Abstract
Implant-associated infection is a serious complication in orthopedic surgery, and endowing implant surfaces with antibacterial properties could be one of the most promising approaches for preventing such infection. In this study, we developed cefazolin loaded biodegradable polypeptide multilayer nanofilms on orthopedic implants. We found that the amount of cefazolin released could be tuned. A high local concentration of cefazolin was achieved within the first a few hours and therefore may inhibit bacterial colonization in the critical post-implantation period. The developed cefazolin loaded nanofilms showed their in vitro efficacy against Staphylococcus aureus (S. aureus); the more antibiotics loaded, the longer the nanocoated implant had antibacterial properties. More interestingly, antibiotic-loaded polypeptide multilayer nanofilms also improved osteoblast bioactivity including cell viability and proliferation. These findings suggested that biodegradable polypeptide multilayer nanofilms as antibiotic carriers at the implant/tissue interface are compatible with human cells such as osteoblasts and bactericidal to bacteria such as S. aureus. These characteristics could be promising for preventing implant-associated infection and potentially improving bone healing.
Keywords: Implant-associated infection, local antibiotic delivery, electrostatic layer-by-layer self-assembly, antibiotic, polypeptide
INTRODUCTION
Implant-associated infection is one of the most serious complications in orthopedic surgery. Of the more than two million orthopedic implants used in patients annually in the United States, approximately 4% get infected1. The number of implant-associated infections will continue to rise as more baby boomers receive biomedical implants. Bone infections associated with foreign body materials are especially difficult to treat. Removal of the infected implants2,3, long-term systemic antibiotic therapy, and multiple revisions with radical debridement are frequently required1,4,5. The consequences of infection can be devastating and may lead to prolonged hospitalization, poor functional outcome, sepsis, and even amputation6.
The most probable reason that implant-associated infection is difficult to treat is that pathogens, primarily S. aureus and S. epidermidis, colonize on the implant surface and form a bio-film7–10. Bio-films are resistant to both the immune response and systemic antibiotic therapies1. It is, therefore, of great importance to prevent the initial bacterial adhesion thereby preventing bio-film formation on implants. Antibiotic coatings on implants could be an effective approach to reduce bacterial colonization in vitro and bio-film formation in vivo11–13.
Many approaches for surface coating, such as dip coating, spin coating, and plasma spray, have been developed to achieve an antibacterial surface14–16. Among these techniques, electrostatic layer-by-layer (LBL) self-assembly nanotechnology is one of the most promising methods17. This method is simply based on the alternative deposition of oppositely charged polyelectrolyte layers. The driving force for film construction is the alternating charges (positive and negative) that appear after each layer of polyelectrolyte deposition. This technique offers multiple advantages over other approaches. First, the buildup process may be easily performed by simple adsorption procedures on any surfaces of devices used in clinical applications. Second, the formed films may possibly be used as carriers of proteins and other biologically active molecules that can be incorporated without losing their bioactivity18–20. Using this technique, a biodegradable coating may be developed to deliver ideal drug dosages over a desirable time period leaving no residual materials on implants21,22.
The objectives of this study were: (i) to develop biodegradable polypeptide multilayer nanofilms potentially serving as antibiotic carriers at the implant/tissue interface; (ii) to evaluate the efficacy of cefazolin loaded poly(L-lysine)/poly(L-glutamic acid) (PLL/PLGA) nanofilms on stainless steel disks (SSDs), in killing S. aureus; and (iii) to evaluate the effects of cefazolin loaded nanofilms on osteoblast cell behavior.
MATERIALS AND METHODS
Layer-by-layer Nanocoating and Cefazolin Loading and Release
SSDs of 10 mm in diameter and 0.25 mm thick were thoroughly cleaned and polypeptide multilayer nanofilms were developed using the LBL technique. Details of the LBL process were reported earlier23. The thickness, measured by ellipsometry24, of 40 layers of PLL and PLGA or PLL/PLGA20 nanofilms was about 240 nm.
PLL/PLGA20 nanofilms were loaded with cefazolin by immersing them in a cefazolin solution (pH 7) of 2.5, 5, and 10 mg/ml for 20 min. In the in vitro release studies, cefazolin-loaded PLL/PLGA20 nanocoated SSDs were incubated in 10 ml phosphate buffered saline or PBS (pH 7) at 37°C. 0.6 ml of PBS aliquot solution was taken to determine cefazolin concentration at predetermined time points and 0.6 ml fresh PBS was added afterward.
In vitro Bacterial Inhibition Study
Therapeutic activities of PLL/PLGA20 nanofilms were assessed against proliferation of S. aureus, which was isolated from a patient with chronic osteomyelitis. A freshly cultured S. aureus suspension was centrifuged, then washed and diluted with PBS to contain ~1 × 108 colony forming units (CFU)/ml. The suspension was used for two types of bacterial inhibition assays (see Supplementary Materials): zone of inhibition (ZOI) test and a modified bacterial killing assay25.
In vitro Bacterial Adhesion Study
A S. aureus suspension, containing ~1 × 107 CFU/ml of S. aureus was prepared. A highly lipophilic carocyanine dye, i.e. Dio, was used to stain S. aureus before seeding onto SSDs. A 1 ml S. aureus suspension was then added to a 24-well plate containing bare SSDs or PLL/PLGA20 nanocoated SSDs (without cefazolin) and cultured at 37°C for 2 h. After washing with PBS three times and fixing using 10% formalin, the SSDs were glued onto glass slides and observed under confocal fluorescent microscopy (LSM 700, Carl Zeiss, Germany) with excitation/emission wavelengths of 480 nm/505 nm.
Osteoblast Cell Adhesion and Visualization
CRL-11372 human osteoblast cell line (American Type Culture Collection or ATCC, Manassas, VA) was routinely grown In Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F-12, 1:1 medium (DMEM: F-12 medium, ATCC) with 10% fetal bovine serum (ATCC), 100 I.U./ml penicillin, and 100 µg/ml streptomycin (ATCC) in a 5% CO2 and 95% air atmosphere incubator at 37°C. Cells were also cultured in the absence of penicillin and streptomycin, and used to study osteoblast adhesion and viability (See Supplementary Materials). Cells were seeded on SSD samples in a 24-well plate at 1 × 105 cells/well and incubated at 37°C in a 5% CO2 humidified incubator. After culturing for 2 and 24 h, the number of adherent cells were examined using hemocytometry. The SSDs were rinsed three times with PBS and transferred to a new 24-well plate. The cells on the SSD samples were then detached with 0.25% Trypsin/0.53 mM EDTA solution (ATCC) followed by rinsing with culture medium. The rinsing media was collected and the number of detached cells was determined using a hemocytometer.
