Abstract
Background and objectives
Endemic renal insufficiency (RI) of unknown etiology is a major public health issue with high mortality in the Pacific coastal regions of Central America. We studied RI in León and Chinandega, Nicaragua, evaluating associations with known risk factors and hypothesized exposures.
Methods
A cross-sectional survey was conducted with assessment of medical, social, and occupational history and exposures in conjunction with measurement of serum creatinine. Cases were defined by an estimated glomerular filtration rate (eGFR) ≤60 mL/min/1.73 m2 using the modified four-variable Modification of Diet in Renal Disease (MDRD) study equation for non-African Americans. Logistic regression models controlling for known risk factors of kidney disease were used to evaluate associations between exposures and RI.
Results
A total of 124 RI cases were compared to 873 persons without RI. Cases had no significant differences in the odds of having a systolic blood pressure (SBP) > 140 or diastolic blood pressure (DBP) > 90 mmHg, or in reporting diabetes. Agricultural labor was associated with RI (OR = 2.48, 95%CI: 1.59, 3.89, p < 0.0001). There was no association with agricultural non-field work (OR = 0.91, 95%CI: 0.60, 1.38, p = 0.65). Consumption of unregulated alcohol (“lija”) was associated with RI (OR = 2.10, 95%CI: 1.31, 3.39, p = 0.0023), as was drinking 5 L or more of water per day (OR = 3.59 vs. 1 L 95%CI: 1.52, 4.46, p = 0.0035).
Conclusions
Agricultural field labor and lija consumption were associated with RI in this region. Water intake may also be important. Identifying specific risk factors for RI within these exposures, such as individual pesticides or lija ingredients, may facilitate prevention in a setting where dialysis and transplantation are limited.
Keywords: chronic kidney disease, Nicaragua, alcohol, pesticides, renal failure
INTRODUCTION
Endemic renal insufficiency (RI) of unknown etiology is reported in the Pacific coastal regions of Central America where access to dialysis and transplantation are limited. In Nicaragua, RI is a national health priority. Available surveillance data reported by the Nicaraguan Ministry of Health (Ministerio De Salud Republica de Nicaragua – MINSA) list RI as the seventh cause of death nationwide, accounting for 4.2% of deaths.1–3 Of particular interest are the western states of León and Chinandega with populations of 389,628 and 441,308, respectively,4 where RI has been reported as the number one and two causes of death, accounting for 12.6% and 11.7% of mortality in these regions, respectively.1–3 In the United States during this same period, 5.7% of deaths were attributed to nephritis, nephrosis, diabetes, essential hypertension, or hypertensive kidney disease.5
Studies are limited regarding RI in Nicaragua. Unpublished data collected by La Universidad Nacional Autonoma de Nicaragua (UNAN) in León suggest a high prevalence of RI among males and those less than 60 years of age6,7 as well as an association between RI, agricultural labor, and alcohol consumption. These data and the regional distribution of renal-failure-associated mortality have suggested a contribution of environmental exposures (e.g., pesticides or contaminated water). Public health professionals are also concerned about consumption of homemade alcohol, or “lija,” liquor prepared by an unregulated process in the region. The public health concern and lack of peer-reviewed data for Nicaragua prompted the current community-based investigation.
MATERIALS AND METHODS
Study design and recruitment
A community-based, cross-sectional survey was conducted in nine municipalities in western Nicaragua. Municipalities (counties) within the departments (states) of León and Chinandega were selected from those containing local health centers. Six were chosen from Chinandega (El Viejo, Corinto, Posoltega, El Realejo, Chichigalpa, and Somotillo) and three from León (Telica, Malpaisillo, and León). Health centers advertised screenings to the public. Screenings were conducted from July to November 2003. All volunteers were initially included. However, after preliminary data analysis revealed few female cases, women were excluded from subsequent screenings. Female subjects were included in the exploratory data analysis for completeness, but removed from the prespecified matched nested case–control analyses. Both are presented.
Data collection
Participants’ height and weight were recorded. Blood pressures were measured manually by sphygmoma-nometer by a trained technician. At each site a subset of blood pressures were repeated by a physician to assure agreement. Blood was collected and analyzed for serum creatinine content the same day. Participants were invited back for their results and education.
Study questionnaires assessed demographics (e.g., age, gender), known risk factors for kidney disease (e.g., hypertension, diabetes, family history of renal disease), occupational exposures (e.g., agricultural field labor and work with specific crops), and non-occupational exposures (e.g., water intake and alcohol use). Questionnaires were piloted with approximately 25 volunteers with revisions incorporated for study participants. Final administration was done one-on-one by physicians familiar with the questionnaire (LC and CDA). Agricultural workers were asked about the types of labor they had performed in the past, including planting or harvesting specific types of crops. Evaluation of alcohol consumption included type, drinks per month, and years of consumption. Consumption of lija was also estimated. Water intake (liters per day) and cumulative pesticide exposure were solicited.
Definitions for and selection of cases and controls
Data were collected from 1002 participants, with 997 available for analysis after five serum samples were lost. Data analysis was performed in two phases: an exploratory phase and a matched nested case–control phase, using different definitions for cases and controls. In each phase, cases and controls were defined by estimated glomerular filtration rate (eGFR) calculated using the modified four-variable MDRD equation for non-African Americans,8 and the frequency of known risk factors for kidney disease, occupational, and non-occupational exposures was examined.
In the exploratory phase, including univariate and multivariable analyses, cases were defined as those with an eGFR ≤ 60 mL/min/1.73 m2 (n = 124), and controls as those with an eGFR >60 mL/min/1.73 m2 (n = 873). These definitions were chosen to describe the entire study population (n = 997).
During the second phase, a prespecified matched nested case–control design was used, with cases defined as having an eGFR ≤60 mL/min/1.73 m2 (n = 124), and controls as having a eGFR ≥80 mL/min/1.73 m2 (n = 232). Subjects with eGFRs 61–79 mL/min/1.73 m2 (n = 176) were not included. These definitions were chosen to minimize case–control misclassifications by the MDRD equation, which lacks precision above eGFR > 60 mL/min/1.73 m2. In this phase, cases and controls were stratified by gender and age group (aged <27, 27–36, 36–46, and ≥ 46 years for male, 32–71 for female) and frequency matched 1:2. Matching was performed to minimize confounding from differences in age and age-associated risk factors for kidney disease. Because the population of interest in this study was adults, and because women were excluded from later screenings, only results for the analysis that included men >18 years of age are reported. A separate analysis was performed including women (not shown). No significant differences were identified between the results of this analysis and the main findings of the analysis presented.
