Abstract
We have previously shown that simple N-acyl or N-alkyl polyamines bind to and sequester Gram-negative bacterial lipopolysaccharide, affording protection against lethality in animal models of endotoxicosis. Several iterative design-and-test cycles of SAR studies, including high-throughput screens, had converged on compounds with polyamine scaffolds which have been investigated extensively with reference to the number, position, and length of acyl or alkyl appendages. However, the polyamine backbone itself had not been explored sufficiently, and it was not known if incremental variations on the polymethylene spacing would affect LPS-binding and neutralization properties. We have now systematically explored the relationship between variously elongated spermidine [NH2-(CH2)3-NH-(CH2)4-NH2] and norspermidine [NH2-(CH2)3-NH-(CH2)3-NH2] backbones, with the N-alkyl group being held constant at C16 in order to examine if changing the spacing between the inner secondary amines may yield additional SAR information. We find that the norspermine-type compounds consistently showed higher activity compared to corresponding spermine homologues.
Endotoxins, or lipopolysaccharides (LPS), a structural component of the outer membrane of Gram-negative bacteria,1 play a pivotal role in septic shock, a syndrome of systemic toxicity which occurs frequently as a sequel to serious systemic Gram-negative infections.2 The activation by LPS of the innate immune response, mediated via toll-like receptor-4,3 leads to an uncontrolled production of numerous inflammatory mediators, including tumor necrosis factor-α (TNF-α), interleukin-1 β (IL-1β), and interleukin-6 (IL-6),4 precipitating a systemic inflammatory response, culminating in the frequently fatal syndrome of multiple system organ failure.5