The distribution and morphology of osteoblasts adhered on the SSDs were examined using scanning electron microscopy (SEM) and confocal fluorescent microscopy. After culturing for 2 and 24 h, the cell-seeded SSDs were rinsed with PBS, fixed in 2.5% glutaraldehyde, and post-fixed in 4% osmium tetroxide. The fixed specimens were dehydrated by immersing them into increasing concentrations of ethanol (70%, 85%, 95% and 100%). Then all specimens were dried using a critical point dryer (CPD030, Bal-Tec, Carlsbad, CA). The specimens were observed under SEM (S-4700, Hitachi, Japan). For confocal fluorescent microscopy, cells were stained with a highly lipophilic carocyanine dye Dil before seeding onto the SSDs. Nanocoated and bare SSDs were incubated with the labeled cells for 2 and 24 h. The adherent osteoblasts were observed under confocal fluorescent microscopy with excitation/emission wavelengths of 540 nm/560 nm.
Cell Viability
Cell viability was determined by MTT assay using an in vitro toxicology kit (Sigma-Aldrich, St. Louis, MO). Osteoblasts were seeded on SSDs, incubated at 37°C for 4 days, and 200 µl of MTT solution was added to each well and incubated for 2 h. Then, 200 µl of MTT solubilization solution was added to each well and the dissolved solution was transferred to a 96-well plate. Absorbance at 570 nm was measured using a micro-plate reader (µQuant, Bio-Tek, Winooski, VT). The background absorbances of multiwell plates were measured at 690 nm and subtracted from the 570 nm measurements.
Cell Proliferation
Cells were seeded on SSDs at a density of 2×105 cells/well. Sample solutions were collected on days 1, 3, and 5. Briefly, osteoblasts adhered to the surface of SSDs were gently rinsed with PBS, overlaid with 1 ml Trypsin/EDTA solution per well and incubated at 37°C for 5 min. The loosely detached cell layer was then scraped off the SSD substrate, centrifuged at 1,200 rpm for 7 min, and re-suspended in 1 ml distilled water with 1% Triton X-100. Then the cell suspensions underwent three freeze-thaw cycles. The triton lysates were stored at −80°C until testing for DNA content. A 30 µl aliquot of cell suspension was used to determine dsDNA content, which was measured by a fluorometric quantification method using a Quant-iT dsDNA high-sensitivity assay kit (Invitrogen, Carlsbad, CA).
Statistical Methods
Data were expressed as the mean ± standard deviation (SD). Statistical differences were analyzed using the one-way ANOVA analysis. p<0.05 was considered statistically significant. SPSS software 11.0 was used for statistical analysis.
RESULTS
Cefazolin Release from Polypeptide Nanofilms
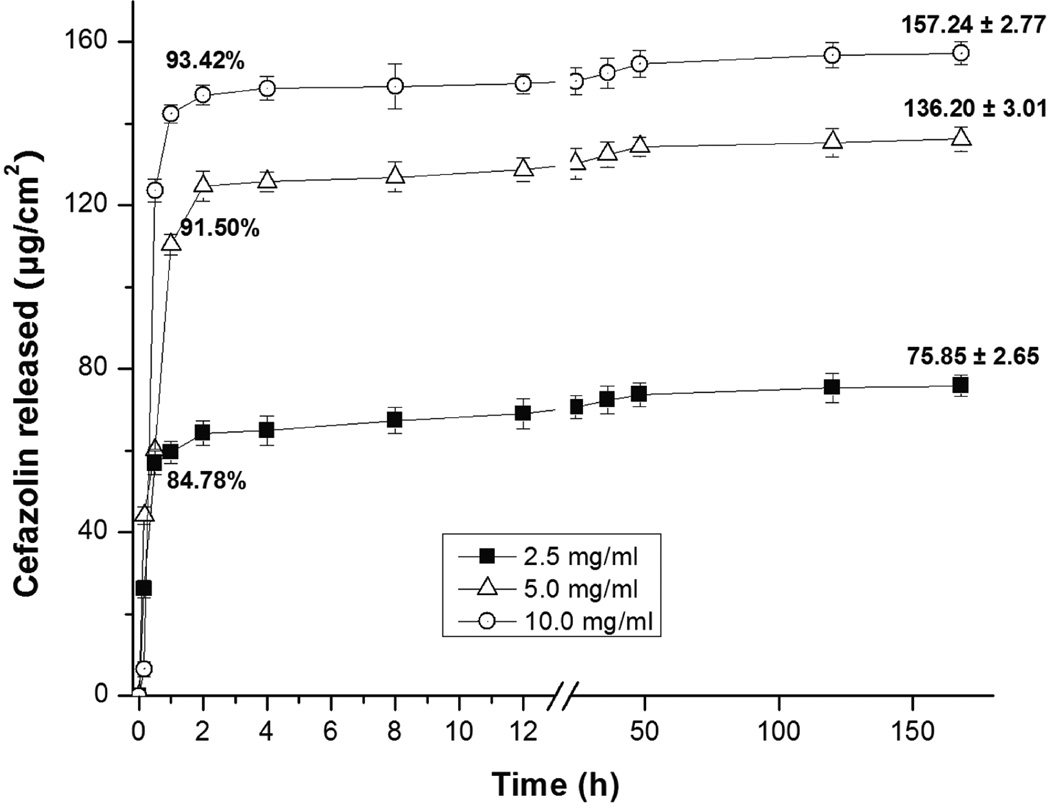
Different amounts of cefazolin were loaded into PLL/PLGA20 nanofilms; all the nanofilms had similar pharmacokinetics (Fig. 1). All nanofilms had a burst release of cefazolin in the first 2 h followed by a release of 16% or less afterwards. The PLL/PLGA20 nanofilms loaded with cefazolin at a higher antibiotic concentration contained a higher amount of antibiotic and subsequently eluted more antibiotics. The PLL/PLGA20 nanofilms on SSDs loaded in 2.5, 5.0, and 10.0 mg/ml cefazolin solutions released 64 (84% of total), 125 (91% of total), and 147 µg/cm2 (93% of total), respectively, of cefazolin at 2 h, and increased to 76, 136, and 157 µg/cm2, respectively, at the end of the time period studied (i.e. 7 days). In addition, under the same conditions, much more cefazolin was released from PLL/PLGA20 nanofilms on quartz slides (~250 µg/cm2)24 than on SSDs (~150 µg/cm2) as the surface properties vary.
Figure 1.
Release profiles of PLL/PLGA20 nanocoated SSDs loaded with cefazolin at three different concentrations. Three samples were tested at each cefazolin concentration.
