Abstract
Biomedical device-associated infection is one of the most common and problematic complications faced by millions of patients worldwide. The current antibiotic therapy strategies face challenges, the most serious of which is antibiotic resistance. Studies have shown that the systemic level of interleukin 12 (IL-12) decreases following major injuries resulting in decreased cell-mediated immune response. Here we report the development of IL-12 nanoscale coatings using electrostatic layer-by-layer self-assembly nanotechnology. We found that IL-12 nanoscale coatings at the implant/tissue interface substantially decrease infections in vivo, and IL-12 nanoscale coatings are advantageous over traditional treatments. This approach could be a revolutionary step toward preventing device-associated infections using a non-antibiotic approach.
1. Introduction
Biomedical device-associated infection is one of the most common and problematic complications faced by millions of patients worldwide. It is estimated that approximately two million fracture fixation devices are implanted annually in the U.S. alone [1] The infection rates are 7–9% for elbow replacements and 1–2% for hip replacements [2], and the incidence of infection for Gustilo grade III open fracture is 10–50% [3,4]. The current antibiotic therapy strategies face challenges; the most serious challenge is antibiotic resistance. Overuse of antibiotics may lead to undesirable side effects and antibiotic resistance which has become a worldwide issue [5,6]. Strains of Staphylococcus aureus (S. aureus) have emerged that resist or have reduced susceptibility to antibiotics such as methicillin, vancomycin, and others [5,7,8]. According to the U.S. Centers for Disease Control and Prevention, more than 70% of bacteria that cause hospital-acquired infections are resistant to at least one of the drugs most commonly used to treat them, and thousands of patients died in hospitals in 1992 from infections caused by antibiotic-resistant bacteria. Therefore, in today’s era of emerging multidrug-resistant bacteria and increased number of infections encountered in severe open fractures and contaminated wounds, there is a vital need for advanced strategies other than antibiotic therapies to prevent and treat infections associated with open fractures.
IL-12 (also termed IL-12 p70 or IL-12 p75 but commonly designated IL-12) is an immunoregulatory cytokine that promotes cell mediated immunity [9,10]. In the cytokine family [10], IL-12 is composed of a p35 and a p40 subunit, and it is reported to have therapeutic potential as a stimulator of cell-mediated immune responses to metastatic cancer and viral infections [11−13]. IL-12 is the major cytokine that influences T helper (Th) cells to secrete Th1 cytokines [14], which instruct the cell-mediated immune system to produce cells, e.g. macrophages, that are especially effective against bacterial invaders. The Th1 cytokines, including interferon-γ or IFN-γ (which primes macrophages), are a potent package to help defend against a bacterial attack in tissue and blood. Studies (including human patients) have also shown that the systemic level of IL-12 decreased following major injuries (e.g. trauma or burn) and resulted in decreased and delayed cell-mediated immune responses [15−18]. Meanwhile, it is known that patients who have weakened immune systems and those who have open fractures are at greater risk of infection [19].
Here we report on a new approach (i.e. stimulating the body’s natural defense system using IL-12) that can effectively prevent biomedical device associated infections and may also provide a resolution to antibiotic resistance. IL-12 nanoscale coatings were prepared at the implant/tissue interface using electrostatic layer-by-layer self-assembly (i.e. LBL) [20], which is based on electrostatic attraction of oppositely charged molecules. The efficacy of IL-12 nanoscale coatings on infection prevention was evaluated in an open fracture rat model.
2. Materials and methods
2.1. Preparation of multilayer coatings using electrostatic layer-by-layer self-assembly
Nanoscale coatings were prepared on three types of substrates. Quartz slides and stainless steel discs were used to monitor the formation of polypeptide multilayer nanoscale coatings using UV-vis spectroscopy and ellipsometry, respectively. Stainless steel Kirschner wires (K-wires) were used to prepare samples for the IL-12 (i.e. IL-12 p70) release study and also for in vivo studies. Prior to use, the substrates were cleaned. The quartz slides, 25 mm × 10 mm × 1 mm, were cleaned by immersing them in a piranha solution (3:1 H2SO4/H2O) for 2 h at 80°C, and rinsed with deionized water. Stainless steel K-wires, 1.1 mm in diameter and 63.5 mm in length (Smith & Nephew, Inc., Memphis, TN), and stainless steel discs, 10 mm in diameter and 0.25 mm in thickness, were ultrasonicated in 70%ethanol/30%H2O for 0.5 h and rinsed with deionized water. Dulbecco’s phosphate buffer solution (PBS, pH 7.0, Invitrogen, Carlsbad, CA) was used as buffer solution. IL-12 was dissolved in a bovine serum albumin (BSA) solution (pH 7.0, PBS buffered) and referred to as BSA(IL-12). Poly(L-lysine) or PLL (5 mg/mL) and poly(Lglutamic acid) or PLGA (5 mg/mL) solutions were prepared by dissolving them in PBS buffer solution and mixing.
Polypeptide multilayer nanoscale coatings were prepared in a closed sterile chamber. A pre-cleaned substrate was immersed in a positively-charged solution, PLL, for 20 min, rinsed with buffer solution for 3 min, followed by N2 drying. The sample was then immersed in a negatively-charged solution, BSA mixed with IL-12, BSA(IL-12), or PLGA, for 20 min, rinsed with buffer solution for 3 min, and dried with N2. The process was repeated to achieve the desired coating structures. For in vivo studies, all aqueous solutions were filtered using a 0.2 µm filter and all the K-wires were sterilized.