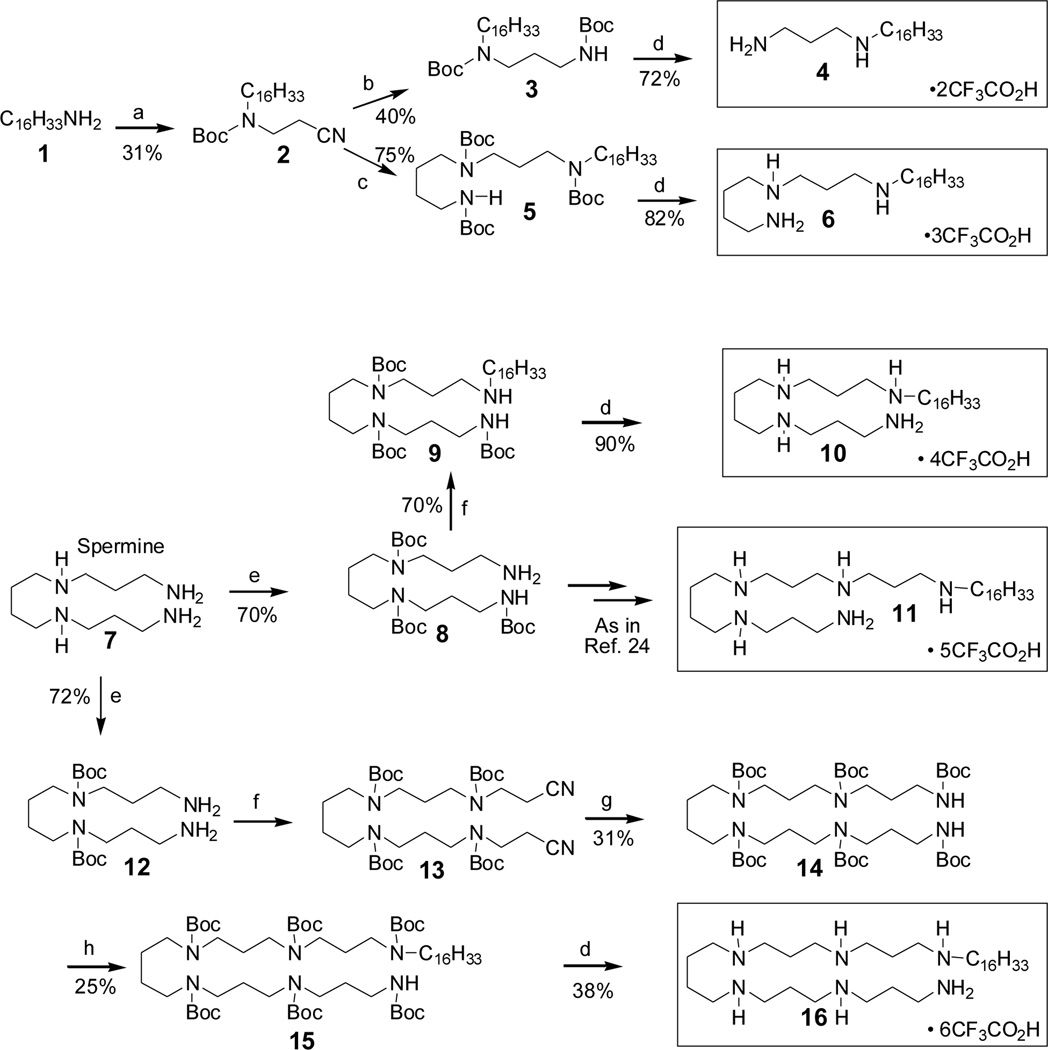
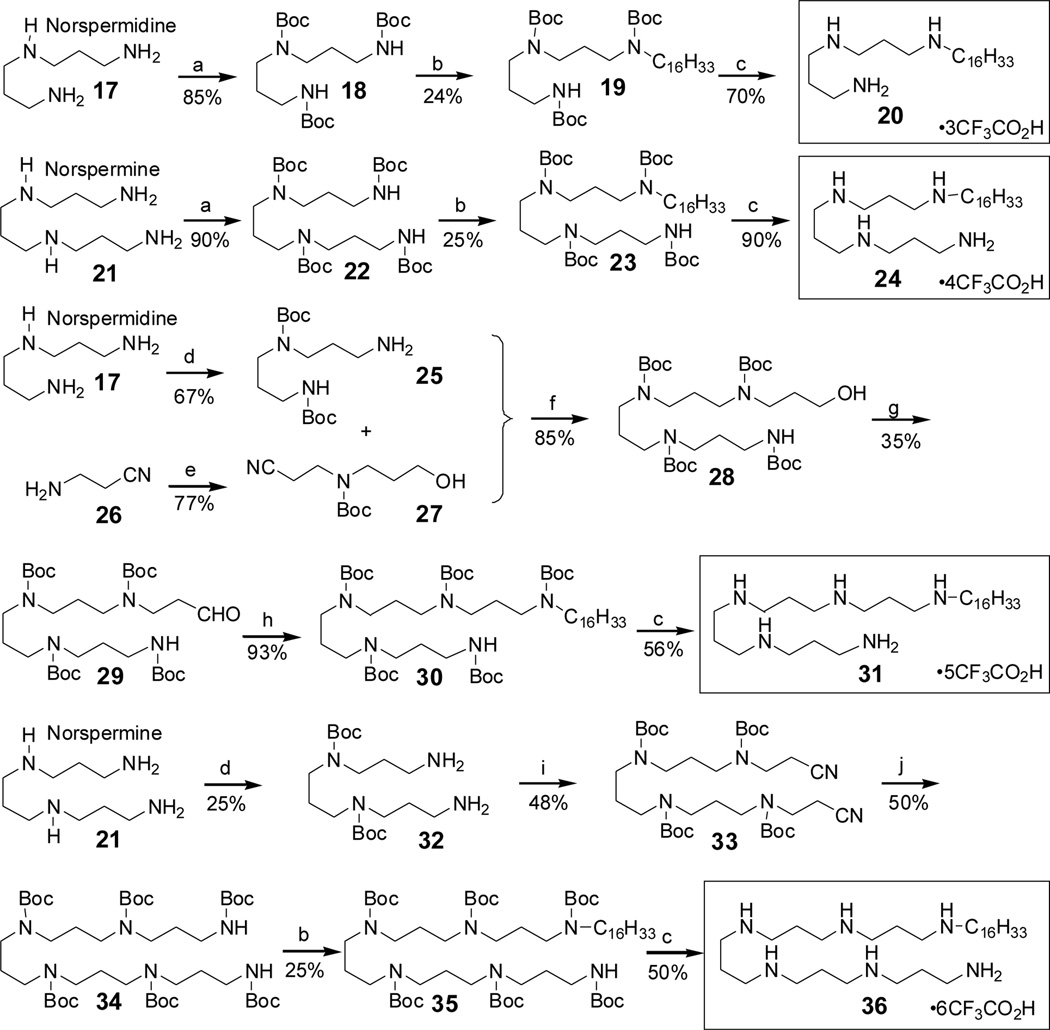
The toxicity of LPS resides in its structurally highly conserved glycolipid component called Lipid A,6 which is composed of a hydrophilic, bis-phosphorylated diglucosamine backbone, and a hydrophobic domain comprised of acyl chains in amide and ester linkages7 (Fig. 1). We had identified the pharmacophore necessary for optimal recognition and neutralization of lipid A,8 and iterative cycles of pharmacophore-based ligand synthesis and screening exploring various chemotypes with differing number, position, and length of acyl or alkyl appendages9–19 exploring had converged on an N1,mono-alkyl-mono-homologated spermine derivative, DS-96, which sequestered LPS and neutralized its toxicity with high potency, affording complete protection in a murine model of LPS-induced lethality.20 However, the polyamine backbone [spermine: NH2-(CH2)3-NH-(CH2)4-NH-(CH2)3-NH2], itself, had not been explored sufficiently, and it was not known if incremental variations on the polymethylene spacing would affect LPS-binding and neutralization properties. Furthermore, our original hypothesis pertaining to the NH2↔NH2 pharmacophore allowing for simultaneous interactions with the lipid A phosphate groups/KDO carboxylates8,17,21 was empirical, and only the effect of acyl/and or alkyl chain lengths had been examined. Two recent crystal structures of LPS indicate that the distance between the P atoms in the phosphate groups of lipid A is 12.45 Å (Fig. 1).22,23 The Cartesian distance between the centroids of the charge-delocalized