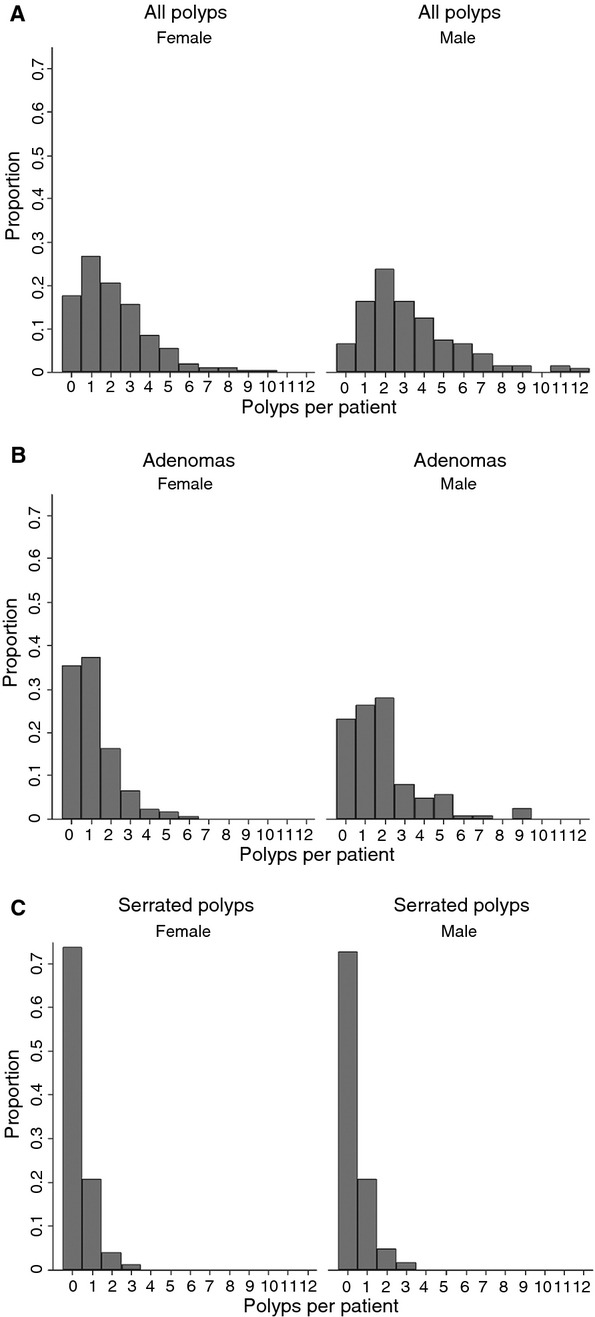
Distribution of polyps per patient for men and women for all polyps (A), adenomas (B), and serrated polyps (C).
Adenoma detection
The adenoma detection rate was 60% overall and 71% in men and 53% in women. Figure 1B shows that the mean number of adenomas per patient was 1.4 (1.2, 1.6). For men and women, the mean numbers of adenomas detected were 1.9 (1.5, 2.2) and 1.1 (0.9, 1.3), respectively (p < 0.0001; Fig. 2B). The mean number of adenomas per patient positive for precancerous lesions (adenomas or serrated lesions) was 1.9 (1.7, 2.1).
Serrated lesion and precancerous lesion detection
Serrated lesions were detected in 79 patients (serrated lesion detection rate: 23%), and the detection rate remained the same in men and women (Fig. 2C). Among the 100 serrated lesions diagnosed in 79 patients, 43 were serrated adenomas, and 57 were hyperplastic polyps proximal to splenic flexure. The serrated lesions were located in the cecum (n = 22), ascending colon (n = 32), transverse colon (n = 34), descending colon (n = 7), sigmoid colon (n = 5), and rectum (n = 4), respectively. Majority of the polyps were non-polypoid (36/40; the Paris Classification was used during the later part of the study).
The precancerous lesion (adenoma and serrated lesion) detection rate was 66% (men: 78% and women: 58%; P = 0.01). The mean number of adenomas or serrated lesions per screening colonoscopy was 1.6 (1.4, 1.8).