Statistical analysis
In the exploratory phase and matched nested case– control phase of analysis, cases and controls were compared using Student’s t-test for continuous variables and chi-square tests for categorical measures. When assumptions for these parametric tests were not met, nonparametric corollaries were used: Wilcoxon rank tests for continuous variables and continuity-adjusted chi-square tests for categorical measures. Logistic regression was used to evaluate the association between risk factors and case–control status. Results are presented as odds ratios, 95% confidence intervals, and p-values. Multivariable logistic regression models were adjusted for known risk factors of kidney disease including age, gender (where applicable), hypertension (systolic blood pressure or SBP >140, diastolic blood pressure or DBP >90 mmHg), a reported history of diabetes, a family history of end-stage kidney disease (ESKD), and body mass index (BMI, as a continuous variable). No exposures were controlled for in the multivariable analysis, as there were no preanalysis hypotheses regarding the causal relationships within the exposures and RI.
We assessed for a dose-dependent relationship between RI and pesticide use, water and lija consumption. Pesticide use was evaluated across quartiles based on reported years of exposure (>0–2 years, >2–8 years, >8–13 years, and >13 years). Lija use was quantified by multiplying reported years of consumption by reported frequency of monthly use (arbitrary units), using non-lija users as the referent group. Water consumption was assessed in five groups consuming 1–5 L per day, with 1 L/day as the referent group.
RESULTS
Exploratory analysis: demographics, known risk factors for kidney disease, and exposures of interest
The exploratory analysis included all members of the study population (n = 997). The univariate analysis presented describes their demographics and key characteristics, cases versus controls (Table 1). The prevalence of RI was 12.4% in the study population (124 cases in 997 enrolled subjects) and by definition, cases had worse renal function than controls (eGFR = 39.4 mL/min/1.73 m2 vs. 103.7 mL/min/1.73 m2). The average age of cases was approximately 14 years older than that of controls (51 ± 13 vs. 37 ± 14, p < 0.0001). Both groups were predominantly male, with a higher proportion of males among cases (96% vs. 84%, p = 0.0003).
TABLE 1.
Univariate analysis of demographics, known risk factors for kidney disease, and exposure frequencies between cases and controls for the entire study population.
| Mean ± SD or N (%) |
N** 997 |
Cases (eGFR ≤ 60) n = 124 |
Cases (eGFR > 60) n = 873 |
p-Value* |
|---|---|---|---|---|
| Demographics and known risk factors for kidney disease | ||||
| Age | 997 | 51.1 2 ± 13.47 |
37.13 ± 13.85 | <0.0001 |
| Gender | 0.0003 | |||
| Male | 848 | 119 (95.97%) | 729 (83.51%) | |
| Female | 149 | 5 (4.03%) | 144 (16.49%) | |
| Systolic blood pressure >140 | 0.0436 | |||
| Yes | 45 | 10 (8.06%) | 35 (4.03%) | |
| No | 947 | 114 (91.94%) | 833 (95.97%) | |
| Diastolic blood pressure >90 | 0.1164# | |||
| Yes | 36 | 8 (6.45%) | 28 (3.23%) | |
| No | 956 | 116 (93.55%) | 840 (96.77%) | |
| eGFR (mL/min/1.73 m2) | 997 | 39.40 ± 14.23 | 103.68 ± 29.62 | <0.0001 |
| Self-reported history of diabetes | 0.0406# | |||
| Yes | 30 | 8 (6.45%) | 22 (2.52%) | |
| No | 967 | 116 (93.55%) | 851 (97.48%) | |
| Family history of end-stage kidney disease | 0.0197 | |||
| Yes | 89 | 18 (14.52%) | 71 (8.13%) | |
| No | 908 | 106 (85.48%) | 802 (91.87%) | |
| Body mass index (BMI kg/m2, continuous) | 997 | 24.60 ± 4.72 | 24.72 ± 4.35 | 0.9670 |
| BMI (categorical) | 0.5772 | |||
| BMI ≤ 25 | 577 | 73 (58.87%) | 504 (57.73%) | |
| 25 < BMI ≤ 30 | 304 | 40 (32.26%) | 264 (30.24%) | |
| BMI > 30 | 116 | 11 (8.87%) | 105 (12.03%) | |
| Occupational history/exposures | ||||
| Agricultural field work | <0.0001 | |||
| Yes | 506 | 88 (70.97%) | 418 (47.88%) | |
| No | 491 | 36 (29.03%) | 455 (52.12%) | |
| Agricultural non-field work | 0.8253 | |||
| Yes | 409 | 52 (41.94%) | 357 (40.89%) | |
| No | 588 | 72 (58.06%) | 516 (59.11%) | |
| Sugar mill work | 0.1059 | |||
| Yes | 307 | 46 (37.10%) | 261 (29.93%) | |
| No | 689 | 78 (62.90%) | 611 (70.07%) | |
| Work with rice | <0.0001 | |||
| Yes | 101 | 28 (22.58%) | 73 (8.36%) | |
| No | 896 | 96 (77.42%) | 800 (91.64%) | |
| Work with corn | 0.0001 | |||
| Yes | 345 | 62 (50.00%) | 283 (32.42%) | |
| No | 652 | 62 (50.00%) | 590 (67.58%) | |
| Work with banana | <0.0001 | |||
| Yes | 48 | 17 (13.71%) | 31 (3.55%) | |
| No | 949 | 107 (86.29%) | 842 (96.45%) | |
| Work with sesame seed | 0.1227 | |||
| Yes | 140 | 23 (18.55%) | 117 (13.40%) | |
| No | 857 | 101 (81.45%) | 756 (86.60%) | |
| Work with cotton | 0.0164 | |||
| Yes | 146 | 27 (21.77%) | 119 (13.63%) | |
| No | 851 | 97 (78.23%) | 754 (86.37%) | |
| Work with beans | 0.0022 | |||
| Yes | 88 | 20 (16.13%) | 68 (7.79%) | |
| No | 909 | 104 (83.87%) | 805 (92.21%) | |
| Work with “other crops” | 0.0041 | |||
| Yes | 68 | 16 (12.90%) | 52 (5.96%) | |
| No | 929 | 108 (87.10%) | 821 (94.04%) | |
| Work with cattle | 0.0090 | |||
| Yes | 72 | 16 (12.90%) | 56 (6.41%) | |
| No | 925 | 108 (87.10%) | 817 (93.59%) | |
| Work with or exposure to pesticides | <0.0001 | |||
| Yes | 363 | 66 (53.23%) | 297 (34.02%) | |
| No | 634 | 58 (46.77%) | 576 (65.98%) | |
| Non-occupational exposures | ||||
| Beer consumption | 0.0144 | |||
| Yes | 633 | 91 (73.39%) | 542 (62.08%) | |
| No | 364 | 33 (26.61%) | 331 (37.92%) | |
| Lija (moonshine) consumption | <0.0001 | |||
| Yes | 452 | 92 (74.19%) | 360 (41.24%) | |
| No | 545 | 32 (25.81%) | 513 (58.76%) | |
| Water consumption groups (liters per day) | 0.0010 | |||
| 1 | 124 | 8 (6.56%) | 116 (13.57%) | |
| 2 | 257 | 22 (18.03%) | 235 (27.49%) | |
| 3 | 196 | 22 (18.03%) | 174 (20.35%) | |
| 4 | 221 | 35 (28.69%) | 186 (21.75%) | |
| 5 | 179 | 35 (28.69%) | 144 (16.84%) | |
Notes: Univariate analysis associated between eGFR as dichotomous variable ≤60/>60 mL/min/1.73 m2 and all known risk factors for kidney disease and exposures.