In Vitro Antibacterial Activity against S. aureus
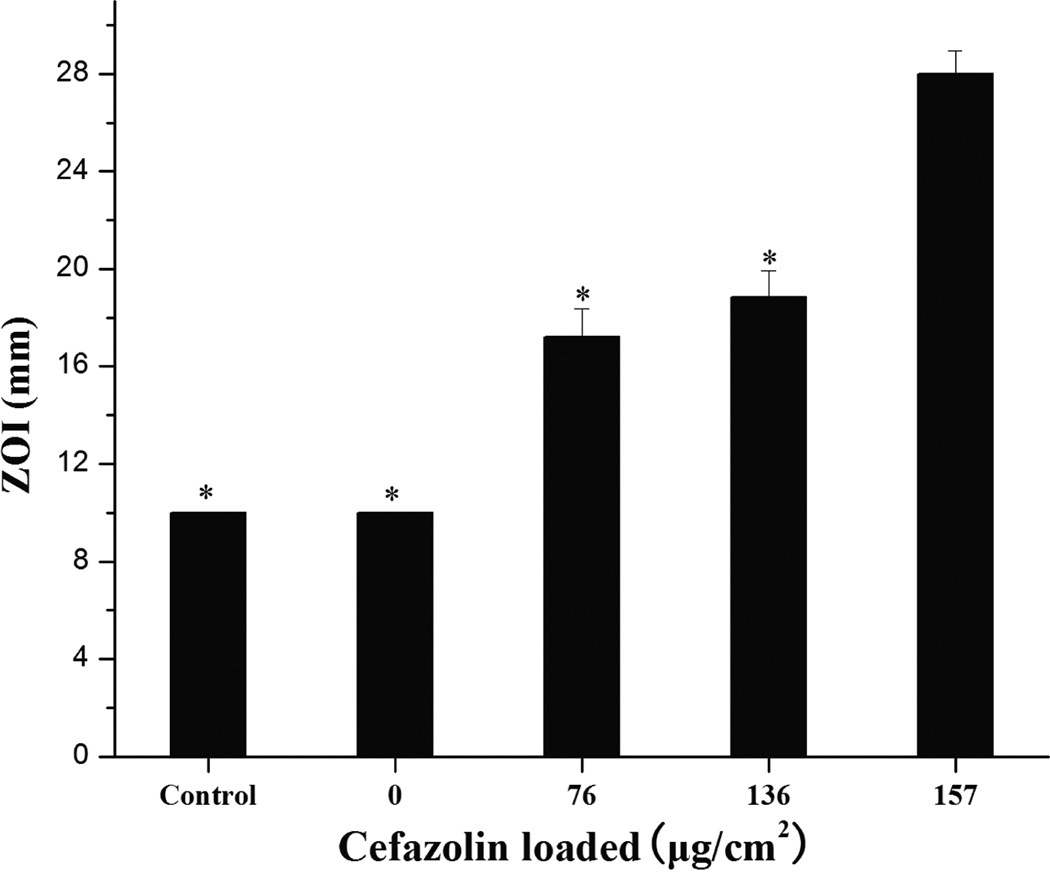
ZOI data show that the antibacterial activity of cefazolin loaded PLL/PLGA20 nanocoated SSDs increased with increasing amounts of cefazolin (Fig. 2). No antibacterial activity was observed on either the bare SSDs (control) or the PLL/PLGA20 coated SSDs without cefazolin. The ZOI diameters increased from a mean of 17.2 ± 1.2 mm of PLL/PLGA20 nanocoated samples at 76 µg/cm2 cefazolin to 28.0 ± 0.9 mm of PLL/PLGA20 nanocoated samples at 157 µg/cm2 cefazolin.
Figure 2.
ZOI data vs. amount of cefazolin loaded in PLL/PLGA20 nanofilms. Bare SSDs were used as a control. The diameter of SSDs was 10 mm. *p<0.05 compared with 157 µg/cm2 group.
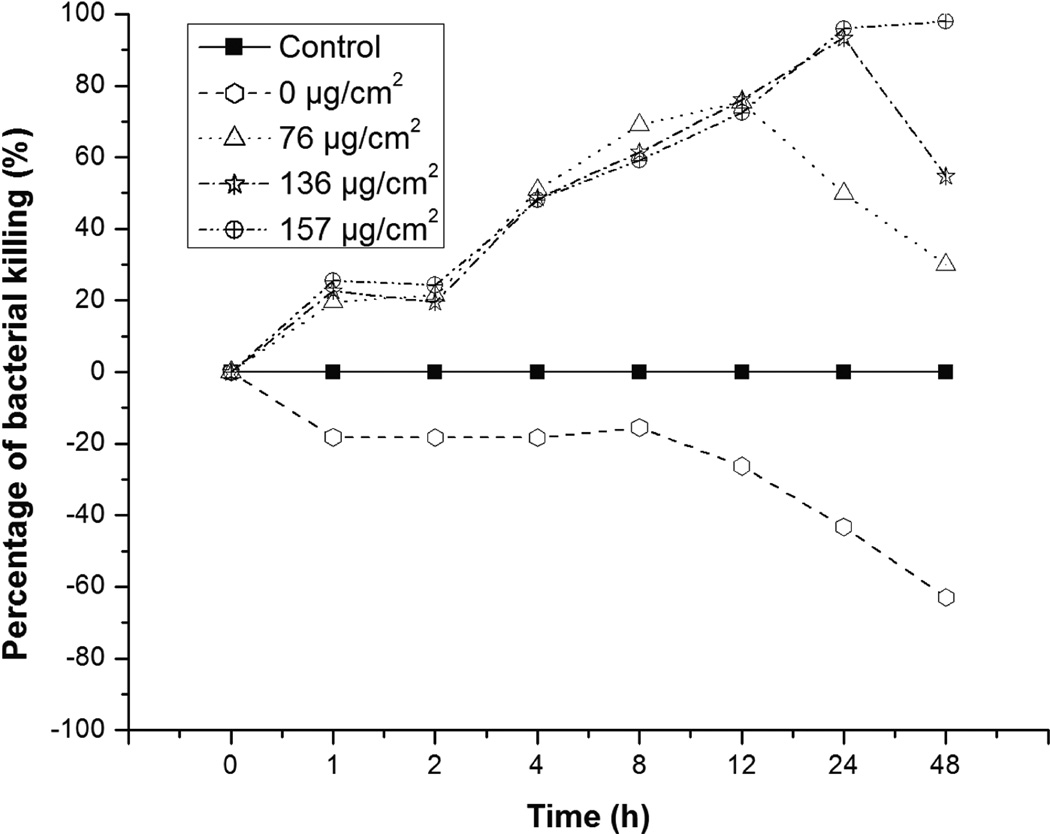
The bacterial killing studies in Mueller Hinton (MH) broth show that, with increasing loading of cefazolin in the polypeptide nanofilms, the ability of the PLL/PLGA20 nanofilms to inhibit the growth of S. aureus increased (Fig. 3). The PLL/PLGA20 nanocoated SSDs with 76, 136, and 157 µg/cm2 cefazolin kept inhibiting the growth of S. aureus at 37°C in MH broth for up to 12, 24, and 48 h, respectively. The PLL/PLGA20 nanocoated SSDs without cefazolin, however, stimulated growth of S. aureus to some extent. Polypeptide nanocoatings with high cefazolin loading inhibited S. aureus growth for a long time (e.g. 7 days, see Supplementary Materials).
Figure 3.
Percentage of S. aureus killed in MH broth based on OD measurements. 1 × 107 CFU/ml of S. aureus was used. Percentage of killing = {1 − [(OD of sample − OD of MH broth)/(OD of control − OD of MH broth)]} × 100%.