Advanced adenoma and multiple adenoma detection
The advanced adenoma detection rate was 3. The mean number of adenomas in patients with advanced adenomas was 4.2 (2.2, 6.2).
The multiple adenoma (≥3 adenomas) detection rate was 14%. The detection rate of three or more adenomas or serrated lesions was 16%.
There were no statistically significant differences between the cancer and no cancer groups for adenoma (P = 0.63), serrated lesion (P = 0.47), or advanced adenoma (P = 0.56) or multiple adenoma (P = 0.51) detection rates, even though some mild trends appear in Table 6.
Discussion
This study demonstrates that a comprehensive colonoscopy screening program designed to optimize quality parameters results in a high adenoma and serrated polyp detection rate which exceeds current quality standards.
The adenoma detection rate is the most widely used metric for assessing quality of colonoscopy screening. In our study, the comprehensive colonoscopy screening resulted in adenoma detection rate of 60%. This rate was higher than that reported in a large meta-analysis (22–58.2%) [50], chromoendoscopy studies (45–59%) [51–54], and wide-angle colonoscopy (27%) studies [55].
The comprehensive colonoscopy screening program was associated with an advanced adenoma prevalence of 3%. The prevalence of advanced adenomas reported in a large meta-analysis varied between 2.48% and 9.67% [50].
Another emerging quality indicator of screening colonoscopy is that the serrated lesion detection rate and the miss rates and variability in detection for serrated lesions are greater than those for adenomas [56, 57]. The prevalence of serrated lesions in patients undergoing comprehensive colonoscopy screening program was 23% compared to those reported in the literature 13% ± 7.8% (1–18%) [56–58], and closer to those reported in the autopsy studies (25–50%) [59–61].
The ultimate goal of screening colonoscopy is to prevent colorectal cancer by detecting and removing all precancerous lesions, not only adenomas but also serrated lesions [43]. Although the mean number of adenomas per patient provides additional information about the performance of the colonoscopist [10], reporting on precancerous lesion detection rate (adenoma + serrated lesion detection rate) and the mean number of precancerous lesions per patient may serve as a better bench mark [10]. We report 66% precancerous lesion detection rate and 1.4 precancerous lesions per patient. This could be due to the type of population screened or the impact of a comprehensive colonoscopy screening program.
We doubt that the high prevalence of adenomas and serrated lesions in our study population is due to the population selected for screening at a cancer center, because there was no difference between the cancer and no cancer groups of patients in the adenoma detection rates.
Age is the single most important independent determinant of the prevalence of adenomas, advanced adenomas, and cancer, with twofold and sevenfold higher rates, respectively, among older cohorts (≥65 years) [50]. In contrast to the advanced adenoma prevalence rates of 8.89% and 9.67% in cohorts with a mean age of 65 years and 68.6 years, respectively [62, 63], the advanced adenoma detection rate in our study population (mean age of 58.2 years) is 3%, suggesting additional benefit of a comprehensive colonoscopy screening program [26, 33].
We believe that the high adenoma detection rate as well as the high serrated lesion detection rate was due to the additive effect of multiple factors incorporated in the comprehensive colonoscopy screening protocol each of which were clearly demonstrated to improve adenoma detection, such as ensuring high-quality colon preparation [16, 34, 35], routine use of a cap fitted colonoscope to improve the examination of inter-haustral area [38, 39, 64], adequate distension of the colon with the use of patient position change as needed [37], and use of a screening protocol to detect flat lesions by a trained endoscopy team [36, 40, 65–68]. Although the importance of position of the video monitor and slight overcorrection of the eye glasses for better visual acuity has not been formally addressed, the role of gaze pattern on adenoma detection has been found to improve adenoma detection [69]. In addition, recording the videos of subtle flat lesions routinely as part of our practice and reviewing them may help train the eye for pattern recognition of serrated lesions.
An important strength of the study is the use of a comprehensive colonoscopy screening program in all patients undergoing their first exam, with over 98% cecal intubation, excellent quality of bowel cleansing (Boston Bowel Preparation Scale of 8.97), examination by an endoscopy team trained in the detection of subtle flat lesions, and recording of the data based on the recommendations of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable [9, 70, 71].