2.2. In vitro IL-12 release from polypeptide multilayer coatings
Stainless steel K-wires incorporated with IL-12 were immersed in 10 mL of PBS. A 0.6 mL sample of PBS solution was taken at designated time points and analyzed by a standard enzyme-linked immunosorbent assay (ELISA) kit (Invitrogen Corp., Camarillo, CA). Meanwhile, 0.6 mL of fresh PBS solution of the same pH was added to keep a constant volume of release medium. The ELISA procedures were performed according to the manufacturer’s directions. IL-12 levels were determined with known concentrations of IL-12 standards that came with the ELISA kits. The minimum detectable concentration was <3 pg/mL.
2.3. Evaluation of the stability of polypeptide multilayer nanoscale coatings in a rat model
Stability of the nanoscale coatings during the implantation process was tested in an open femur fracture model (see supplementary data). Six K-wires with nanoscale coatings were prepared, three of them were used as controls. The remaining three were implanted and then analyzed using SEM to document the thickness, regularity, and damage to the coating during the implantation process by comparing to the control samples.
2.4. Rat surgery and bacterial challenge
Approval for in vivo studies was obtained from the West Virginia University Animal Care and Use Committee. Rats were prepared for surgery as follows: each rat was anesthetized using Nembutal 50 mg/mL (Ovation Pharmaceuticals, Deerfield, IL) at a dose of 0.1 mL/100 grams of body weight for an initial dose and then a booster of 0.2 mL one hour following the initial dose. Buprenorphine HCl 0.3 mg/mL (Reckitt Benckiser Pharmaceuticals, Inc., Richmond, VA) was used for post-operative pain control at a dose of 0.03 mg/kg. The first dose was given pre-operatively and then every 12 h through the first post-operative day. The operative hindquarter was shaved and placed over the platforms of the fracture device (ventral side up) with a blunted blade placed at the midshaft of the femur. A weight of 0.94 kg was dropped from 15.3 cm to the point of impact with the blunted blade delivering a calculated force of 104.8 Newtons (this force was calculated using the spring method).
The rat was then prepped with Betadine solution and draped in a sterile fashion. An incision was made on the dorsolateral surface of the femur from the area of the greater trochanter to the epicondyles of the femur through the gluteus superficialis. The fracture ends were exposed. The rats were infected at this time by injecting 100 µL of bacterial suspension containing 102 CFU/0.1mL S. aureus with a sterile pipette directly at the fracture site. The fracture was left open for one hour to mimic the “golden hour” of a trauma patient. Then the fracture was fixed using an intramedullary stainless steel K-wire, 1.1 mm in diameter and 63.5 mm in length. First we drilled in a retrograde fashion using a K-wire starting at the fracture site and coming out in the piriformis fossa. The K-wire was then placed in an antegrade fashion through that hole behind the abductors into the distal fragment and partially into the epiphysis for distal fixation. The excess part of the K-wire was cut off at the site of entry. The wound was closed. The rats received no further intervention. At certain time periods following injury, the animals were euthanized by administering 1 mL of Euthasol (Virbac AH, Inc., Fort Worth, TX) solution via intracardiac puncture following anesthetization using Nembutal.
2.5. Quantitative culturing of the tissue homogenates
S. aureus is the most common cause of osteomyelitis in humans [19] and was chosen to induce infection in our open fracture rat model (see supplementary data). S. aureus was provided by West Virginia University Hospital Clinical Laboratory (John G. Thomas, PhD, Professor, Department of Pathology). The bacterium was an isolate from a clinical operative wound infection.
Quantitative culturing of bone tissue homogenates was used to determine infection rates [21,22]. Upon sacrifice, animals were prepped with Betadine solution and the surgical femur was sterilely removed. The K-wire was removed from the intramedullary canal and the femur was used to determine the presence of infection. For each specimen, approximately 500 mg (weights were recorded) of femur was homogenized in 5 mL of brain heart infusion broth; the ratio of bone to broth was kept at 100 mg to 1 mL. Then 0.1 mL of the homogenized bone broth was plated onto blood agar plates and placed in a 37°C incubator for 48 h. Infection was defined as the presence of >2–5 bacterial colonies per plate (corresponding to 200–500 CFU/gram of tissue). Part of the femur was placed in a sterile peel pack and shipped to Antech Diagnostics, Inc. (Southaven, MS) for identification of organisms as a referee culture.
2.6. Statistical analysis
Statistical analyses were conducted using a Type I error rate of 0.05 (p) and JMP Statistical Discovery Software (Version 6.0, SAS Institute, Inc., Cary, NC). Data on coating thickness and IL-12 release were expressed as the mean ± standard deviation.
The determination of sample size was made using the JMP statistical computer package. The procedure employs an algorithm based on the Noncentral F-distribution to determine the minimum sample size needed to detect, as statistically significant, a specified difference among treatment groups. The power analysis was conducted specifying a significance level of 0.05 and a minimum power of 0.80, assuming a one-sided alternate hypothesis (decrease in infection rate of the treatment groups). Under these conditions, we determined that a minimum of six rats were required for each study group.