p-values were calculated by chi-square test for category variables; and Wilcoxon two-sample test for continues variables unless otherwise indicated.
p-values were calculated by Fisher exact test.
Due to missing responses, the total number of respondents in each category, n, may be less than the total population surveyed, N = 997.
Known risk factors for kidney disease, occupational, and non-occupational exposures of interest were assessed for cases versus controls using univariate and multivariable analyses (Tables 1 and 2, respectively). In the univariate analysis, all known risk factors for kidney disease examined were associated with RI, with the exception of BMI (kg/m2) and DBP > 90 mmHg the day of the screening. Cases reported a diagnosis of hypertension more often than controls (23% vs. 11%, p < 0.0001), more often had a SBP >140 mmHg the day of evaluation (8% vs. 4%, p = 0.048), as well as a DBP >90 (6.5% vs. 3.2, p = 0.0783), though this did not reach statistical significance. Cases more often reported a history of diabetes (6.5% vs. 2.5%, p = 0.0208) and family history of ESKD (14.5% vs. 8.1%, p = 0.0215). No significant difference was found in the average BMI between the two groups (BMI 24.6 vs. 24.7 kg/m2, p = 0.967), nor was any significant difference found in the frequency of individuals with a BMI > 30 kg/m2. In the multivariable analysis, only age remained significantly associated with RI (p < 0.0001) among the known risk factors. BMI measured as a continuous variable became significantly associated with disease status, but the relationship was opposite that anticipated; each unit of higher BMI (kg/m2) was associated with lower odds of having an eGFR < 60 mL/min/1.73 m2 (OR = 0.944, p = 0.0196).
TABLE 2.
Multivariable analysis of demographics, known risk factors for kidney disease, and exposure frequencies between cases and controls.
| Mean ± SD or N (%) |
N 997 |
Odds ratio* (95%CI) |
p-Values* |
|---|---|---|---|
| Demographics and known risk factors for kidney diseasea | |||
| Age | 997 | 1.071 (1.055,1.088) | <0.0001 |
| Gender | 0.0042 | ||
| Male | 848 | 4.002 (1.548,10.344) | |
| Female | 149 | (Reference) | |
| Systolic blood pressure>140 |
0.7639 | ||
| Yes | 45 | 0.863 (0.329,2.262) | |
| No | 947 | (Reference) | |
| Diastolic blood pressure>90 |
0.4519 | ||
| Yes | 36 | 1.496 (0.524,4.277) | |
| No | 956 | (Reference) | |
| Self-reported history of diabetes |
0.5005 | ||
| Yes | 30 | 1.367 (0.551,3.392) | |
| No | 967 | (Reference) | |
| Family history of end-stage kidney disease |
0.0540 | ||
| Yes | 89 | 1.814 (0.990,3.324) | |
| No | 908 | (Reference) | |
| Body mass index (BMI kg/m2, continuous) |
997 | 0.944 (0.9,0.991) | 0.0196 |
| Occupational history/exposuresb | |||
| Agricultural field work | <0.0001 | ||
| Yes | 506 | 2.483 (1.587,3.886) | |
| No | 491 | (Reference) | |
| Agricultural non-field work |
0.6511 | ||
| Yes | 409 | 0.908 (0.598,1.379) | |
| No | 588 | (Reference) | |
| Sugar mill work | 0.0003 | ||
| Yes | 307 | 2.334 (1.478,3.685) | |
| No | 689 | (Reference) | |
| Work with rice | <0.0001 | ||
| Yes | 101 | 3.144 (1.847,5.353) | |
| No | 896 | (Reference) | |
| Work with corn | 0.0272 | ||
| Yes | 345 | 1.602 (1.054,2.435) | |
| No | 652 | (Reference) | |
| Work with banana | 0.0009 | ||
| Yes | 48 | 3.185 (1.605,6.323) | |
| No | 949 | (Reference) | |
| Work with sesame seed | 0.7636 | ||
| Yes | 140 | 0.919 (0.531,1.592) | |
| No | 857 | (Reference) | |
| Work with cotton | 0.9882 | ||
| Yes | 146 | 0.996 (0.599,1.656) | |
| No | 851 | (Reference) | |
| Work with beans | 0.1748 | ||
| Yes | 88 | 1.518 (0.831,2.773) | |
| No | 909 | (Reference) | |
| Work with “other crops” |
0.0854 | ||
| Yes | 68 | 1.786 (0.922,3.460) | |
| No | 929 | (Reference) | |
| Work with cattle | 0.5025 | ||
| Yes | 72 | 1.253 (0.648,2.425) | |
| No | 925 | (Reference) | |
| Work with or exposure to pesticides |
0.1441 | ||
| Yes | 363 | 1.375 (0.897,2.108) | |
| No | 634 | (Reference) | |
| Non-occupational exposuresb | |||
| Beer consumption | 0.8936 | ||
| Yes | 633 | 0.967 (0.589,1.586) | |
| No | 364 | (Reference) | |
| Lija consumption | 0.0023 | ||
| Yes | 452 | 2.102 (1.305,3.386) | |
| No | 545 | (Reference) | |
| Water consumption groups (liters per day) |
|||
| 1 | 124 | (Reference) | |
| 2 | 257 | 1.603 (0.662,3.881) | 0.2956 |
| 3 | 196 | 1.905 (0.781,4.649) | 0.1568 |
| 4 | 221 | 2.141 (0.911,5.031) | 0.0806 |
| 5 | 179 | 3.588 (1.52,8.467) | 0.0035 |
Logistic model: eGFR = Age + Gender + SBP > 140 + DBP > 90 + Diabetes + Family history of ESKD + BMI (continuous). eGFR was dichotomous variable ≤60/>60 mL/min/ 1.73 m2.