There are several limitations to this study that need to be emphasized. A major drawback is that this report is the experience of a single endoscopist. Whether or not various measures utilized in the protocol based on the ASGE/American College of Gastroenterology task force recommendations to improve the quality of colonoscopy could be widely adopted is not clear, given the structure of pay for procedure modus of operation in the United States [72]. However, the Institute of Medicine's initiative in improving the quality of care in the United States is an opportunity to implement several strategies similar to those implemented in the United Kingdom – ensuring colonoscopists meet high standard before starting screening and through ongoing quality assurance [10, 73]. Another drawback was the retrospective nature of the study; however, the detailed endoscopy data recording minimizes some of the drawbacks of a retrospective study [41, 49]. The total duration of the colonoscope insertion and withdrawal in our study may be considered impractical in community practice by some practitioners. Several factors account for the prolonged duration of procedure in this study, such as the use of conscious sedation of patients, routine use of simethicone flush to clear the bubbles followed by suction of all the luminal contents of the colon during endoscope insertion for better examination of a dry clean colon during the withdrawal, removal of polyps during insertion, higher adenoma detection rate, higher number of polyps removed per patient, routine use of endoscopic mucosal resection (EMR) of all flat lesions larger than 5 mm (as margins of serrated lesions are hard to define without submucosal injection of Indigo Carmine), and clearance of all polyps including EMR of large and giant lesions in the same endoscopy session.
Although the adenoma detection rate is an independent predictor of the risk of interval cancer [6], whether or not the higher adenoma detection rate observed after comprehensive colonoscopy screening protocol has any clinical benefit on limiting interval cancers or is a function of lead-time or length bias needs to be defined by long-term cohort studies. The paper by Kaminski et al. [6] does not support the notion that there is a plateau to the benefit of adenoma detection. We believe that high adenoma detection as noted in the study may have a protective effect based on a personal audit of the author's interval colorectal cancer development at another institution [74].
In summary, the comprehensive colonoscopy screening program results in high-quality screening of adenomas, advanced adenomas, serrated lesions, and multiple adenomas. The applicability of this program in improving adenoma detection across the nation needs further investigation. In the interim, many aspects of a comprehensive colonoscopy program can be readily adopted and include adequacy of the preparation, use of a cap on the endoscope, positioning of the monitor, and establishing a credentialing or continuing education related to polyp identification for all physicians and support staff involved in the performance of colonoscopy, and sufficient time for instrument withdrawal. These measures are not projected to encumber cost or insert inconvenience when performing procedures and can be differentiated from other aspects that are a function of quality assurance monitoring and documentation.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Video S1. Colon preparation education hyperlink (http://www3.mdanderson.org/streams/FullVideoPlayer.cfm?xml=patientEd%2Fconfig%2Fmda_Colon4--cfg).
Video S2. http://www.youtube.com/user/GottumukkalaSRaju?feature=guide.
References