Influence of Polypeptide Nanofilms on S. aureus Adhesion
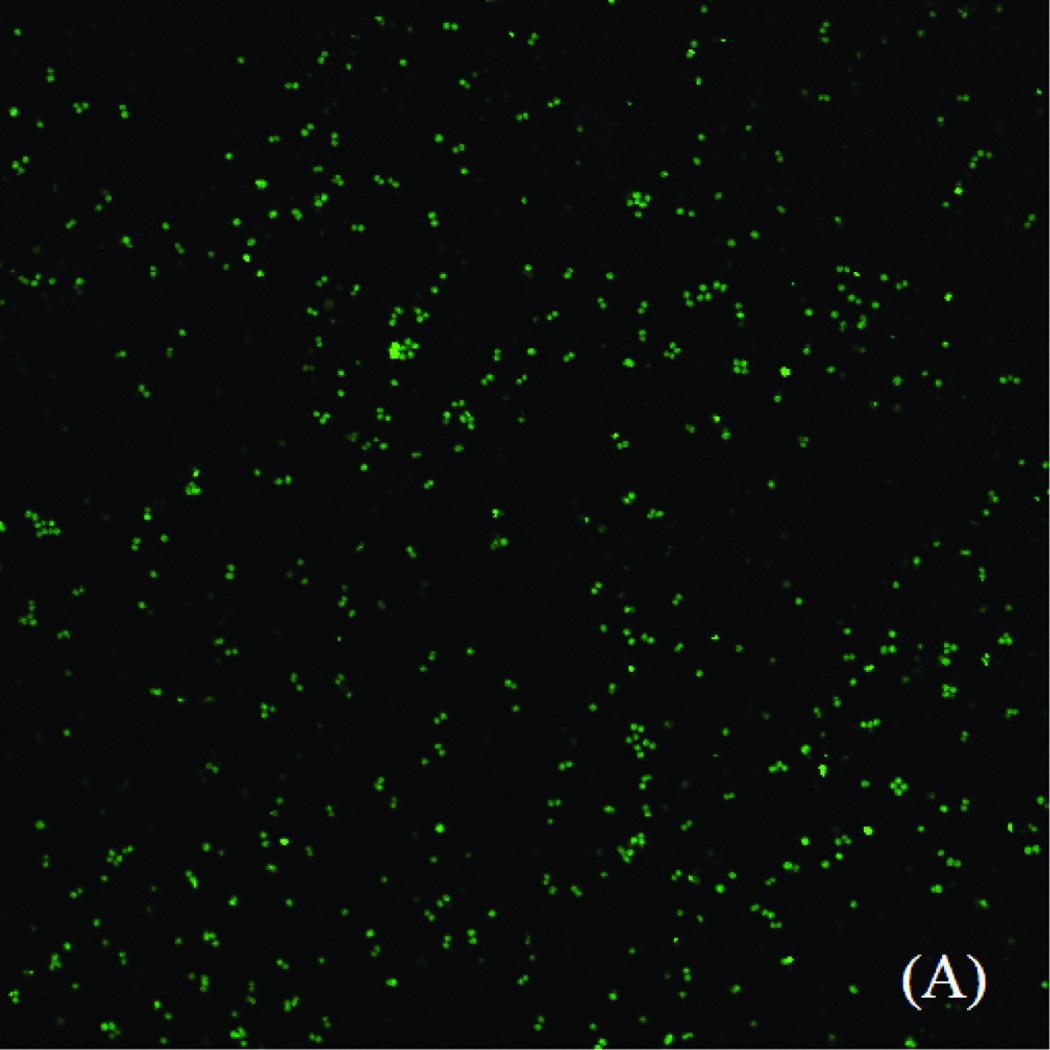
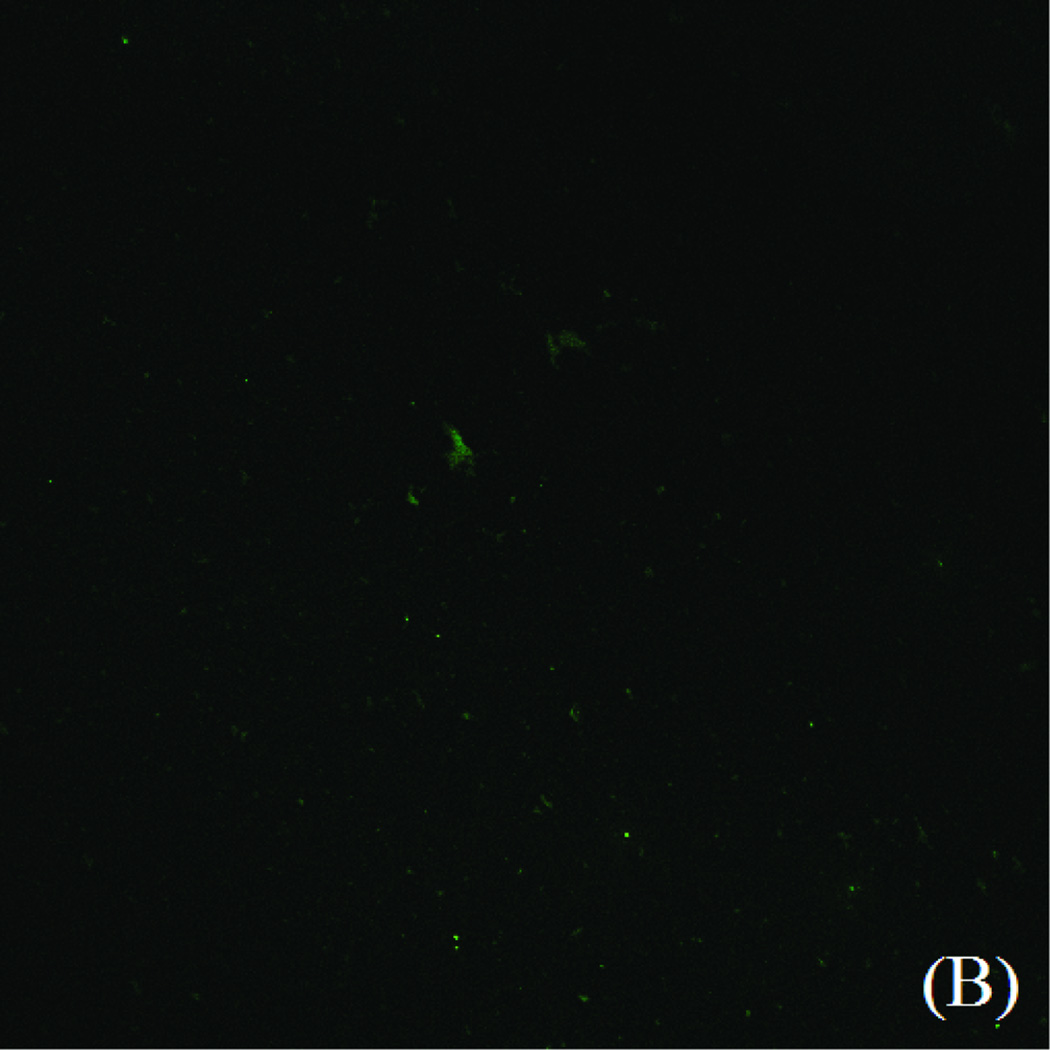
PLL/PLGA20 nanofilms were found to substantially reduce S. aureus adhesion. A large quantity of S. aureus was adhered on the bare SSDs, while substantially fewer S. aureus was observed on PLL/PLGA20 nanocoated SSDs (Fig. 4).