3. Results
3.1. Developing IL-12 polypeptide multilayer coatings using electrostatic layer-by-layer self-assembly
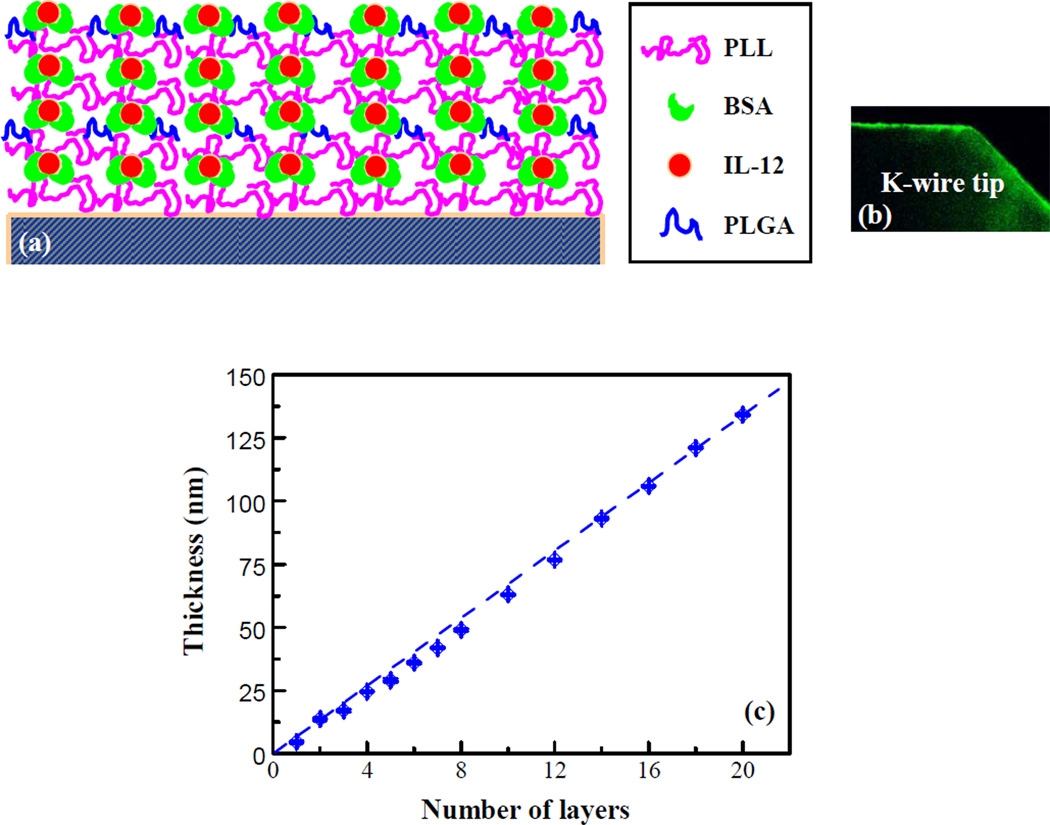
To develop IL-12 nanoscale coatings, we modified a recently evolved nanotechnology, i.e. electrostatic layer-by-layer self-assembly or LBL. In our study, BSA was a carrier protein used to assist in the incorporation of IL-12, and also to control the release rate of IL-12. A schematic diagram is shown in Fig. 1a and fluorescent labeled coatings on K-wires are shown in Fig. 1b. The formation of the multilayers on stainless steel K-wires was simulated by preparing multilayer nanoscale coatings on stainless steel discs that were monitored using ellipsometry. Fig. 1c shows that the thickness of the nanoscale coating increased almost linearly with an increasing number of layers. The average thickness was about 7 nm, and was consistent with our observations using scanning electron microcopy (SEM).
Figure 1.
(a) A schematic diagram of the nanoscale coating structure. BSA, as a carrier of IL-12, was used as a coating component. PLL was used as a “glue” coating component. (b) Fluorescence labeled coating on a K-wire. (c) Formation of the multilayer nanoscale coating monitored by ellipsometry.