electron cloud of the PO42- ↔PO42- was computed to about 14.5 Å. It was of interest, therefore, to experimentally test this premise of our pharmacophore model in a congeneric series of compounds wherein only the length of the polyamine backbone, and not the hydrophobic appendage, was systematically varied. We have systematically explored the relationship between variously elongated spermidine [NH2-(CH2)3-NH-(CH2)4-NH2] and norspermidine [NH2-(CH2)3-NH-(CH2)3-NH2] backbones, with the N-alkyl group being held constant at C16. We elected to examine these two sets of analogues which vary one from the other by only one methylene unit in order to examine if changing the spacing between the inner secondary amines may yield additional SAR data. The spermidine/spermine and norspermidine/norspermine series were synthesized (Schemes 1 and 2, respectively) using procedures reported earlier by us.16,17,19–21,24,25 Compound 11 represents DS-96.20,24
Figure 1.
Atomic structure of lipid A showing inter-P-P distance of 12.45Å. From the crystal structure of LPS (PDB entry 2e59).
Scheme 1.
Synthesis of the Spermidine/Spermine series of N-alkylpolyamines
Reagents:
a. (i)CH2=CHCN, MeOH, r.t, 15h; (ii) Boc2O (excess), MeOH, r.t, 12h.
b. AcOH, Pd(OH)2/C, H2, 50 psi; Boc2O (excess), MeOH, r.t, 12h.
c. (i) 1,4-Diaminobutane (excess), Pd(OH)2/C, H2, 60 psi; (ii) Boc2O (excess), MeOH, r.t, 12 h.
d. CF3CO2H (excess), r.t.
e. (i) CF3COOEt (1or 2 Eq.), MeOH. −78°C to 0° C, 1h; (ii) Boc2O (excess); (iii) LiOH, THF, r.t.
f. (i) CH2=CHCN (2 Eq.), MeOH, r.t, 15 h; (ii) Boc2O (excess), MeOH, r.t, 12h.
g. AcOH, Pd(OH)2/C, H2, 50 psi; Boc2O (excess), MeOH, r.t, 12 h.
h. NaH, C16H33I, DMF, −15°C-r.t, 24h.