Figure 4.
S. aureus adhesion on (A) bare SSDs and (B) PLL/PLGA20 nanocoated SSDs (without cefazolin) after 2 h incubation.
Influence of Cefazolin Loaded Polypeptide Nanofilms on Osteoblast Cell Adhesion
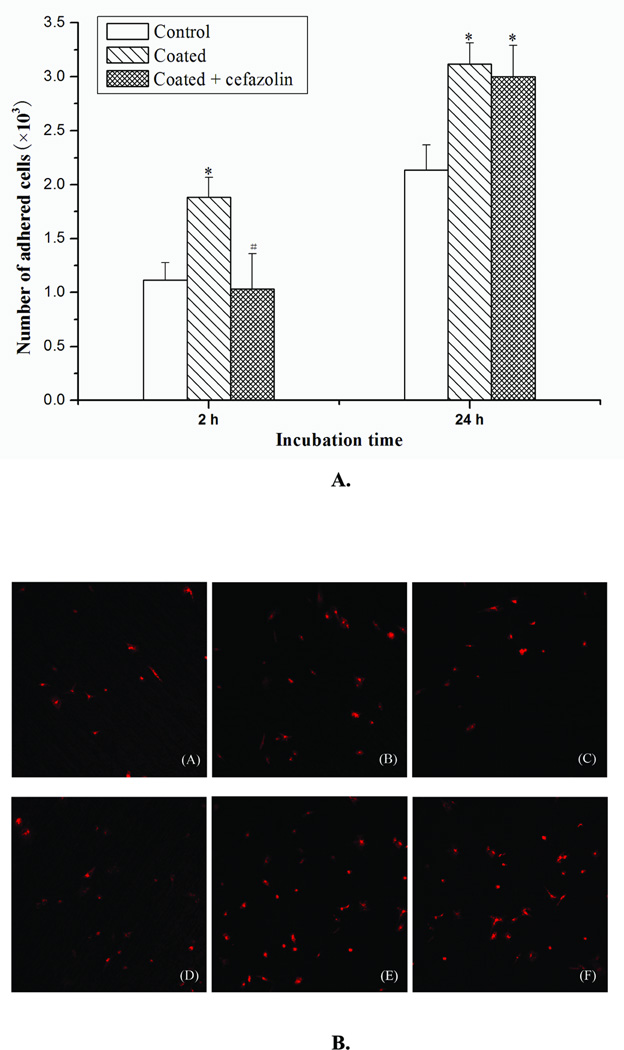
Osteoblast cells were cultured and seeded on SSD samples (Fig. 5A). At 2 h, significantly more osteoblast cells were attached to the PLL/PLGA20 nanocoated surfaces than to bare SSDs, while dramatically fewer osteoblast cells were adhered to cefazolin loaded PLL/PLGA20 nanofilms compared to PLL/PLGA20 nanofilms without cefazolin. The numbers of osteoblasts on bare SSDs and on cefazolin loaded PLL/PLGA20 nanofilms were about the same. At 24 h, significantly more osteoblasts were observed on both the PLL/PLGA20 nanocoated surfaces and the cefazolin loaded PLL/PLGA20 nanocoated surfaces compared to the bare SSD samples, while no significant difference occurred between the PLL/PLGA20 nanocoated samples and cefazolin loaded PLL/PLGA20 nanocoated samples. This result was confirmed by examining the cells under confocal fluorescent microscopy. Fig. 5B shows that more osteoblast cells were adhered at 2 h on the PLL/PLGA20 nanocoated SSDs compared to both bare SSDs and PLL/PLGA20 nanocoated SSDs with cefazolin. At 24 h, the number of adhered osteoblasts on coated and coated with cefazolin SSDs are more than that adhered on non-coated SSDs.
Figure 5.
A. Influences of PLL/PLGA20 nanofilms and cefazolin (157 µg/cm2) loaded PLL/PLGA20 nanofilms on osteoblast cell adhesion. *p<0.05 compared to non-coated group; #p<0.05 compared to coated group.
B. Fluorescence labeling of adhered osteoblasts on bare SSDs (A and D), PLL/PLGA20 nanocoated SSDs (B and E), and PLL/PLGA20 nanocoated SSDs with 157 µg/cm2 cefazolin (C and F) after 2 (A, B, and C) and 24 h (D, E, and F) cell seeding.
Influence of Cefazolin Loaded Polypeptide Nanofilms on Osteoblast Cell Morphology
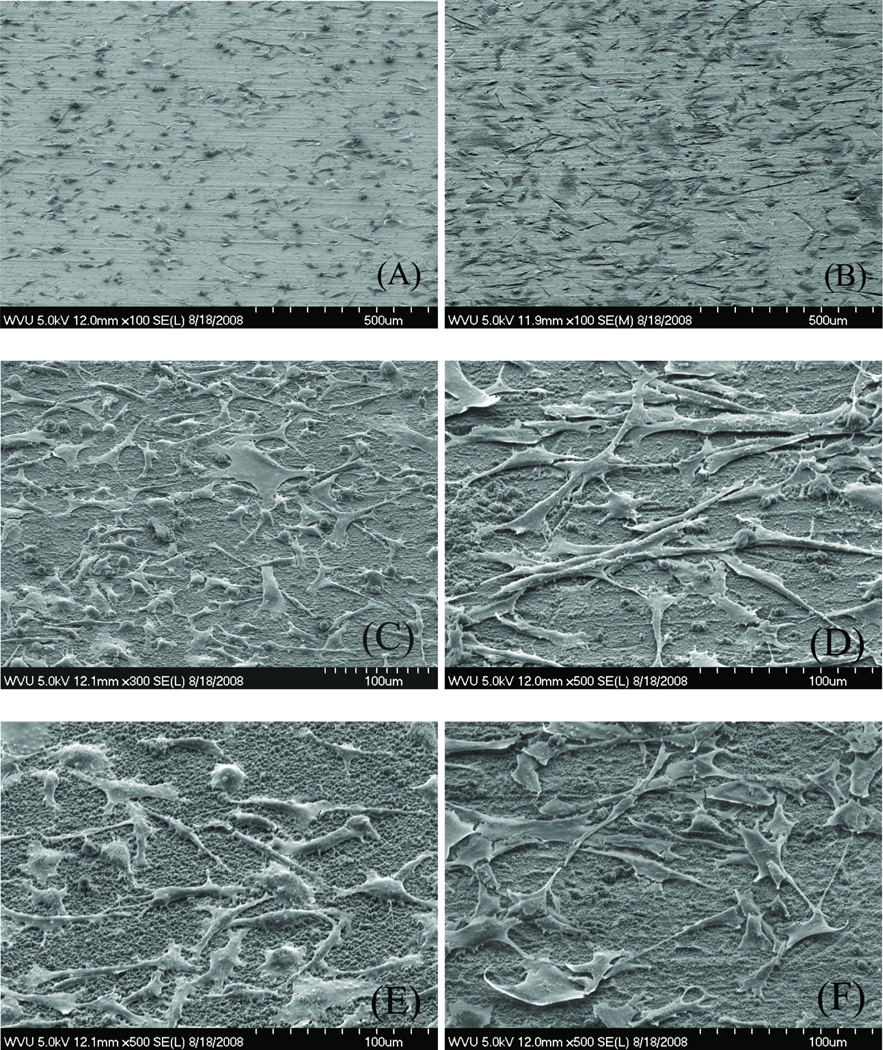
SEM observations show that, at 2 h incubation, the osteoblast cells that attached to PLL/PLGA20 nanocoated SSDs and PLL/PLGA20 nanocoated SSDs with cefazolin flattened and showed relatively smooth cell surfaces with a few long slender cell processes (Fig. 6). In contrast, cells on the bare SSDs were not spread out as much and were more round in shape. At 24 h incubation, osteoblasts on the three types of surfaces were all spread out and formed many long slender cell processes. Most cells were connected to their neighboring cells. The cells on PLL/PLGA20 nanocoated SSDs still showed better spreading than those on bare SSDs.