Logistic model: eGFR = Age + Gender + SBP > 140 + DBP > 90 + Diabetes + Family history of ESKD + BMI (continuous) + each exposure. eGFR was dichotomous variable ≤60/>60 mL/ min/1.73 m2.
Odds ratios and p-values were calculated using logistic regression models.
The univariate analysis revealed numerous statistically significant differences in the frequency of reported exposures between cases and controls (Table 1). Past performance of any agricultural field labor (OR = 2.48, p < 0.0001), having worked with rice (OR = 3.14, p < 0.0001), having worked with corn (OR = 1.60, p = 0.027), and having worked with bananas (OR = 3.19, p = 0.0009) (Table 2) remained statistically significant after controlling for known risk factors of kidney disease. Also of note, having worked in a sugar mill (OR = 2.33, p = 0.0003) became statistically significant in the multivariable analysis. Having performed agricultural non-field work was not significantly associated with RI (OR = 0.91, p = 0.651). Report of exposure to pesticides was significantly related to RI on univariate analysis (p < 0.0001), but not in multi-variable analysis (OR = 1.38, p = 0.144).
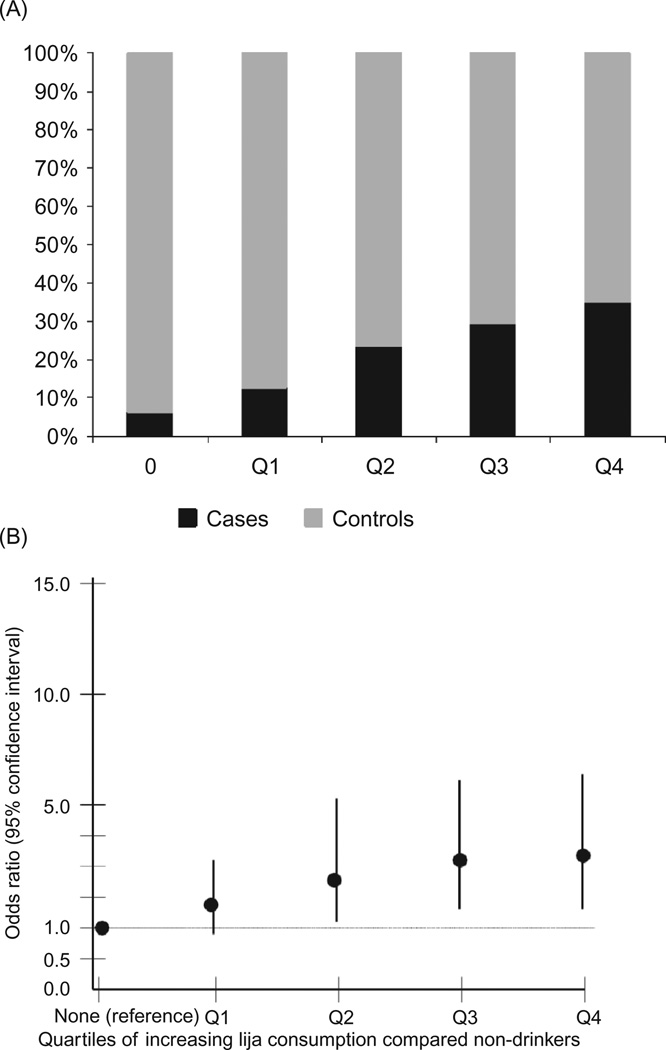
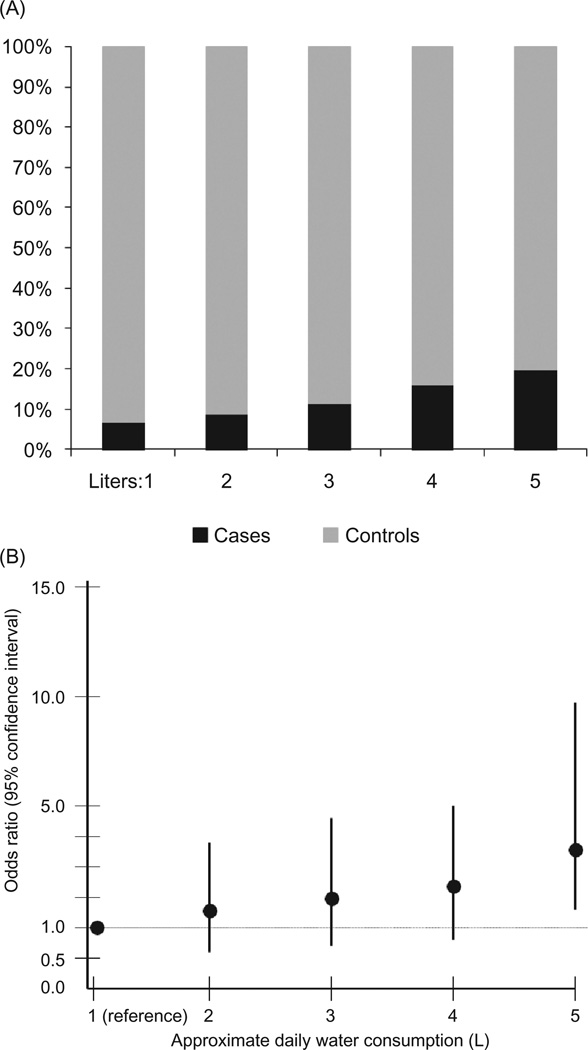
Regarding the non-occupational exposures of interest, consumption of lija and ingesting more than 5 L of water per day (compared to drinking 1 L per day) were associated with RI by a statistically significant degree in both univariate and multivariable analyses. Those ingesting lija had 2.10 times the odds of having an eGFR ≤ 60 mL/min/1.73 m2 (p = 0.0023) compared to those who did not drink lija. Those ingesting 5 L or more of water per day had 3.59 times the odds of having RI (p = 0.004) compared to those drinking 1 L of water per day. A general linear increase in the odds of RI was found with increasing consumption of lija and water, though the difference for water only reached statistical significance at 5 L (Figures 1A, B and 2A, B).