3.2. Fine-tuning IL-12 release from polypeptide multilayer nanoscale coatings on K-wires
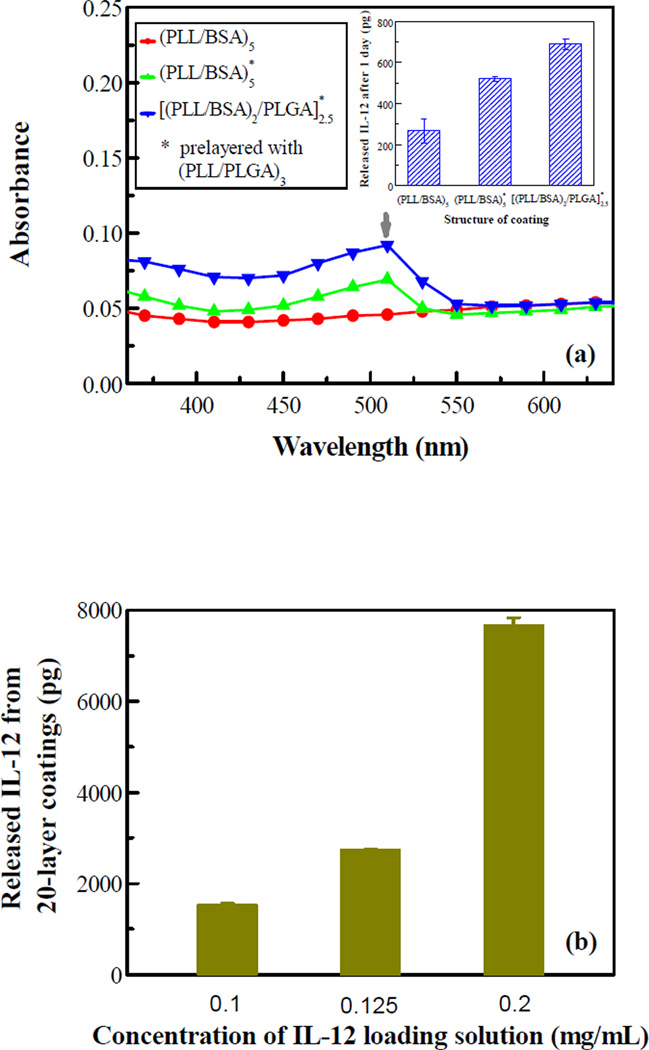
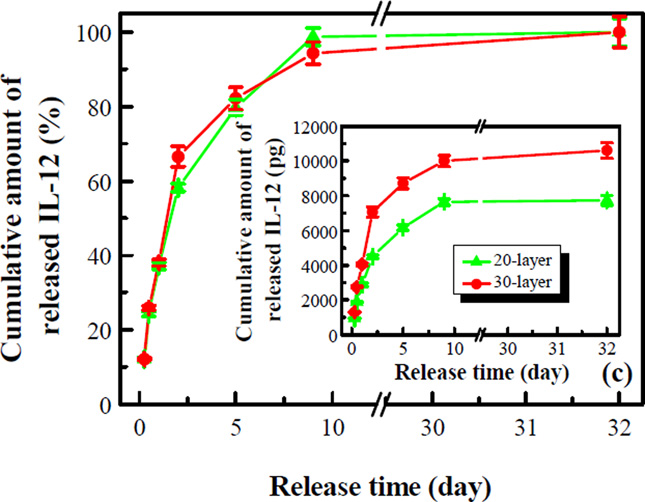
The structure of multilayer nanoscale coatings and the incorporation and release of IL-12 were engineered. Fig. 2a shows that the absorbance of the nanoscale coating could be tuned with the coating structure, and the pre-layers of (PLL/PLGA)3 increased absorbance of the (PLL/BSA)5 coating as well as the release of IL-12. The in vitro release study showed that the [(PLL/BSA)2/PLGA]2.5 coating had the highest release of IL-12 (Fig. 2a inset). The incorporation of IL-12 could also be tuned through IL-12 concentration in the BSA solution; more IL-12 was released with increasing IL-12 concentration (Fig. 2b). Incorporation of IL-12 was further enhanced by simply increasing the number of coating layers (Fig. 2c inset). It was found that much more IL-12 was released from the 30-layer nanoscale coating than from the 20-layer sample; 7.1 ng vs. 4.5 ng at day 2 (57% more). Fig. 2c also shows that the release profiles in percentages are very similar for the 20- and 30-layer samples. In all IL-12 release studies, a burst release in the beginning was observed, and almost all the IL-12 was released within 9 days.
Figure 2.
(a) Preparation of a nanoscale coating and (b-c) release of IL-12 from the coating. (a) coating absorbance vs. wavelength; the inset shows the amounts of IL-12 released from IL-12 nanoscale coatings of different coating structures. (b) IL-12 release vs. IL-12 concentration in the BSA solution at day 9. (c) IL-12 release vs. time; the inset shows the actual amounts of IL-12 released. Data shown is an average of three samples.
3.3. Evaluating the stability of IL-12 nanoscale coatings in an open fracture rat model
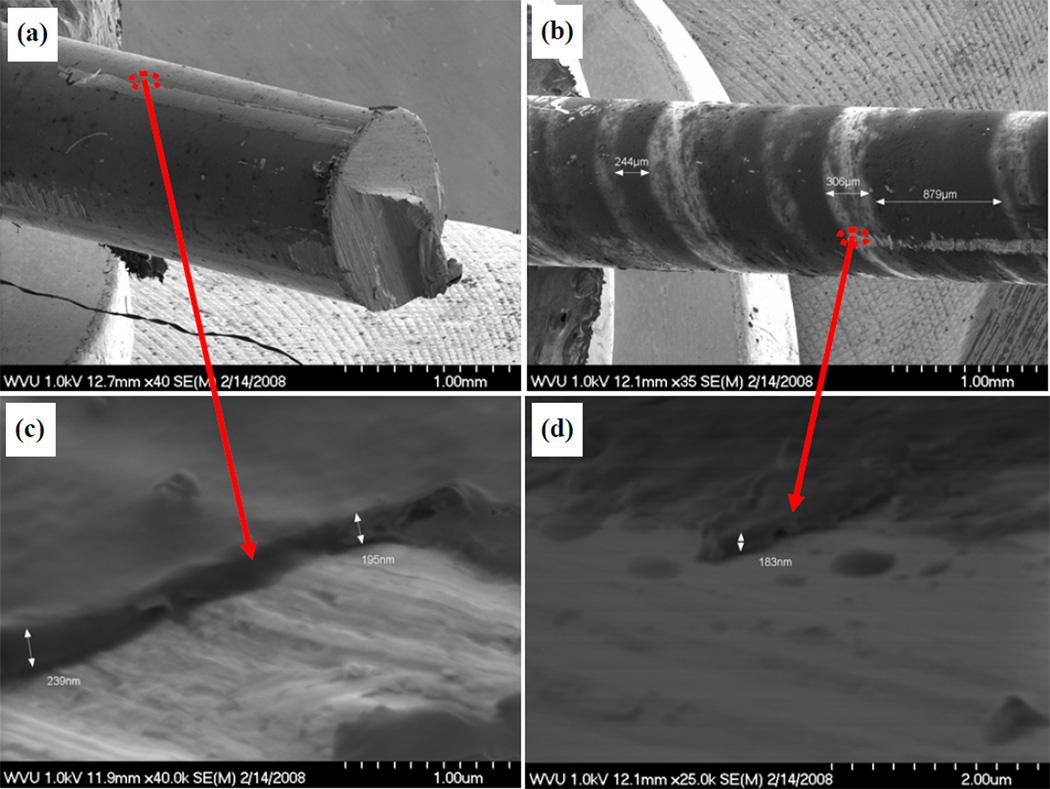
The stability of the nanoscale coatings was tested using an in vivo rat model. After analyzing the thickness, regularity and damage to the nanoscale coatings on K-wires after implantation and comparing them to the control samples before implantation (Fig. 3), we found that approximately 70–95% of the nanoscale coatings withstood the implantation process in our in vivo model.