Figure 6.
SEM images of osteoblasts adhered to bare SSDs (A and B), PLL/PLGA20 nanocoated SSDs (C and D), and PLL/PLGA20 nanocoated SSDs with 157 µg/cm2 cefazolin (E and F) at 2 (A, C, and E) and 24 h (B, D, and F) culturing.
Influence of Cefazolin Loaded Polypeptide Nanofilms on Osteoblast Cell Viability
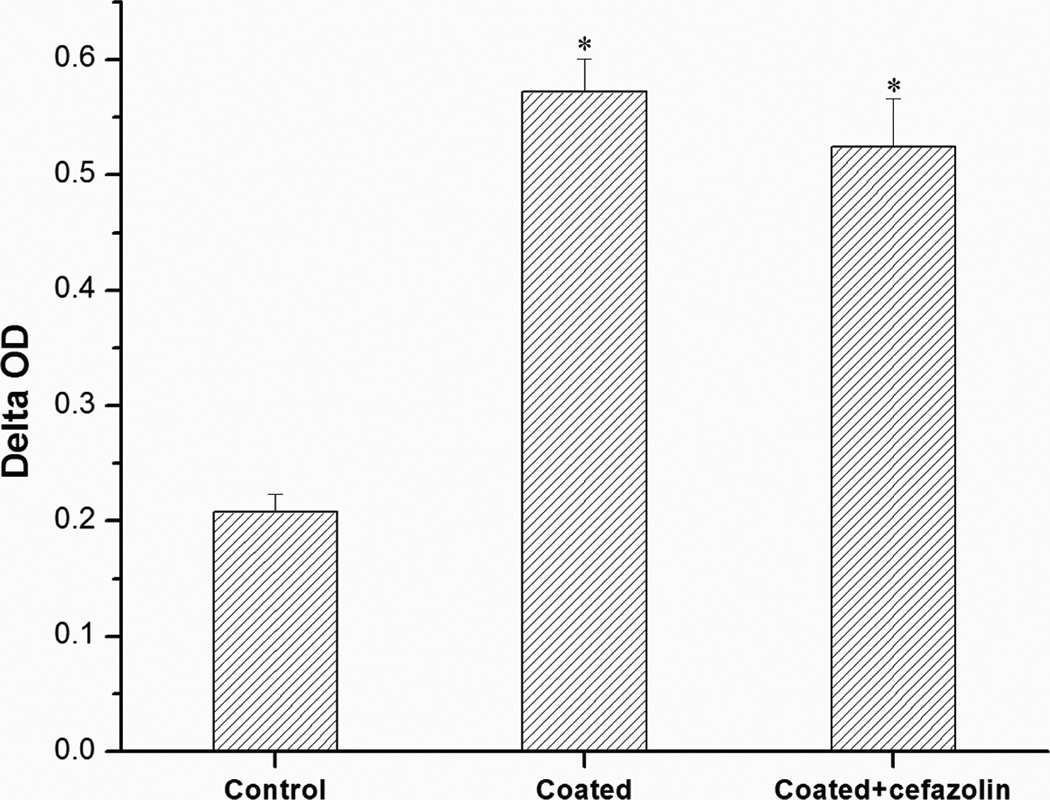
MTT assay studies (Fig. 7) showed that the cell viability of osteoblasts adhered to PLL/PLGA20 nanocoated SSDs, with and without cefazolin, was significantly higher than that of cells adhered to bare SSDs. No significant difference in cell viability was observed for the PLL/PLGA20 nanocoated SSDs and PLL/PLGA20 nanocoated SSDs with cefazolin.
Figure 7.
Influences of PLL/PLGA20 nanofilms and cefazolin (157 µg/cm2) loaded PLL/PLGA20 nanofilms on osteoblast viability. *p<0.05 compared to bare SSD samples.
Influence of Cefazolin Loaded Polypeptide Nanofilms on Osteoblast Cell Proliferation
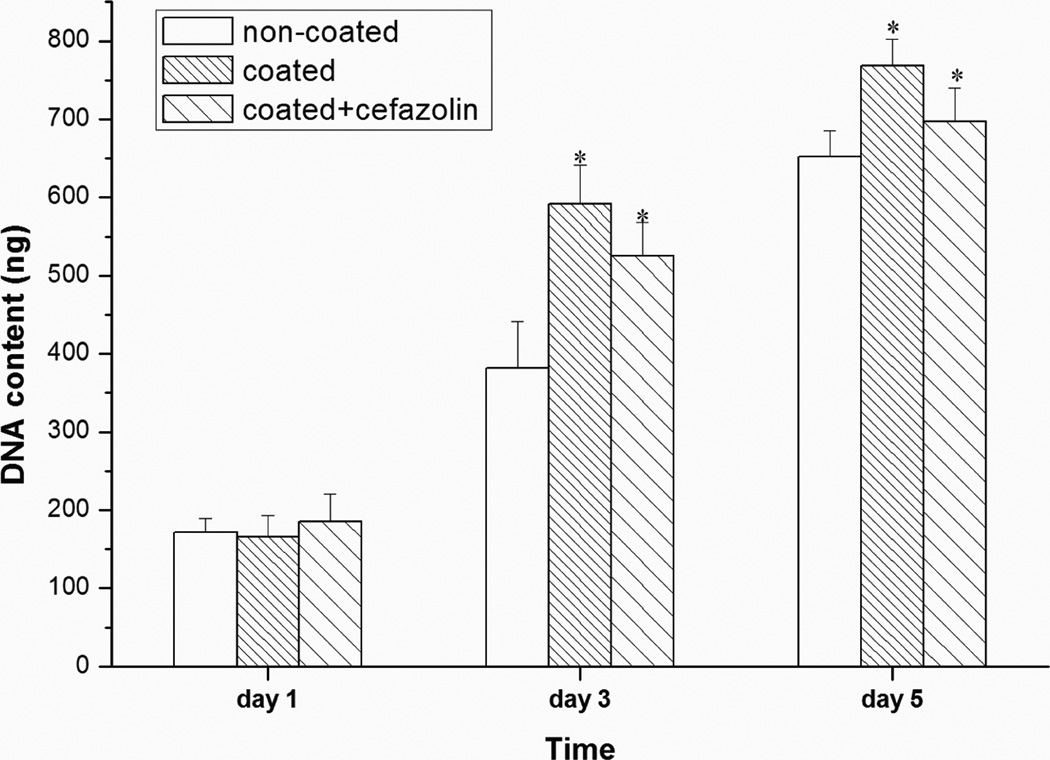
The influence of PLL/PLGA20 nanofilm and PLL/PLGA20 nanofilm with cefazolin on osteoblast cell proliferation was studied at 1, 3, and 5 days (Fig. 8). No differences were observed at day 1. At days 3 and 5, the numbers of osteoblast cells grown on PLL/PLGA20 nanocoated SSDs and PLL/PLGA20 nanocoated SSDs with cefazolin were significantly higher than those on bare SSDs. The average cell numbers at 3 and 5 days on the PLL/PLGA20 nanocoated SSDs with cefazolin was lower than those on the PLL/PLGA20 nanocoated SSDs without cefazolin; however, the difference between these two types of samples was not statistically significant.