FIGURE 1.
(A) Percentage of cases and controls among non-drinkers and across quartiles of increasing lija consumption (years of consumption times frequency of use per month).(B) The odds ratios and 95% confidence intervals from the exploratory multivariable analysis that includes the entire study population (n = 997) for the association of increasing quar-tiles of lija consumption (years of consumption times frequency of use per month) with renal insufficiency (eGFR of ≤ 60 mL/min/1.73 m2 vs. eGFR ≥ 80 mL/min/1.73 m2). Non-lija drinkers are the referent group.
FIGURE 2.
(A) The percentage of cases and controls across reported levels of daily water consumption (in liters). (B) The odds ratios and 95% confidence intervals from the exploratory multivariable analysis that includes the entire study population (n = 997) for the association of increasing levels of water consumption with renal insufficiency (eGFR of ≤60 mL/min/1.73 m2 vs. eGFR ≥ 80 mL/min/1.73 m2). Consumers of 1 L of water per day are the referent group.
Matched nested case–control analysis: known risk factors for kidney disease, exposures of interest
In the prespecified nested case–control analysis, only results for men >18 years of age are reported (Table 3) (no significant difference in the results was found when women were included, results not shown). Regarding known risk factors for kidney disease, despite matching for age, increased age remained minimally associated with increased odds of having RI (OR = 1.03, p = 0.022). Increasing BMI (kg/m2) remained negatively associated with RI (OR = 0.94, p = 0.035). Dichotomized blood pressure values measured on the day of the screening, a reported history of diabetes, and a reported family history of ESKD were not associated with an increased risk of RI by a statistically significant amount.
TABLE 3.
Matched nested case–control multivariable analysis of known risk factors for kidney disease and exposure frequencies between cases and controls.
| Mean ± SD or N (%) | N 334 | Case (eGFR ≤ 60) n = 112 |
Control (eGFR ≥ 80) n = 222 |
Odds ratio* (95%CI) |
p-Value* |
|---|---|---|---|---|---|
| Demographics and known risk factors for kidney diseasea | |||||
| Age | 334 | 49.09 ± 11.93 | 46.27 ± 10.80 | 1.025 (1.004,1.047) | 0.0220 |
| Systolic blood pressure >140 | 0.8084 | ||||
| Yes | 22 | 9 (8.04%) | 13 (5.86%) | 1.144 (0.385,3.406) | |
| No | 312 | 103 (91.96%) | 209 (94.14%) | (reference) | |
| Diastolic blood pressure >90 | 0.7212 | ||||
| Yes | 20 | 8 (7.14%) | 12 (5.41%) | 1.227 (0.399,3.774) | |
| No | 314 | 104 (92.86%) | 210 (94.59%) | (reference) | |
| Self-reported diagnosis of diabetes | 0.8502 | ||||
| Yes | 17 | 6 (5.36%) | 11 (4.95%) | 0.903 (0.313,2.605) | |
| No | 106 | 106 (94.64%) | 211 (95.05%) | (reference) | |
| Family history of end-stage kidney disease | 0.1835 | ||||
| Yes | 41 | 17 (15.18%) | 24 (10.81%) | 1.59 (0.803,3.149) | |
| No | 293 | 95 (84.82%) | 198 (89.19%) | (reference) | |
| Body mass index (BMI kg/m2 continuous) |
334 | 24.72 ± 4.83 | 25.71 ± 4.63 | 0.944 (0.895,0.996) | 0.0352 |
| Occupational history/exposuresb | |||||
| Agricultural field work | 0.0008 | ||||
| Yes | 192 | 80 (71.43%) | 112 (50.45%) | 2.375 (1.435,3.931) | |
| No | 142 | 32 (28.57%) | 110 (49.55%) | (reference) | |
| Agricultural non-field work | 0.4138 | ||||
| Yes | 166 | 51 (45.54%) | 115 (51.80%) | 0.823 (0.515,1.314) | |
| No | 168 | 61 (54.46%) | 107 (48.20%) | (reference) | |
| Sugar mill work | 0.0011 | ||||
| Yes | 89 | 42 (37.50%) | 47 (21.17%) | 2.405 (1.42,4.075) | |
| No | 245 | 70 (62.50%) | 175 (78.83%) | (reference) | |
| Work with rice | 0.0021 | ||||
| Yes | 51 | 27 (24.11%) | 24 (10.81%) | 2.657 (1.424,4.957) | |
| No | 283 | 85 (75.89%) | 198 (89.19%) | (reference) | |
| Work with corn | 0.0262 | ||||
| Yes | 133 | 56 (50.00%) | 77 (34.68%) | 1.724 (1.067,2.787) | |
| No | 201 | 56 (50.00%) | 145 (65.32%) | (reference) | |
| Work with banana | 0.0351 | ||||
| Yes | 33 | 16 (14.29%) | 17 (7.66%) | 2.241 (1.058,4.746) | |
| No | 301 | 96 (85.71%) | 205 (92.34%) | (reference) | |
| Work with sesame seed | 0.4880 | ||||
| Yes | 52 | 21 (18.75%) | 31 (13.96%) | 1.249 (0.666,2.343) | |
| No | 282 | 91 (81.25%) | 191 (86.04%) | (reference) | |
| Work with cotton | 0.4037 | ||||
| Yes | 83 | 25 (22.32%) | 58 (26.16%) | 0.788 (0.45,1.379) | |
| No | 251 | 87 (77.68%) | 164 (73.87%) | (reference) | |
| Work with beans | 0.1617 | ||||
| Yes | 41 | 19 (16.96%) | 22 (9.91%) | 1.63 (0.822,3.229) | |
| No | 293 | 93 (83.04%) | 200 (90.09%) | (reference) | |
| Work with “other crops” | 0.0902 | ||||
| Yes | 27 | 14 (12.50%) | 13 (5.86%) | 2.028 (0.898,4.596) | |
| No | 307 | 98 (87.50%) | 209 (94.14%) | (reference) | |
| Work with cattle | 0.8918 | ||||
| Yes | 29 | 11 (9.82%) | 18 (8.11%) | 1.058 (0.472,2.369) | |
| No | 305 | 101 (90.18%) | 204 (91.89%) | (reference) | |
| Exposure to or work with pesticides | 0.0684 | ||||
| Yes | 154 | 62 (55.36%) | 92 (41.44%) | 1.569 (0.967,2.545) | |
| No | 180 | 50 (44.64%) | 130 (58.56%) | (reference) | |