Figure 3.
SEM images of nanoscale coatings on control samples (a and c) and after implantation (b and d).
3.4. Determining the effects of IL-12 on infection prevention in an infection rat model
We evaluated the efficacy of IL-12 nanoscale coatings in preventing S. aureus-induced infection using an open femur fracture rat model we developed (see supplementary data). Twenty-four rats had their femurs fractured, infected, and then fixed using K-wires as intramedullary nails. Twelve of the rats’ fractures were internally fixed with IL-12 (10.6 ng) coated K-wires with 30-layer nanoscale coatings, and euthanized on post-operative days 6 and 21 (six for each time period). The other 12 rats received uncoated K-wires and served as controls, and were euthanized on post-operative days 6 and 21. After sacrifice, bone culture studies were conducted. We found that local IL-12 therapy combined with nanoscale coating systems substantially decreased S. aureus infection rates at day 6 (50% vs. 100%) and 21 (20% vs. 90%) compared to the control study. At sacrifice, gross observation, by three independent orthopaedic surgeons and technicians blinded to the treatments, of callus formation and bone quality showed that IL-12 nanoscale coating treated animals had better bone quality and improved healing compared to the control group. Further evaluation of the effect of IL-12 on bone healing is beyond the scope of this study and will be considered in the future.
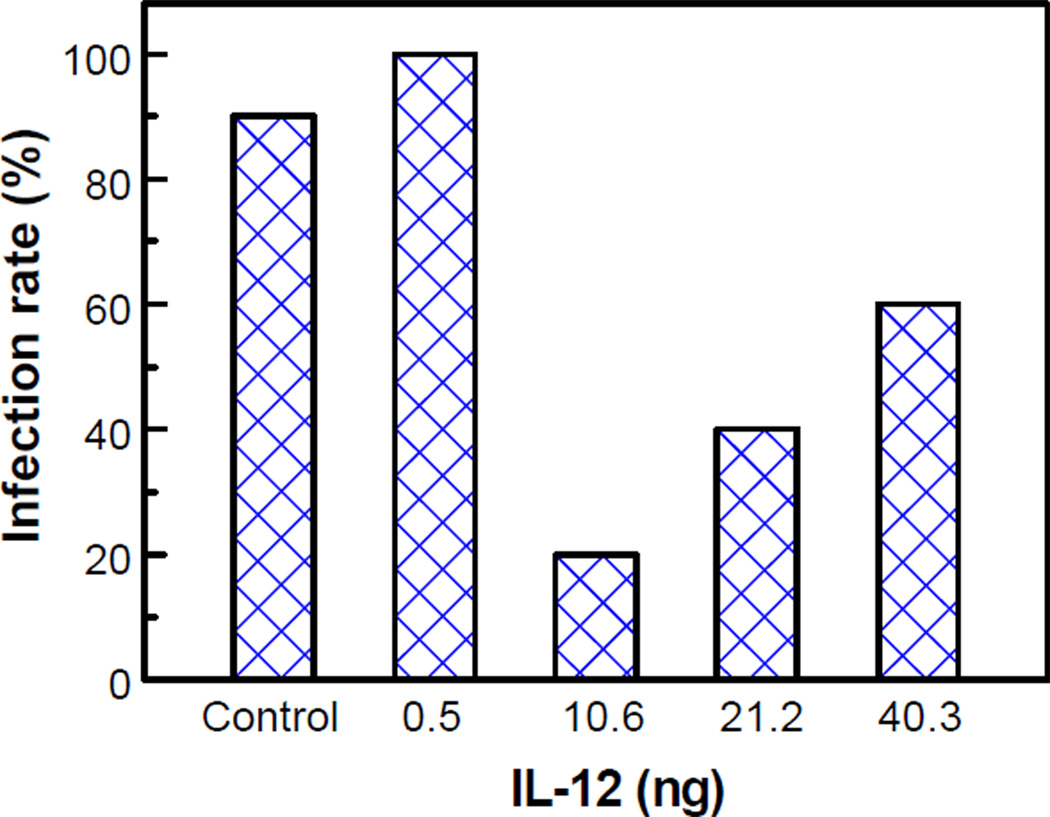
We further confirmed that IL-12 nanoscale coatings at the implant/tissue interface decreased S. aureus infection by studying different doses of IL-12 at post-operative day 21. We found that the effect of IL-12 on infection prevention was dose dependent, and nanoscale coatings of 10.6 ng IL-12 decreased the infection most effectively (Fig. 4).