Figure 8.
Influences of PLL/PLGA20 nanofilms and cefazolin (157 µg/cm2) loaded PLL/PLGA20 nanofilms on osteoblast proliferation. *p<0.05 compared to bare SSD samples.
DISCUSSION
Bacteria are known to colonize on metal implants and form adherent bio-films26. Additionally, osteomyelitis is found to develop preferentially beneath the surfaces of fixation devices adjacent to bone26,27. Coating metal implants with a bactericidal film would inhibit bacteria from colonizing implant surfaces and provide a high antibiotic concentration in a local region commonly found as a nidus for bacterial infection. In this study, we developed polypeptide multilayer nanofilms as a biodegradable carrier for antibiotic delivery. Cefazolin, a widely used antibiotic in orthopedics, is negatively charged and was incorporated in PLL/PLGA20 nanofilms based on electrostatic attraction23. The loading of cefazolin in PLL/PLGA20 nanofilms on SSDs was controlled by cefazolin concentration (Fig. 1), and the tuning of cefazolin loading was translated to the tunability of in vitro antibacterial activity (Figs. 2 and 3).
Implant-associated infections are, at the cellular level, the result of bacterial adhesion onto biomaterial surfaces11. Upon implantation, a competition exists between the integration of the implant into surrounding tissue and the adhesion of bacteria to the implant surface28. A post-implantation “decisive period” (6 h), has been identified and is believed to be critical for preventing bacterial adhesion as well as achieving long-term success of implantation29. Implants are particularly susceptible to surface adhesion of bacteria within the decisive period, and once adhered, certain species of bacteria start to form a bio-film at the implant-tissue interface. The bio-films are remarkably resistant to both immune responses and systemic antibiotic therapies, and their formation is thought to be the primary cause of implant-associated infection. Therefore, inhibiting bacterial adhesion is often regarded as the most critical step in preventing implant-associated infection. In this study, polypeptide nanofilms were engineered on implant surfaces, enabling active release of antibacterial agents to reduce bacterial adhesion. The nanofilms were designed to release high fluxes of antibacterial agents during the post-implantation decisive period to effectively inhibit the initial adhesion of bacteria. More specifically, in the developed systems, a high percentage (84–93%) of loaded cefazolin was released within the first 2 h, and the cefazolin loaded PLL/PLGA20 nanocoated samples were effective in inhibiting and killing bacteria for up to 12, 24, and 48 h or longer, depending on the amount of cefazolin loaded (Fig. 3). Moreover, PLL/PLGA20 nanofilms (without cefazolin) could significantly inhibit S. aureus adhesion (Fig. 4) although the reasons for this are not clear. The negative charge of the outermost layer, i.e. PLGA, may play a role in inhibiting S. aureus adhesion and PLL may have some antibacterial activity30. Therefore, the developed cefazolin loaded polypeptide nanofilms may have the potential to prevent implant-associated infection.
Another advantage offered by polypeptide multilayer nanofilms is that their physicochemical properties23 can be easily controlled thereby allowing better control of related cellular responses including cell adhesion, motility, spreading, growth, and differentiation. It has been shown that different parameters such as hydrophobicity and hydrophilicity31, surface charge32, roughness, surface free energy33 and topography34 affect cell adhesion. In the present study, we tested the cellular responses of osteoblasts to cefazolin loaded polypeptide multilayer nanofilms. Cell culture studies at 2 and 24 h showed that polypeptide multilayer nanofilms (without cefazolin) improved the adhesion of osteoblasts dramatically. The incorporation of cefazolin in polypeptide multilayer nanofilms decreased the adhesion of osteoblasts at 2 h culturing; this effect vanished at 24 h, which was probably due to the substantial release of cefazolin within the first few hours. As a result, no difference in osteoblast cell adhesion was observed at 24 h between PLL/PLGA20 nanocoated samples and PLL/PLGA20 nanocoated samples with cefazolin; cefazolin loaded polypeptide nanofilms have similar (2 h) or enhanced (24 h) osteoblast cell adhesion compared to the control (Fig. 5A). Our results also showed that PLL/PLGA20 nanofilms, with and without cefazolin, could improve the viability and proliferation of osteoblasts, and no differences at a relatively long time period (a few days) were found in viability and proliferation of osteoblasts between the PLL/PLGA20 nanofilms and PLL/PLGA20 nanofilms with cefazolin (Figs. 7 and 8). These results suggested, for the first time, that the developed antibiotic loaded polypeptide multilayer nanofilms, used as surface coatings, are biocompatible with osteoblasts and promote osteoblast proliferation.
In summary, this study showed that a biodegradable implant nanofilms can be engineered to have antibacterial activity against organisms frequently associated with osteomyelitis. Polypeptide multilayer nanofilms, with or without cefazolin, were found to be biocompatible with osteoblasts. The developed polypeptide nanocoated implants could be used prophylactically to reduce the incidence of soft tissue and bone infection that frequently complicate open fractures. Meanwhile, the nanocoatings may improve bone healing through improving osteoblast cell adhesion, viability, and proliferation. In the future, the effects of cefazolin loaded polypeptide nanofilms on osseointegration will be studied.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge financial support from the AO Foundation, NSF (Grant Number OISE-0737735), NASA WV EPSCoR, and WVU. Project S-07-43L was supported by the AO Research Fund of the AO Foundation. The authors appreciate the use of confocal laser scanning microscope at the Microscopic Imaging Facilities at West Virginia University Health Sciences Center. We acknowledge John G. Thomas, PhD (Department of Pathology) for the contribution of S. aureus and consultation on bacterial culture. We thank Karen Martin for assisting in confocal imaging, Suzanne Smith for proofreading, Nina Clovis for laboratory training, and Vincent Kish for assistance in SEM sample preparation. MH also thanks the E.J. Van Liere fellowship.