| Non-occupational exposuresb | |||||
| Beer consumption | 0.2252 | ||||
| Yes | 266 | 84 (75.00%) | 182 (81.98%) | 0.704 (0.4,1.241) | |
| No | 68 | 28 (25.00%) | 40 (18.02%) | (reference) | |
| Lija (moonshine) consumption | 0.0060 | ||||
| Yes | 215 | 85 (75.89%) | 130 (58.56%) | 2.072 (1.232,3.484) | |
| No | 119 | 27 (24.11%) | 92 (41.44%) | (reference) | |
| Water consumption groups (liters per day) | |||||
| 1 | 42 | 7 (6.36%) | 35 (16.06%) | (reference) | |
| 2 | 75 | 20 (18.18%) | 55 (16.06%) | 1.648 (0.62,4.379) | 0.3164 |
| 3 | 63 | 19 (17.27%) | 44 (2.018%) | 1.923 (0.709,5.217) | 0.1989 |
| 4 | 80 | 31 (28.18%) | 49 (22.48%) | 2.585 (1.002,6.672) | 0.0495 |
| 5 | 68 | 33 (30.00%) | 35 (16.06%) | 4.585 (1.762,11.929) | 0.0018 |
Notes: Logistic regression evaluated the association of all known risk with eGFR. Cases were defined as those with an eGFR of ≤60 mL/min/1.73 m2 and controls had to have an eGFR ≥80 mL/min/1.73 m2, and were frequency matched by gender and age group 1:2.
Logistic model: eGFR = Age + Gender + SBP > 140 + DBP > 90 + Diabetes + Family history of ESKD + BMI (continuous).
Logistic model: eGFR = Age + Gender + SBP > 140 + DBP > 90 + Diabetes + Family history of ESKD + BMI (continuous) + each exposure
Odds ratios and p-values were calculated using logistic regression models.
Regarding occupational exposures, despite the difference in methodology and smaller sample in this analysis, the findings paralleled those of the multivariable exploratory analysis. Reporting past performance of any agricultural field labor (OR = 2.38, p = 0.0008), having worked with rice (OR = 2.66, p = 0.0021), corn (OR = 1.72, p = 0.0262), and bananas (OR = 2.24, p = 0.0351) were all associated with increased odds of having RI. Having worked in a sugar mill was similarly associated with an increase risk of RI (OR = 2.41, p = 0.001). Reporting exposure to pesticides was associated with an increased odds of RI, though this did not reach a level of statistical significance (OR = 1.57, p = 0.068).
Finally, with regards to non-occupational exposures, consumption of lija and more than 4 L of water per day (compared to drinking 1 L per day) were associated with RI (lija consumption, OR = 2.07, p = 0.0060; ingesting >4 L water per day, OR = 2.59, p = 0.05; ingesting >5 L water per day, OR = 4.59, p = 0.0018) with the same linear trend as noted above.
DISCUSSION
Our results support concerns for a regionally prevalent kidney disease in western Nicaragua that is independent of traditional risk factors, and associated with environmental exposures, particularly agricultural work, consumption of locally made alcohol, and water intake. To the best of our knowledge, this is the first peer-reviewed epidemiologic investigation of RI and associated risk factors in Nicaragua. Important design and methodological considerations highlight the need for further investigations, which are currently underway.
Traditional risk factors for kidney disease
An important finding is the lack of an association between diabetes and RI in both the exploratory (6.5% of cases and 2.5% of controls) and matched nested case–control (5.4% of cases and 5.0% of controls) multivariable analyses. By comparison, in the United States, 6.8% of adults over 20 years of age report having diabetes,9 whereas approximately 21.5% of those with a GFR between 15 and 60 mL/min/1.73 m2 report having diabetes.10 In Morelia, Mexico, an urban region with an estimated population of 600,000, screening demonstrated a 10.9% general prevalence of diabetes and a 25% prevalence among those with a GFR <60 mL/min/1.73 m2.11 Self-reporting likely underestimates the prevalence of diabetes in our study population,11 but we expect that underreporting occurred equally between cases and controls, and therefore did not mask a positive association with RI.
Cases more often reported having hypertension than controls and were more likely to have SBP > 140 mmHg on the day of the screening. Elevated SBP > 140 mmHg was statistically significant in the exploratory univariate analysis, but became statistically insignificant in the multivariable analyses. Because hypertension is both a cause and effect of RI, and because we did not review participants’ use of antihy-pertensive medications, the significance of these findings is uncertain, but does not likely play a primary role in the development of RI in this region.
In multivariable analysis, lower BMI values were associated with greater odds of having RI. We included BMI in the multivariable analyses to control for differences in serum creatinine associated with body mass. The concern was that a higher BMI might reflect greater muscle mass, therefore incorrectly categorizing participants with greater muscle mass as cases. Our finding is opposite from what was expected. One explanation for this is that those with RI are in a catabolic state, reflected by a lower BMI. Definitive conclusions cannot be made without a more detailed description of participants’ body composition.