Figure 4.
Infection rate vs. IL-12 dose. Six rats were studied in each group and the control group was repeated once (total 36 rats). The different doses of IL-12 were obtained by manipulating the concentration of IL-12 loading solution and the number of coating layers, as shown in Figs. 2b and c.
In addition, we investigated the effect of systemic IL-12 treatment in preventing S. aureus induced infections. All rats had a femur fractured, inoculated with S. aureus, and fixed. Rats (six in each group) were systemically injected with a dose (0, 10, 200, or 1000 ng) of IL-12 daily for 10 days (except the 6 day group), and euthanized on post-operative days 6, 10, 14, or 21. We found that systemic IL-12 therapy did not show a significant impact on the infection rate; all the rats were infected.
4. Discussion
The objective of this study was to explore a non-antibiotic approach and to evaluate the effects of IL-12 nanocoated K-wires in preventing osteomyelitis or infection in open fractures. IL-12 is a cytokine that has profound effects on both T and natural killer (NK) cells, including the ability to stimulate proliferation, cytolytic activity, and cytokine induction [23,24]. It has been suggested that IL-12 has therapeutic potential as a stimulator of cell-mediated immune responses to metastatic cancer and viral infections [12,13,25,26]. There are a few reports on systemic delivery of IL-12. IL-12 was shown to be active in vivo against lymphocytic choriomeningitis virus infections [27], and to induce a Th1 type response and protective immunity [28,29]. Local delivery of IL-12 based on phase-inversion encapsulation, which may denature the drug, was used for tumor treatment, and was shown to promote an antitumor immune response, and to induce complete regression of established tumors [30,31]. Moreover, studies have shown that the systemic level of IL-12 decreases following trauma and results in decreased and delayed cell-mediated immune response and decreased resistance to infection [15−18].
Therefore, there is a potential that exogenous IL-12 can restore and enhance the decreased IL-12 level and the decreased cell mediated immunity thereby preventing infection. In this study, we developed IL-12 nanoscale coatings at the implant/tissue interface using LBL technology, which was developed in the early 1990s by Decher and co-workers [20]. PLL, PLGA, and BSA(IL-12) were used to develop the IL-12 nanoscale coatings. PLL is positivelycharged, and BSA(IL-12) and PLGA are negatively-charged around neutral pH; these polymers are biocompatible and biodegradable [32]. BSA was used to facilitate and control the loading and release of IL-12, and the use of linear polymers, such as PLL and PLGA, were to assist the coating preparation. The nanoscale coating system may prevent IL-12 from travelling beyond the injury site. LBL is currently one of the most powerful methods for preparation of multilayer nanoscale coatings of controlled molecular architecture. The physical basis of LBL is mainly electrostatic attraction [20]. However, other forces, e.g. hydrophobic, van der Waals, and hydrogen bonding, may also play a significant role under certain conditions, and these forces could be used to tune drug loading and release [33]. Note that we have also prepared polypeptide multilayer coatings on other substrate materials such as titanium.
Based on ellipsometry observation (Fig. 1c), the thickness per layer of the developed IL-12 coatings was several nanometers. Since the risk of foreign body reactions increases with the amount of polymers placed into the tissue [34], the developed coatings in the nanometer scale thickness may have an advantage compared to coatings in µm or mm thickness.
Using LBL technology, we were able to tune the loading and release of IL-12 from nanoscale coatings (Fig. 2). Fig. 2c shows that a sustained, up to 9 days, release of IL-12 was obtained. Approximately 38% of IL-12 was released within one day and 60% within two days. The amount of IL-12 released varied from samples with different numbers of layers. More IL-12 was released from the samples of 30-layers (~ 10.6 ng IL-12 per K-wire) than those of 20-layers (~ 7.7 ng IL-12 per K-wire). In addition, we found that more IL-12 was released from samples fabricated with higher IL-12 concentrations in the BSA coating solution (Fig. 2b). Therefore, we can tune the incorporation and release of IL-12 using the LBL process including the number of layers and the concentration of IL-12 in the BSA coating solution. The release profiles in percentages for nanoscale coatings of different layers are similar (Fig. 2c); therefore, the release from the nanoscale coatings of varying layers is predictable. IL-12 was released almost completely within 9 days (Fig. 2c); such a release characteristic could be desirable since it was reported that the first 10 days were critical because resistance to infection is maximally suppressed during this period following traumatic injuries [15].