REFERENCES
- 1.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350(14):1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 2.Sanderson PJ. Preventing infection in orthopaedic implants. J Antimicrob Chemother. 1989;24(3):277–280. doi: 10.1093/jac/24.3.277. [DOI] [PubMed] [Google Scholar]
- 3.Taylor GJ, Bannister GC, Calder S. Perioperative wound infection in elective orthopaedic surgery. J Hosp Infect. 1990;16(3):241–247. doi: 10.1016/0195-6701(90)90112-2. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JM. Biological responses to materials. Ann Rev Mater Res. 2001;31:81–110. [Google Scholar]
- 5.An YH, Friedman RJ. Prevention of sepsis in total joint arthroplasty. J Hosp Infect. 1996;33(2):93–108. doi: 10.1016/s0195-6701(96)90094-8. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun JH, Manring MM. Adult osteomyelitis. Infect Dis Clin North Am. 2005;19(4):765–786. doi: 10.1016/j.idc.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Barton AJ, Sagers RD, Pitt WG. Bacterial adhesion to orthopedic implant polymers. J Biomed Mater Res. 1996;30(3):403–410. doi: 10.1002/(SICI)1097-4636(199603)30:3<403::AID-JBM15>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 8.Gotz F. Staphylococcus and biofilms. Mol Microbiol. 2002;43(6):1367–1378. doi: 10.1046/j.1365-2958.2002.02827.x. [DOI] [PubMed] [Google Scholar]
- 9.Gristina AG. Implant failure and the immuno-incompetent fibro-inflammatory zone. Clin Orthop Relat Res. 1994;298:106–118. [PubMed] [Google Scholar]
- 10.Khardori N, Yassien M. Biofilms in device-related infections. J Ind Microbiol. 1995;15(3):141–147. doi: 10.1007/BF01569817. [DOI] [PubMed] [Google Scholar]
- 11.Hetrick EM, Schoenfisch MH. Reducing implant-related infections: active release strategies. Chem Soc Rev. 2006;35(9):780–789. doi: 10.1039/b515219b. [DOI] [PubMed] [Google Scholar]
- 12.Price JS, Tencer AF, Arm DM, Bohach GA. Controlled release of antibiotics from coated orthopedic implants. J Biomed Mater Res. 1996;30(3):281–286. doi: 10.1002/(SICI)1097-4636(199603)30:3<281::AID-JBM2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 13.Lucke M, Schmidmaier G, Sadoni S, et al. Gentamicin coating of metallic implants reduces implant-related osteomyelitis in rats. Bone. 2003;32(5):521–531. doi: 10.1016/s8756-3282(03)00050-4. [DOI] [PubMed] [Google Scholar]
- 14.Stigter M, Bezemer J, de Groot K, et al. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J Control Release. 2004;99(1):127–137. doi: 10.1016/j.jconrel.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey JS, Mehta S, Seaber AV, et al. Pharmacokinetics of a degradable drug delivery system in bone. Clin Orthop Relat Res. 1998;349:218–224. doi: 10.1097/00003086-199804000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Nablo BJ, Schoenfisch MH. Poly(vinyl chloride)-coated sol-gels for studying the effects of nitric oxide release on bacterial adhesion. Biomacromolecules. 2004;5(5):2034–2041. doi: 10.1021/bm049727w. [DOI] [PubMed] [Google Scholar]
- 17.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277(5330):1232–1237. [Google Scholar]
- 18.Lvov Y, Ariga K, Ichinose I, et al. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J Am Chem Soc. 1995;117(22):6117–6123. [Google Scholar]
- 19.Kong W, Wang LP, Gao ML, et al. Immobilized Bilayer Glucose-Isomerase in Porous Trimethylamine Polystyrene-Based on Molecular Deposition. Chem Commun. 1994;1994(11):1297–1298. [Google Scholar]
- 20.Ladam G, Schaaf P, Cuisinier FJG, et al. Protein adsorption onto auto-assembled polyelectrolyte films. Langmuir. 2001;17:878–882. doi: 10.1016/s1389-0344(02)00031-x. [DOI] [PubMed] [Google Scholar]
- 21.Ladam G, Schaad P, Voegel JC, et al. In situ determination of the structural properties of initially deposited polyelectrolyte multilayers. Langmuir. 2000;16:1249–1255. [Google Scholar]
- 22.Li B, Jiang B, Boyce B, Lindsey B. Multilayer polypeptide nanoscale coatings for the prevention of biomedical device associated infections. Biomaterials. 2009;30:2552–2558. doi: 10.1016/j.biomaterials.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang B, Li B. Polypeptide nanocoatings for preventing dental and orthopaedic device-associated infection: pH-induced antibiotic capture, release, and antibiotic efficacy. J Biomed Mater Res B. 2009;88(2):332–338. doi: 10.1002/jbm.b.31021. [DOI] [PubMed] [Google Scholar]
- 24.Jiang B, Li B. Tunable drug loading and release from polypeptide multilayer nanofilms. Int J Nanomedicine. 2009;4(1):37–53. doi: 10.2147/ijn.s4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmerhorst EJ, Van't Hof W, Veerman EC, et al. Synthetic histatin analogues with broad-spectrum antimicrobial activity. Biochem J. 1997;326(Pt 1):39–45. doi: 10.1042/bj3260039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb LX, Holman J, de Araujo B, et al. Antibiotic resistance in staphylococci adherent to cortical bone. J Orthop Trauma. 1994;8:28–33. doi: 10.1097/00005131-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Chang CC, Merritt K. Infection at the site of implanted materials with and without preadhered bacteria. J Orthop Res. 1994;12(4):526–531. doi: 10.1002/jor.1100120409. [DOI] [PubMed] [Google Scholar]
- 28.Gristina AG. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science. 1987;237(4822):1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 29.Poelstra KA, Barekzi NA, Rediske AM, et al. Prophylactic treatment of gram-positive and gram-negative abdominal implant infections using locally delivered polyclonal antibodies. J Biomed Mater Res. 2002;60:206–215. doi: 10.1002/jbm.10069. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa M, Ogawa K. Inhibition of epsilon-poly-L-lysine biosynthesis in Streptomycetaceae bacteria by short-chain polyols. Appl Environ Microbiol. 2006;72:2306–2312. doi: 10.1128/AEM.72.4.2306-2312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grinnell F, Feld MK. Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J Biol Chem. 1982;257(9):4888–4893. [PubMed] [Google Scholar]
- 32.Qiu Q, Sayer M, Kawaja M, et al. Attachment, morphology, and protein expression of rat marrow stromal cells cultured on charged substrate surfaces. J Biomed Mater Res. 1998;42:117–127. doi: 10.1002/(sici)1097-4636(199810)42:1<117::aid-jbm15>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 33.Kapur R, Rudolph AS. Cellular and cytoskeleton morphology and strength of adhesion of cells on self-assembled monolayers of organosilanes. Exp Cell Res. 1998;244(1):275–285. doi: 10.1006/excr.1998.4156. [DOI] [PubMed] [Google Scholar]
- 34.Curtis AS, Varde M. Control of cell behavior: Topological factors. J Natl Cancer Inst. 1964;33:15–26. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.