- 1.Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N. Engl. J. Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Zauber AG, Winawer SJ, O'Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N. Engl. J. Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robertson DJ, Greenberg ER, Beach M, Sandler RS, Ahnen D, Haile RW, et al. Colorectal cancer in patients under close colonoscopic surveillance. Gastroenterology. 2005;129:34–41. doi: 10.1053/j.gastro.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 4.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, Dekker SJ, van Deventer E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am. J. Gastroenterol. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 5.Pabby A, Schoen RE, Weissfeld JL, Burt R, Kikendall JW, Lance P, et al. Analysis of colorectal cancer occurrence during surveillance colonoscopy in the dietary Polyp Prevention Trial. Gastrointest. Endosc. 2005;61:385–391. doi: 10.1016/s0016-5107(04)02765-8. [DOI] [PubMed] [Google Scholar]
- 6.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N. Engl. J. Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 7.Williams JE, Faigel DO. Colonoscopy reports and current state of performance measures. Gastrointest. Endosc. Clin. N. Am. 2010;20:685–697. doi: 10.1016/j.giec.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Hewett DG, Rex DK. Improving colonoscopy quality through health-care payment reform. Am. J. Gastroenterol. 2010;105:1925–1933. doi: 10.1038/ajg.2010.247. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Bond JH, Winawer S, Levin TR, Burt RW, Johnson DA, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee TJ, Rutter MD, Blanks RG, Moss SM, Goddard AF, Chilton A, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2011;61:1050–1057. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 11.Schatzkin A, Lanza E, Corle D, Lance P, Iber F, Caan B, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. Polyp Prevention Trial Study Group. N. Engl. J. Med. 2000;342:1149–1155. doi: 10.1056/NEJM200004203421601. [DOI] [PubMed] [Google Scholar]
- 12.Alberts DS, Martinez ME, Roe DJ, Guillen-Rodriguez JM, Marshall JR, van Leeuwen JB, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N. Engl. J. Med. 2000;342:1156–1162. doi: 10.1056/NEJM200004203421602. [DOI] [PubMed] [Google Scholar]
- 13.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest. Endosc. 2005;61:378–384. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 14.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest. Endosc. 2003;58:76–79. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 15.Lebwohl B, Kastrinos F, Glick M, Rosenbaum AJ, Wang T, Neugut AI. The impact of suboptimal bowel preparation on adenoma miss rates and the factors associated with early repeat colonoscopy. Gastrointest. Endosc. 2011;73:1207–1214. doi: 10.1016/j.gie.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ibanez M, Parra-Blanco A, Zaballa P, Jimenez A, Fernandez-Velazquez R, Fernandez-Sordo JO, et al. Usefulness of an intensive bowel cleansing strategy for repeat colonoscopy after preparation failure. Dis. Colon Rectum. 2011;54:1578–1584. doi: 10.1097/DCR.0b013e31823434c8. [DOI] [PubMed] [Google Scholar]
- 17.Aslinia F, Uradomo L, Steele A, Greenwald BD, Raufman JP. Quality assessment of colonoscopic cecal intubation: an analysis of 6 years of continuous practice at a university hospital. Am. J. Gastroenterol. 2006;101:721–731. doi: 10.1111/j.1572-0241.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 18.Rex DK. Quality in colonoscopy: cecal intubation first, then what? Am. J. Gastroenterol. 2006;101:732–734. doi: 10.1111/j.1572-0241.2006.00483.x. [DOI] [PubMed] [Google Scholar]
- 19.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N. Engl. J. Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 20.Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointest. Endosc. 2000;51:33–36. doi: 10.1016/s0016-5107(00)70383-x. [DOI] [PubMed] [Google Scholar]