Meanwhile, a limiting factor of an effective IL-12 prophylaxis is the method of delivering IL-12 to the interface between an implant and tissue. By incorporating IL-12 at the implant/tissue interface, we expect that sustained local delivery of IL-12 will enhance cell-mediated immunity thereby preventing infection or osteomyelitis. Our in vivo studies showed that IL-12 nanoscale coatings at the implant/tissue interface substantially decreased infection rates compared to the control, and the effect of IL-12 on infection prevention is dose dependent (Fig. 4). Moreover, we found that IL-12 nanoscale coating is advantageous in preventing infection compared to the traditional systemic IL-12 injection, since our studies showed that the latter treatment had little effect in decreasing the infection rates. IL-12 has a short in vivo half-life of a few hours [35]; therefore, IL-12 applied by systemic administration may undergo rapid metabolization. The developed IL-12 nanoscale coatings can deliver IL-12 directly at the fracture site for an appropriate sustained time period (Fig. 2c).
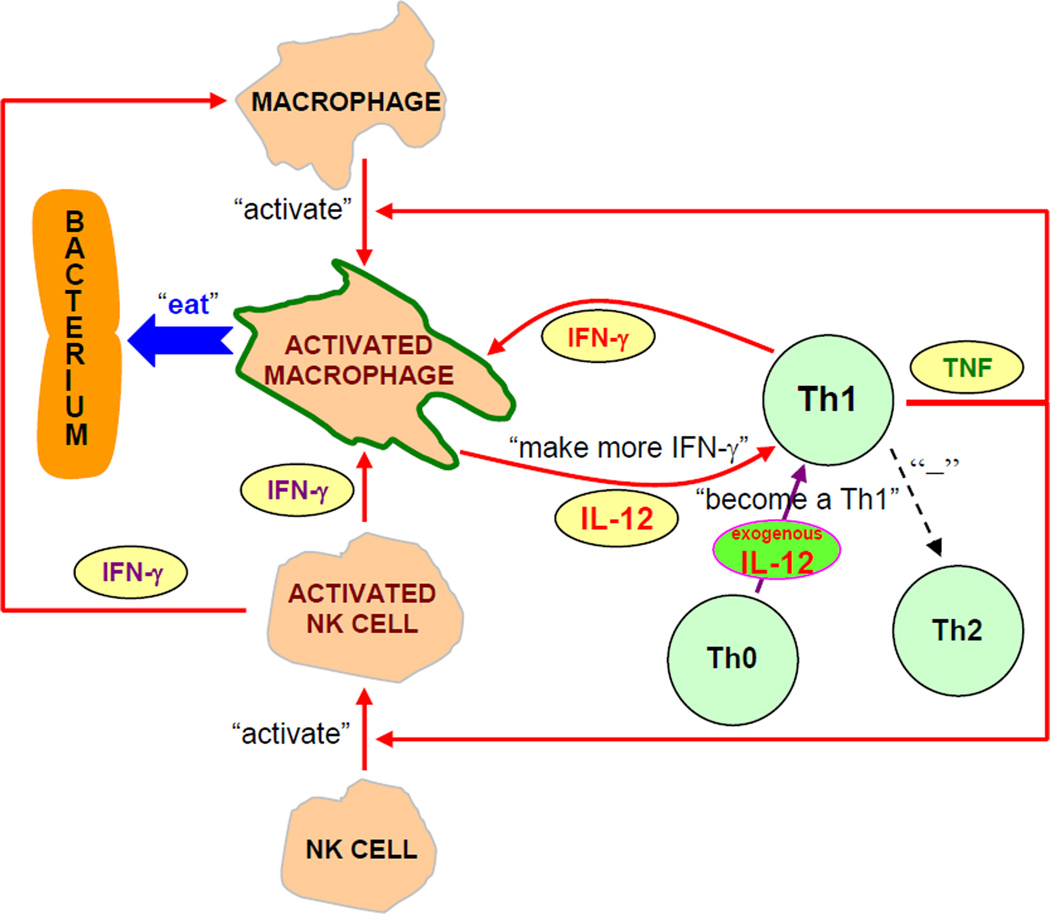
An underlying mechanism of IL-12 nanoscale coating therapy is proposed in Fig. 5. An IL-12 nanoscale coating at the implant/tissue interface creates an environment rich in IL-12 around the fracture site. When newly activated Th cells, responding to the presence of bacteria such as S. aureus, exit the blood in such a local environment, uncommitted Th (Th0) cells “realize” what type of battle is being fought and are influenced to produce more Th1 cytokines. Moreover, the high concentration of IL-12 at the battle site, i.e. fracture site, may strengthen the commitment of Th cells that have already decided to secrete Th1 cytokines (e.g. IFN-γ) which will activate macrophages. Activated macrophages will produce more IL-12, therefore, via positive feedback (Fig. 5), Th1 response is stabilized and the local level of Th1 cytokines increases and the macrophages are more effective in phagocytizing bacteria such as S. aureus thereby leading to the prevention of infection. Through stimulating the cell-mediated immune response, IL-12 nanoscale coatings at the implant/tissue interface may be able to more quickly activate and build the body’s natural defenses, thus jumpstarting the fight against an infection in the early stage. The benefit of this IL-12 nanoscale coating approach is that it maximizes the body’s natural response to an extent where infections can be reduced and possibly prevented without the risk of the offending bacteria developing resistance to the treatment, as is becoming more of a problem with antibiotic therapy alone.
Figure 5.