Scheme 2.
Synthesis of the Norspermidine/Norspermine series.
Reagents: a. Boc2O (excess), MeOH, rt, 12 h. b. NaH (excess), C16H33I, DMF, − 15°C-r.t., 24 h. c. TFA, r.t, 45 min d. (i) CF3COOEt, MeOH, −78 °C to 0 °C, 1h; (ii) Boc2O (excess), MeOH, r.t, 12 h; (iii) LiOH,THF, r.t. e. 3-bromopropan-1-ol, K2CO3, DMF, 60°C (ii) Boc2O (excess), MeOH, r.t, 12h. f. (i) MeOH, Pd(OH)2/C, H2, 60 psi; (ii) Boc2O (excess), MeOH, r.t, 12h. g. PCC, DCM, r.t. h. (i)C16H33NH2, NaCNBH3, glacial AcOH (5 drops), anhyd. MeOH, r.t, 24h (ii) Boc2O (excess), MeOH, r.t, 12h. i. i) CH2CHCN, MeOH, r.t, 6 h; ii) Boc2O (excess), MeOH, rt, 12 h. j. i) Pd(OH)2/C, Glacial acetic acid, H2, 60 psi; ii) Boc2O (excess), MeOH, rt, 12 h.
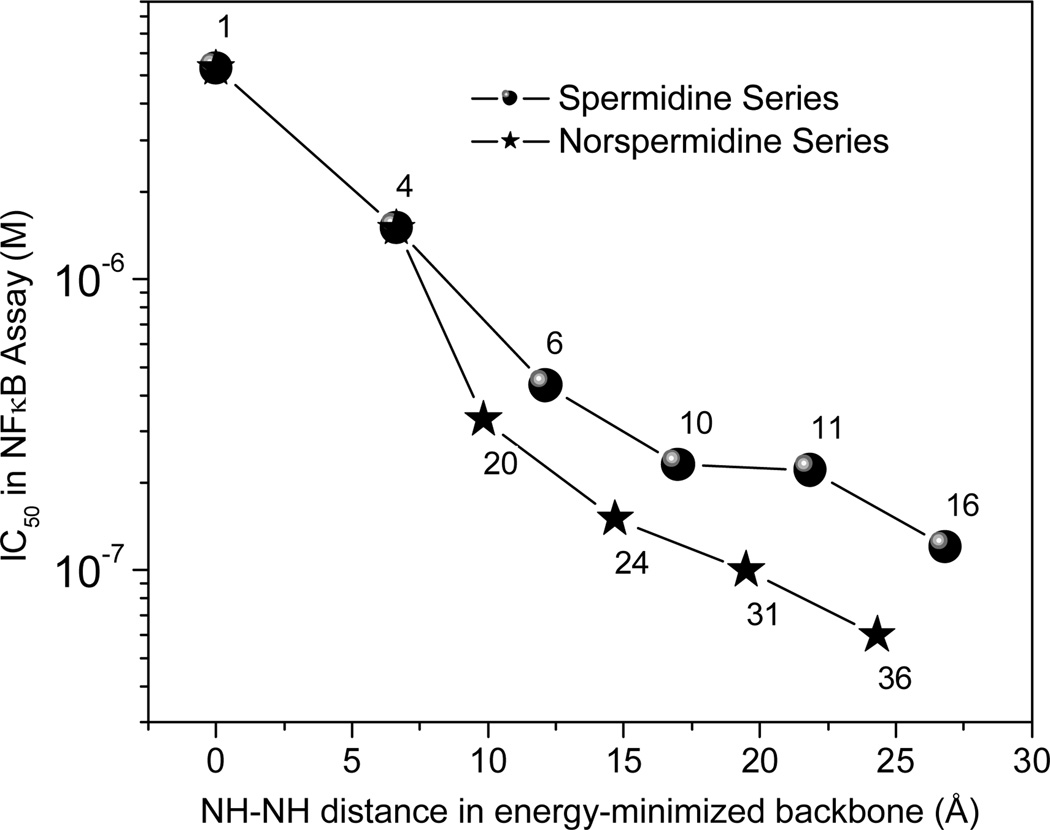
The target compounds were evaluated for LPS sequestration activity in a sensitive NF-κB reporter gene assay using HEK-293 cells stably transfected with human TLR4, MD-2 and CD14 with a secreted alkaline phosphatase readout.17,19,20 As shown in Fig. 2, there is a progressive increase in the potency of both series of compounds with increasing length of the backbone with an inflection point at about 15 Å, which correlates well with the observed inter-phosphate distance. Further elongation of the backbone does not result in a commensurate increase in activity. However, the norspermine-type compounds consistently showed higher activity compared to the spermine homologues. Specifically, the longest 3-3-3-3-3 (bis-homologated N1-alkyl-norspermine) compound 36 exhibited higher potency than the 3-3-4-3-3 bis-homologated spermine analogue, 16, as was also the case for the mono-homologated pair 31 and 11, and the norspermine/spermine compounds, 24 and 10. These data suggest that the propylene spacing of the norspermine series allows for better interactions with LPS, presumably by allowing for more favorable interactions of the inner secondary amines with the carboxylates groups of the anionic KDO sugars of the core glycolipid.20,21
Figure 2.
Correlation of energy-minimized inter-nitrogen distances in spermine/norspermine compounds and LPSsequestering activity in the NF-κB reporter gene assay.
Somewhat unexpectedly, we observed that increasing the backbone length did not result in a ‘saturation’ in the activity profile, but led to incremental enhancements of LPS-neutralizing activity even at about 25 Å. At first sight this may seem to be incongruent with the original hypothesis of an optimal distance of ~15 Å, but it is to be noted that these higher homologues have also increased aqueous solubility (and lower binding to