- 21.DeMarco DC, Odstrcil E, Lara LF, Bass D, Herdman C, Kinney T, et al. Impact of experience with a retrograde-viewing device on adenoma detection rates and withdrawal times during colonoscopy: the Third Eye Retroscope study group. Gastrointest. Endosc. 2010;71:542–550. doi: 10.1016/j.gie.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 22.Leufkens AM, DeMarco DC, Rastogi A, Akerman PA, Azzouzi K, Rothstein RI, et al. Effect of a retrograde-viewing device on adenoma detection rate during colonoscopy: the TERRACE study. Gastrointest. Endosc. 2011;73:480–489. doi: 10.1016/j.gie.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Waye JD, Heigh RI, Fleischer DE, Leighton JA, Gurudu S, Aldrich LB, et al. A retrograde-viewing device improves detection of adenomas in the colon: a prospective efficacy evaluation (with videos) Gastrointest. Endosc. 2010;71:551–556. doi: 10.1016/j.gie.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 24.Soetikno RM, Kaltenbach T, Rouse RV, Park W, Maheshwari A, Sato T, et al. Prevalence of nonpolypoid (flat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–1035. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 25.Pohl J, Schneider A, Vogell H, Mayer G, Kaiser G, Ell C. Pancolonic chromoendoscopy with indigo carmine versus standard colonoscopy for detection of neoplastic lesions: a randomised two-centre trial. Gut. 2011;60:485–490. doi: 10.1136/gut.2010.229534. [DOI] [PubMed] [Google Scholar]
- 26.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am. J. Gastroenterol. 2007;102:856–861. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 27.Leung K, Pinsky P, Laiyemo AO, Lanza E, Schatzkin A, Schoen RE. Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointest. Endosc. 2010;71:111–117. doi: 10.1016/j.gie.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rex DK, Bond JH, Feld AD. Medical-legal risks of incident cancers after clearing colonoscopy. Am. J. Gastroenterol. 2001;96:952–957. doi: 10.1111/j.1572-0241.2001.03677.x. [DOI] [PubMed] [Google Scholar]
- 29.Rex DK. Maximizing detection of adenomas and cancers during colonoscopy. Am. J. Gastroenterol. 2006;101:2866–2877. doi: 10.1111/j.1572-0241.2006.00905.x. [DOI] [PubMed] [Google Scholar]
- 30.Rex DK. Improving detection during colonoscopy: multiple pathways for investigation. J. Clin. Gastroenterol. 2011;45:207–209. doi: 10.1097/MCG.0b013e31820a83c0. [DOI] [PubMed] [Google Scholar]
- 31.Aoun E, Abdul-Baki H, Azar C, Mourad F, Barada K, Berro Z, et al. A randomized single-blind trial of split-dose PEG-electrolyte solution without dietary restriction compared with whole dose PEG-electrolyte solution with dietary restriction for colonoscopy preparation. Gastrointest. Endosc. 2005;62:213–218. doi: 10.1016/s0016-5107(05)00371-8. [DOI] [PubMed] [Google Scholar]
- 32.El Sayed AM, Kanafani ZA, Mourad FH, Barada K, Berro Z, Tarchichi M, et al. A randomized single-blind trial of whole versus split-dose polyethylene glycol-electrolyte solution for colonoscopy preparation. Gastrointest. Endosc. 2003;58:36–40. doi: 10.1067/mge.2003.318. [DOI] [PubMed] [Google Scholar]
- 33.Chokshi RV, Hovis CE, Hollander T, Early DS, Wang JS. Prevalence of missed adenomas in patients with inadequate bowel preparation on screening colonoscopy. Gastrointest. Endosc. 2012;75:1197–1203. doi: 10.1016/j.gie.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest. Endosc. 2010;72:686–692. doi: 10.1016/j.gie.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest. Endosc. 2009;69:620–625. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee RH, Tang RS, Muthusamy VR, Ho SB, Shah NK, Wetzel L, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos) Gastrointest. Endosc. 2011;74:128–134. doi: 10.1016/j.gie.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 37.East JE, Bassett P, Arebi N, Thomas-Gibson S, Guenther T, Saunders BP. Dynamic patient position changes during colonoscope withdrawal increase adenoma detection: a randomized, crossover trial. Gastrointest. Endosc. 2011;73:456–463. doi: 10.1016/j.gie.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 38.Hewett DG, Rex DK. Cap-fitted colonoscopy: a randomized, tandem colonoscopy study of adenoma miss rates. Gastrointest. Endosc. 2010;72:775–781. doi: 10.1016/j.gie.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Horiuchi A, Nakayama Y, Kato N, Ichise Y, Kajiyama M, Tanaka N. Hood-assisted colonoscopy is more effective in detection of colorectal adenomas than narrow-band imaging. Clin. Gastroenterol. Hepatol. 2010;8:379–383. doi: 10.1016/j.cgh.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 40.Saitoh Y, Waxman I, West AB, Popnikolov NK, Gatalica Z, Watari J, et al. Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology. 2001;120:1657–1665. doi: 10.1053/gast.2001.24886. [DOI] [PubMed] [Google Scholar]