Agricultural field work
Consistent with regional reports, we found a positive association between agricultural field labor and RI throughout all analyses. Additionally, those specifically reporting having worked in a sugar mill or with rice, corn, or bananas had statistically significant increased odds of RI. This may reflect a specific exposure and warrants further investigation beyond the limits of this study.
Kidney disease among farmers and farm workers
Health researchers consider farmers and farm workers distinct subpopulations, recognizing their work-related morbidity and mortality and unique risk factors for disease, including contact with pesticides and strenuous working conditions.12 We found no studies of these populations describing an elevated mortality rate from kidney disease, but note that most mortality data come from death certificates in developed countries where renal replacement therapy is available. We found no prevalence estimate of CKD or ESKD among farmers or farm workers. The Agricultural Health Study, a prospective cohort study of nearly 60,000 pesticide applicators in the United States, holds promise for a reliable estimate of ESKD among agricultural workers, as it has previously expressed its intent to cross-reference its membership with the United States Renal Data Survey.13
Studies of morbidity and mortality among farmers and/or farm workers in Latin America are also limited. Garcia-Trabanino et al. published a case series of 205 incident dialysis patients presenting to a referral hospital in El Salvador between November 1999 and March 2000. Only 33% of cases had traditional risk factors for kidney disease (e.g., diabetes, hypertension, consumption of NSAIDS). The remaining cases were largely male farmers.14 In a second publication, results from a volunteer screening conducted in El Salvador, Garcia-Trabanino found 45.7% of 291 male coastland residents, largely farm workers, with significant levels of proteinuria.15
Specific exposures: pesticides, alcohol, water Pesticides
It is important to note that pesticide use is of particular concern in this region. Several studies describe local environmental contamination from common pesticides, as well as pesticide residues in human milk,16 and in the urine of pesticide applicators and their family members.17,18 Large numbers of farm workers have implicated pesticide use in the reported epidemic of kidney disease.19 Allegations of farm worker poisoning in the 1970s and 1980s were recently the subject of litigation.20
In this study, cases reported pesticide exposure more often than controls. This difference was close to, but did not reach statistical significance in multi-variable analyses. Study limitations and the complexity of assessing pesticide exposure from multiple sources prevent drawing definitive conclusions. For example, local kidney disease may be a product of several risk factors (e.g., crystalline silica, heavy metal, and pesticide exposure). Further, our use of a convenience sample and reliance upon self-reporting pesticide exposure may introduce selection and reporting errors biasing our results toward the null. The literature contains few studies examining the relationship between pesticide exposure and kidney disease in humans. These studies are small and have limited designs, preventing any speculation regarding the significance of our results. Further study is needed to understand the effects of chronic, multiple pesticide exposures on kidney function.
Alcohol
Lija consumption was associated with a significantly increased, dose-related, odds of having RI. Lija is locally produced alcohol, manufactured and distributed without regulation. In 2006, 788 cases of methanol poisoning (44 deaths) were reported in western Nicaragua due to lija contamination.21 Public health officials have observed lija being prepared and stored in industrial metal containers previously holding pesticides raising concerns that consumers may be exposed to nephrotoxins such as lead or other heavy metals.
In the United States, more than two or four alcoholic drinks per day have been associated with an increased risk of losing kidney function in the general population.22,23 This level of consumption was not associated with CKD diagnosed through hospital ICD-9 discharge diagnoses, but moonshine consumption was associated with nephrosclerosis, interstitial nephritis, and RI.24 Moonshine stills are often constructed with automotive parts that contain lead,24 and persistent lead levels have been found in consumers,25,26 leading to the presumption that lead ingestion is the culprit for renal damage.24 Further, it has been suggested that lead may act as a cofactor with more established renal risk factors.27 The same principle may apply to the production and storage of lija.
Water consumption
The positive association of water consumption with RI was surprising. We assumed that those drinking less water were more likely to experience cyclic dehydration, potentially harmful to the kidneys. Alternative explanations include the following: (1) those suffering repeated bouts of dehydration consume more water rather than less; (2) increased water intake is indicative of a urine concentrating defect, perhaps due to tubular injury, leading to obligatory water loss and the need for increased water intake; (3) water contamination is a contributing factor and those consuming more water have greater exposure to the offending agent; and (4) water consumption analysis may be confounded by behavioral and societal factors. For example, limited educational campaigns promoting water consumption have targeted those at perceived increased risk for RI, such as agricultural laborers.
Strengths and limitations
An important strength of this study is the replication of findings in the exploratory and matched case– control analysis, supporting the validity of the associations found between exposures and RI. Notably, the case–control design prevents drawing any definitive conclusions regarding cause and effect, and the homogeneity of the study population, as evidenced by the predominance of young males, limits generalizability of our data, though it may have implications regarding the etiology of disease, as well as its social and economic impacts.
It is important to consider several additional limitations. First, our recruitment and use of volunteers may introduce selection bias precluding a valid prevalence estimate. Those at highest risk for disease may have been more likely to volunteer, leading to an overestimate of disease prevalence. Dependence on a single serum creatinine measurement for the detection of disease increases the risk of measurement bias from con-founders such as medications and dehydration, more likely to provide false positives than negatives. Misclas-sification may have occurred, as self-reporting was used to assess diabetes, for example.
We used the MDRD GFR estimating formula, a conservative definition of disease (GFR < 60 mL/ min/1.73 m2), and controlled for BMI in multivari-able analyses to reduce the risk of false positives from daily fluctuations in serum creatinine (e.g., from diet or dehydration) and muscle mass, to minimize the impact of imprecision from using the MDRD estimation at higher levels of GFR, and to reduce error from the lack of calibration of our creatinine assay with the MDRD lab in the Cleveland Clinic. As a consequence of this, and of not testing for proteinuria, our study lacked sensitivity for the detection of early disease.
SUMMARY
Our findings support concerns of public health professionals in Nicaragua for an endemic kidney disease among young agricultural workers in the regions of León and Chinandega. This appears to be independent of diabetes and likely hypertension and associated with occupational and environmental exposures, including agricultural field work, lija alcohol and water consumption. We suggest that the etiology is likely multifactorial. Our data highlight the critical need for further investigation in a region of the world where access to dialysis and transplantation is severely limited, and prevention is imperative.