human serum albumin26) due to the presence of additional amino groups. It is possible, therefore, that the introduction of highly polar functional groups on the molecule that would substantially increase water solubility may result in more potent analogues. Such functional groups may be, for instance, hydroxyls. Anionic groups such as phosphates or sulfonates would render the molecule zwitterionic and may also hinder ionic interactions with the internal KDO carboxylates on the core glycolipid. These hypotheses remain to be tested.
Supplementary Material
Acknowledgements
Funding from the NIH (1U01AI077947) is gratefully acknowledged.
Footnotes
Supplemental data
Synthetic procedures and characterization data of target compounds and precursors.
References
- 1.Rietschel ET, Kirikae T, Schade FU, Mamat U, Schmidt G, Loppnow H, Ulmer AJ, Zähringer U, Seydel U, Di Padova F, et al. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 2.Hurley JC. Drug Saf. 1995;12:183–195. doi: 10.2165/00002018-199512030-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ulevitch RJ. Immunol. Res. 2000;21:49–54. doi: 10.1385/IR:21:2-3:49. [DOI] [PubMed] [Google Scholar]
- 4.Dinarello CA. Curr. Top. Microbiol. Immunol. 1996;216:133–165. doi: 10.1007/978-3-642-80186-0_7. [DOI] [PubMed] [Google Scholar]
- 5.Bone RC. Clin. Chest Med. 1996;17:175–181. doi: 10.1016/s0272-5231(05)70307-5. [DOI] [PubMed] [Google Scholar]
- 6.Holst O, Ulmer AJ, Brade H, Rietschel ET. On the chemistry and biology of bacterial endotoxic lipopolysaccharides. In: Masihi N, editor. Immunotherapy of infections. Vol. 94. New York, Basel, Hong Kong: Marcel Dekker, Inc.; pp. 281–308. [Google Scholar]
- 7.Ferguson AD, Hofmann E, Coulton J, Diedrichs K, Welte W. Science. 1998:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 8.David SA. J. Molec. Recognition. 2001;14:370–387. doi: 10.1002/jmr.549. [DOI] [PubMed] [Google Scholar]
- 9.Blagbrough IS, Geall AJ, David SA. Bioorg. Med.Chem. Lett. 2000;10:1959–1962. doi: 10.1016/s0960-894x(00)00380-2. [DOI] [PubMed] [Google Scholar]
- 10.Burns MR, Wood SJ, Miller KA, Nguyen T, Cromer JR, David SA. Bioorg. Med. Chem. 2005;13:2523–2536. doi: 10.1016/j.bmc.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Burns MR, Jenkins SA, Kimbrell MR, Balakrishna R, Nguyen TB, Abbo BG, David SA. J. Med. Chem. 2007;50:877–888. doi: 10.1021/jm061198m. [DOI] [PubMed] [Google Scholar]
- 12.Burns MR, Jenkins SA, Vermeulen NM, Balakrishna R, Nguyen TB, Kimbrell MR, David SA. Bioorg. Med. Chem. Lett. 2006;16:6209–6212. doi: 10.1016/j.bmcl.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns MR, Jenkins SA, Wood SJ, Miller K, David SA. J. Comb. Chem. 2006;8:32–43. doi: 10.1021/cc0500755. [DOI] [PubMed] [Google Scholar]