- 41.de Jonge V, Sint Nicolaas J, Cahen DL, Moolenaar W, Ouwendijk RJ, Tang TJ, et al. Quality evaluation of colonoscopy reporting and colonoscopy performance in daily clinical practice. Gastrointest. Endosc. 2012;75:98–106. doi: 10.1016/j.gie.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 42.Gawande A. Better: a surgeon's notes on performance. New York, NY: Metropolitan Books; 2008. [Google Scholar]
- 43.Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am. J. Gastroenterol. 2012;107:1315–1329. doi: 10.1038/ajg.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shergill AK, McQuaid KR, Rempel D. Ergonomics and GI endoscopy. Gastrointest. Endosc. 2009;70:145–153. doi: 10.1016/j.gie.2008.12.235. [DOI] [PubMed] [Google Scholar]
- 45.Kudo SE. Early colorectal cancer: detection of depressed types of colorectal carcinomas. Philadelphia, PA: Lippincott Williams & Wilkins; 1996. [Google Scholar]
- 46.Soetikno R, Kaltenbach T. Non-polypoid (flat and depressed) colorectal neoplasms. Gastrointest. Endosc. Clin. N. Am. 2010;20:407–592. doi: 10.1016/j.giec.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 47.Soetikno R, Barro J, Friedland S, Matsui S, Rouse R, Fujii T. 2004. Diagnosis of flat and depressed colorectal neoplasms. In Endoscopy learning library: American society of gastrointestinal endoscopy online learning center. Available at https://www.extendmed.com/asge/
- 48.Kaltenbach T, McGill SK, Kalidindi V, Friedland S, Soetikno R. Proficiency in the diagnosis of nonpolypoid colorectal neoplasm yields high adenoma detection rates. Dig. Dis. Sci. 2012;57:764–770. doi: 10.1007/s10620-011-1921-6. [DOI] [PubMed] [Google Scholar]
- 49.Lieberman D, Nadel M, Smith RA, Atkin W, Duggirala SB, Fletcher R, et al. Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest. Endosc. 2007;65:757–766. doi: 10.1016/j.gie.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 50.Heitman SJ, Ronksley PE, Hilsden RJ, Manns BJ, Rostom A, Hemmelgarn BR. Prevalence of adenomas and colorectal cancer in average risk individuals: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2009;7:1272–1278. doi: 10.1016/j.cgh.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 51.Kahi CJ, Anderson JC, Waxman I, Kessler WR, Imperiale TF, Li X, et al. High-definition chromocolonoscopy vs. high-definition white light colonoscopy for average-risk colorectal cancer screening. Am. J. Gastroenterol. 2010;105:1301–1307. doi: 10.1038/ajg.2010.51. [DOI] [PubMed] [Google Scholar]
- 52.Brooker JC, Saunders BP, Shah SG, Thapar CJ, Thomas HJ, Atkin WS, et al. Total colonic dye-spray increases the detection of diminutive adenomas during routine colonoscopy: a randomized controlled trial. Gastrointest. Endosc. 2002;56:333–338. doi: 10.1016/s0016-5107(02)70034-5. [DOI] [PubMed] [Google Scholar]
- 53.Hurlstone DP, Cross SS, Slater R, Sanders DS, Brown S. Detecting diminutive colorectal lesions at colonoscopy: a randomised controlled trial of pan-colonic versus targeted chromoscopy. Gut. 2004;53:376–380. doi: 10.1136/gut.2003.029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Le Rhun M, Coron E, Parlier D, Nguyen JM, Canard JM, Alamdari A, et al. High resolution colonoscopy with chromoscopy versus standard colonoscopy for the detection of colonic neoplasia: a randomized study. Clin. Gastroenterol. Hepatol. 2006;4:349–354. doi: 10.1016/j.cgh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Fatima H, Rex DK, Rothstein R, Rahmani E, Nehme O, Dewitt J, et al. Cecal insertion and withdrawal times with wide-angle versus standard colonoscopes: a randomized controlled trial. Clin. Gastroenterol. Hepatol. 2008;6:109–114. doi: 10.1016/j.cgh.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 56.Kahi CJ, Li X, Eckert GJ, Rex DK. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest. Endosc. 2012;75:515–520. doi: 10.1016/j.gie.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 57.Schreiner MA, Weiss DG, Lieberman DA. Proximal and large hyperplastic and nondysplastic serrated polyps detected by colonoscopy are associated with neoplasia. Gastroenterology. 2010;139:1497–1502. doi: 10.1053/j.gastro.2010.06.074. [DOI] [PubMed] [Google Scholar]