Acknowledgments
The authors received no external funding to conduct this study. The authors would like to thank the citizens and health professionals who participated in this study.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
REFERENCES
- 1.Distribucion porcentual de las defunciones por grandes grupos de causes segun SILAIS de residencia. [Accessed April 29, 2008];Nicaragua Ministry of Health. 2004 2006. 4-29-2008. Electronic citation. Available at: http://www.minsa.gob.ni/estadisticas/ind2004/distsilais.html.
- 2.Distribucion porcentual de las defunciones por grandes grupos de causes segun SILAIS de residencia. [Accessed April 29, 2008];Nicaragua Ministry of Health. 2003 2006. 4-29-2008. Electronic citation. Available at: http://www.minsa.gob.ni/estadisticas/ind2003/distsilais.html.
- 3.Distribucion porcentual de las defunciones por grandes grupos de causas segun SILAIS de residencia. [Accessed April 29, 2008];Nicaragua Ministry of Health. 2005 2006. 4-29-2008. Electronic citation. Available at: http://www.minsa.gob.ni/estadisticas/ind2005/distsilais.html.
- 4.Indicadores demagráficos. [Accessed April 29, 2008];Ministry of Health, Republic of Nicaragua. 2005 2007. 4-29-2008. Electronic citation. Available at: http://www.minsa.gob.ni/estadisticas/ind2005/inddem.html.
- 5.Minino AM, Heron MP, Smith BL. Deaths: Preliminary data for 2004. Natl Vital Stat Rep. 2006;54:1–49. [PubMed] [Google Scholar]
- 6.Arteaga Y. Historia laboral agrícola como factor de Riesgo para deterioro de la función renal en al Occidedente del pais, Enero 2003–Enero 2005. 2005 4-29-2008. Unpublished work. [Google Scholar]
- 7.Espinoza CA. Relación entre patrón de ingesta de licor en hombres y deterioro de la función renal, Enero 2003–Enero 2005. 2005 4-29-2008. Unpublished work. [Google Scholar]
- 8.Levey AS, Green T, Kusek JW, Beck GJ MDRD Study Group. A simplified equation to predict glomerular filtration rate from serum creatinine. J Am Soc Nephrol. 2000;11:155A. [Google Scholar]
- 9.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 10.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 11.Amato D, varez-Aguilar C, Castaneda-Limones R, et al. Prevalence of chronic kidney disease in an urban Mexican population. Kidney Int Suppl. 2005;97:S11–S17. doi: 10.1111/j.1523-1755.2005.09702.x. [DOI] [PubMed] [Google Scholar]
- 12.Mobed K, Gold EB, Schenker MB. Occupational health problems among migrant and seasonal farm workers. West J Med. 1992;157:367–373. [PMC free article] [PubMed] [Google Scholar]
- 13.Alavanja MC, Sandler DP, McMaster SB, et al. The agricultural health study. Environ Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trabanino RG, Aguilar R, Silva CR, Mercado MO, Merino RL. End-stage renal disease among patients in a referral hospital in El Salvador. Rev Panam Salud Publica. 2002;12:202–206. doi: 10.1590/s1020-49892002000900009. [DOI] [PubMed] [Google Scholar]
- 15.Gracia-Trabanino R, Dominguez J, Jansa JM, Oliver A. Proteinuria and chronic renal failure in the coast of El Salvador: Detection with low cost methods and associated factors. Nefrologia. 2005;25:31–38. [PubMed] [Google Scholar]
- 16.Romero ML, Dorea JG, Granja AC. Concentrations of organochlorine pesticides in milk of Nicaraguan mothers. Arch Environ Health. 2000;55:274–278. doi: 10.1080/00039890009603418. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez T, Younglove L, Lu C, et al. Biological monitoring of pesticide exposures among applicators and their children in Nicaragua. Int J Occup Environ Health. 2006;12:312–320. doi: 10.1179/oeh.2006.12.4.312. [DOI] [PubMed] [Google Scholar]
- 18.Dowling KC, Blanco LE, Martinez I, Aragon A, Bernard CE, Krieger RI. Urinary 3,5,6-trichloro-2-pyridinol levels of chlorpyrifos in Nicaraguan applicators and small farm families. Bull Environ Contam Toxicol. 2005;74:380–387. doi: 10.1007/s00128-004-0595-6. [DOI] [PubMed] [Google Scholar]
- 19.Reyes G. A deadly mystery. The Miami Herald. 2007 May 6;:1A. [Google Scholar]
- 20.Rogers T. Unwelcome exposure. Lancet. 2004;364:1025–1026. doi: 10.1016/S0140-6736(04)17078-5. [DOI] [PubMed] [Google Scholar]
- 21.Methanol poisoning in Leon, Nicaragua. [Accessed April 29, 2008];Pan American Health Organization. 2006 4-29-2008. Electronic citation. Available at: http://www.paho.org/English/DD/PED/nicaraguaMetanol.htm.
- 22.Perneger TV, Whelton PK, Puddey IB, Klag MJ. Risk of end-stage renal disease associated with alcohol consumption. Am J Epidemiol. 1999;150:1275–1281. doi: 10.1093/oxfordjournals.aje.a009958. [DOI] [PubMed] [Google Scholar]
- 23.Shankar A, Klein R, Klein BE. The association among smoking, heavy drinking, and chronic kidney disease. Am J Epidemiol. 2006;164:263–271. doi: 10.1093/aje/kwj173. [DOI] [PubMed] [Google Scholar]
- 24.Vupputuri S, Sandler DP. Lifestyle risk factors and chronic kidney disease. Ann Epidemiol. 2003;13:712–720. doi: 10.1016/s1047-2797(03)00066-8. [DOI] [PubMed] [Google Scholar]
- 25.Pegues DA, Hughes BJ, Woernle CH. Elevated blood lead levels associated with illegally distilled alcohol. Arch Intern Med. 1993;153:1501–1504. [PubMed] [Google Scholar]
- 26.Ellis T, Lacy R. Illicit alcohol (moonshine) consumption in West Alabama revisited. South Med J. 1998;91:858–860. doi: 10.1097/00007611-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Ekong EB, Jaar BG, Weaver VM. Lead-related nephrotoxicity: A review of the epidemiologic evidence. Kidney Int. 2006;70:2074–2084. doi: 10.1038/sj.ki.5001809. [DOI] [PubMed] [Google Scholar]