- 14.David SA, Silverstein R, Amura CR, Kielian T, Morrison DC. Antimicrob. Agents Chemother. 1999;43:912–919. doi: 10.1128/aac.43.4.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khownium K, Wood SJ, Miller KA, Balakrishna R, Nguyen TB, Kimbrell MR, Georg GI, David SA. Bioorg. Med. Chem. Lett. 2006;16:1305–1308. doi: 10.1016/j.bmcl.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 16.Miller KA, Suresh Kumar EVK, Wood SJ, Cromer JR, Datta A, David SA. J. Med. Chem. 2005;48:2589–2599. doi: 10.1021/jm049449j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen TB, Adisechan A, Suresh Kumar EVK, Balakrishna R, Kimbrell MR, Miller KA, Datta A, David SA. Bioorg. Med. Chem. 2007;15:5694–5709. doi: 10.1016/j.bmc.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood SJ, Miller KA, Lushington GH, Burns MR, David SA. Comb. Chem. High Throughput. Screen. 2006;9:27–36. doi: 10.2174/138620706775213903. [DOI] [PubMed] [Google Scholar]
- 19.Wu W, Sil D, Szostak ML, Malladi SS, Warshakoon HJ, Kimbrell MR, Cromer JR, David SA. Bioorg. Med. Chem. 2008;17:709–715. doi: 10.1016/j.bmc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sil D, Shrestha A, Kimbrell MR, Nguyen TB, Adisechan AK, Balakrishna R, Abbo BG, Malladi S, Miller KA, Short S, Cromer JR, Arora S, Datta A, David SA. Antimicrob. Agents Chemother. 2007;58:2811–2819. doi: 10.1128/AAC.00200-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo JX, Wood SJ, David SA, Lushington GH. Bioorg. Med. Chem. Lett. 2006;16:714–717. doi: 10.1016/j.bmcl.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Ohto U, Fukase K, Miyake K, Satow Y. Science. 2007;316:1632–1634. doi: 10.1126/science.1139111. [DOI] [PubMed] [Google Scholar]
- 23.Ferguson AD, Kodding J, Walker G, Bos C, Coulton JW, Diederichs K, Braun V, Welte W. Structure. 2001;9:707–716. doi: 10.1016/s0969-2126(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 24.Shrestha A, Li R, Sil D, Pardeshi NN, Schwarting N, Schorno KS, Rajewski RA, Datta A, David SA. J. Pharm. Sci. 2008;97:5376–5385. doi: 10.1002/jps.21361. [DOI] [PubMed] [Google Scholar]
- 25.Warshakoon HJ, Burns MR, David SA. Antimicrob. Agents Chemother. 2009;53:57–62. doi: 10.1128/AAC.00812-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen TB, Suresh Kumar EV, Sil D, Wood SJ, Miller KA, Warshakoon HJ, Datta A, David SA. Mol. Pharm. 2008 doi: 10.1021/mp8001123. In Press. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.