- 58.Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin. Gastroenterol. Hepatol. 2011;9:42–46. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 59.Clark JC, Collan Y, Eide TJ, Esteve J, Ewen S, Gibbs NM, et al. Prevalence of polyps in an autopsy series from areas with varying incidence of large-bowel cancer. Int. J. Cancer. 1985;36:179–186. doi: 10.1002/ijc.2910360209. [DOI] [PubMed] [Google Scholar]
- 60.Johannsen LG, Momsen O, Jacobsen NO. Polyps of the large intestine in Aarhus, Denmark. An autopsy study. Scand. J. Gastroenterol. 1989;24:799–806. doi: 10.3109/00365528909089217. [DOI] [PubMed] [Google Scholar]
- 61.Eide TJ, Stalsberg H. Polyps of the large intestine in Northern Norway. Cancer. 1978;42:2839–2848. doi: 10.1002/1097-0142(197812)42:6<2839::aid-cncr2820420645>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 62.Johnson DA, Gurney MS, Volpe RJ, Jones DM, VanNess MM, Chobanian SJ, et al. A prospective study of the prevalence of colonic neoplasms in asymptomatic patients with an age-related risk. Am. J. Gastroenterol. 1990;85:969–974. [PubMed] [Google Scholar]
- 63.Imperiale TF, Ransohoff DF, Itzkowitz SH, Turnbull BA, Ross ME. Fecal DNA versus fecal occult blood for colorectal-cancer screening in an average-risk population. N. Engl. J. Med. 2004;351:2704–2714. doi: 10.1056/NEJMoa033403. [DOI] [PubMed] [Google Scholar]
- 64.Rastogi A, Bansal A, Rao DS, Gupta N, Wani SB, Shipe T, et al. Higher adenoma detection rates with cap-assisted colonoscopy: a randomised controlled trial. Gut. 2012;61:402–408. doi: 10.1136/gutjnl-2011-300187. [DOI] [PubMed] [Google Scholar]
- 65.Adler A, Wegscheider K, Lieberman D, Aminalai A, Aschenbeck J, Drossel R, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12 134 examinations (Berlin colonoscopy project 3, BECOP-3) Gut. 2012;62:236–241. doi: 10.1136/gutjnl-2011-300167. [DOI] [PubMed] [Google Scholar]
- 66.Lee CK, Park DI, Lee SH, Hwangbo Y, Eun CS, Han DS, et al. Participation by experienced endoscopy nurses increases the detection rate of colon polyps during a screening colonoscopy: a multicenter, prospective, randomized study. Gastrointest. Endosc. 2011;74:1094–1102. doi: 10.1016/j.gie.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 67.Gromski MA, Miller CA, Lee SH, Park ES, Lee TH, Park SH, et al. Trainees' adenoma detection rate is higher if >/=10 minutes is spent on withdrawal during colonoscopy. Surg. Endosc. 2011;26:1337–1342. doi: 10.1007/s00464-011-2033-2. [DOI] [PubMed] [Google Scholar]
- 68.Coe SG, Wallace MB. Colonoscopy: new approaches to better outcomes. Curr. Opin. Gastroenterol. 2012;28:70–75. doi: 10.1097/MOG.0b013e32834ddab9. [DOI] [PubMed] [Google Scholar]
- 69.Almansa C, Shahid MW, Heckman MG, Preissler S, Wallace MB. Association between visual gaze patterns and adenoma detection rate during colonoscopy: a preliminary investigation. Am. J. Gastroenterol. 2011;106:1070–1074. doi: 10.1038/ajg.2011.26. [DOI] [PubMed] [Google Scholar]
- 70.American Society for Gastrointestinal Endoscopy. Quality and outcomes assessment in gastrointestinal endoscopy. American Society for Gastrointestinal Endoscopy. Gastrointest. Endosc. 2000;52:827–830. [PubMed] [Google Scholar]
- 71.Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, et al. Quality indicators for colonoscopy. Gastrointest. Endosc. 2006;63:S16–S28. doi: 10.1016/j.gie.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 72.Steinberg EP. Improving the quality of care–can we practice what we preach? N. Engl. J. Med. 2003;348:2681–2683. doi: 10.1056/NEJMe030085. [DOI] [PubMed] [Google Scholar]
- 73.Kappelman MD, Dorn SD, Peterson E, Runge T, Allen JI. Quality of care for gastrointestinal conditions: a primer for gastroenterologists. Am. J. Gastroenterol. 2011;106:1182–1187. doi: 10.1038/ajg.2011.118. [DOI] [PubMed] [Google Scholar]
- 74.Singh A, Freeman DH, Riall TS, Raju GS. Delay in colonoscopy diagnosis of colon cancer. Gastrointest. Endosc. 2008;67:AB311. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