Local IL-12 therapy stimulates Th cells to secrete Th1 cytokines. Via positive feedback, cell-mediated immunity can be promoted to battle bacteria such as S. aureus.
5. Conclusions
In summary, we report that IL-12 nanoscale coatings at the implant/tissue interface substantially decreased open fracture-associated infections in an animal model. We prepared polypeptide nanoscale coatings on orthopaedic implants and we finely tuned the incorporation and release of IL-12. We showed that IL-12 nanoscale coatings at the implant/tissue interface are advantageous over traditional treatments such as systemic IL-12 injections. Given that antibiotic resistance has been a challenge to surgeons for decades, the developed IL-12 nanoscale coatings may represent a revolutionary and exciting approach for preventing open fracture and other device-associated infections while also providing a possible resolution to antibiotic resistance. IL-12 nanoscale coating therapy can be applied to other device-associated infections, as orthopaedic infections share many attributes with other bacterial biofilm infection.
Supplementary Material
Acknowledgements
This work was supported by the AO Foundation, NASA WV EPSCoR, and WVU. Project S-07-43L was supported by the AO Research Fund of the AO Foundation. We thank John G. Thomas, PhD (microbiologist, Department of Pathology, West Virginia University) for biofilm studies, John B. Barnett, PhD (immunologist, Department of Microbiology, Immunology & Cell Biology, West Virginia University) for mentoring BL, Sanford E. Emery, MD (orthopaedic surgeon, Department of Orthopaedics, West Virginia University) for consultation on open fracture and animal model, Nina Clovis and Suzanne Smith (animal support staff, Department of Orthopaedics, West Virginia University) for care, handling and surgeries of the animals, Sydha Salihu, PhD (Postdoctoral Research Associate, Department of Orthopaedics, West Virginia University) for ELISA tests, Vincent Kish, ASEE (senior scientific instrumentation technician, Department of Orthopaedics, West Virginia University) for building the fracture device and a closed chamber for nanoscale coating preparation, and Stanley Wearden, PhD (statistician, Department of Statistics, West Virginia University) for assistance in statistical analysis. BL also thanks the USBJD program and faculty for mentoring. The authors appreciate the use of microscopes at the Microscopic Imaging Facilities at West Virginia University Health Sciences Center.
Footnotes
Conflict of Interest: The authors confirm that there are no known conflicts of interest associated with this publication.
Supplementary data: Supplementary data associated with this article can be found in the online version.
References
- 1.Darouiche RO. Treatment of infections associated with surgical implants. N Engl J Med. 2004;350:1422–1429. doi: 10.1056/NEJMra035415. [DOI] [PubMed] [Google Scholar]
- 2.Garvin K, Feschuk C. Polylactide-polyglycolide antibiotic implants. Clin Orthop Relat Res. 2005;437:105–110. doi: 10.1097/01.blo.0000175720.99118.fe. [DOI] [PubMed] [Google Scholar]
- 3.Zalavras CG, Patzakis MJ, Holtom PD, Sherman R. Management of open fractures. Infect Dis Clin North Am. 2005;19:915–929. doi: 10.1016/j.idc.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Schmidmaier G, Lucke M, Wildemann B, Haas NP, Raschke M. Prophylaxis and treatment of implant-related infections by antibiotic-coated implants: a review. Injury. 2006;37:S105–S112. doi: 10.1016/j.injury.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 5.Nixon M, Jackson B, Varghese P, Jenkins D, Taylor G. Methicillin-resistant Staphylococcus aureus on orthopaedic wards: incidence, spread, mortality, cost and control. J Bone Joint Surg Br. 2006;88:812–817. doi: 10.1302/0301-620X.88B6.17544. [DOI] [PubMed] [Google Scholar]
- 6.Trnobranski P. Are we facing a ‘post-antibiotic era’?--a review of the literature regarding antimicrobial drug resistance. J Clin Nurs. 1998;7(5):392–400. doi: 10.1046/j.1365-2702.1998.00181.x. [DOI] [PubMed] [Google Scholar]
- 7.Macdonald DJ, Gray AJ. Methicillin-resistant Staphylococcus aureus in trauma and orthopaedic practice. J Bone Joint Surg Br. 2006;88:137–138. doi: 10.1302/0301-620X.88B1.17026. [DOI] [PubMed] [Google Scholar]
- 8.Heseltine P. Has resistance spread to the community? Clin Microbiol Infect. 2000;6:11–16. doi: 10.1046/j.1469-0691.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 10.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of Th1 responses. Trends Immunol. 2003;24(4):207–212. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Locksley RM. Interleukin 12 in host defense against microbial pathogens. Proc Natl Acad Sci USA. 1993;90:5879–5880. doi: 10.1073/pnas.90.13.5879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trinchieri G. Interleukin-12 and its role in the generation of TH1 cells. Immunol Today. 1993;14:335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 13.Hall SS. IL-12 holds promise against cancer, glimmer of AIDS hope. Science. 1994;263(5154):1685–1686. doi: 10.1126/science.7907819. [DOI] [PubMed] [Google Scholar]
- 14.Sompayrac L. How the immune system works. 2nd ed. Massachussetts: Blackwell Publishing, Inc.; 2003. [Google Scholar]
- 15.O'Sullivan ST, Lederer JA, Horgan AF, Chin DH, Mannick JA, Rodrick ML. Major injury leads to predominance of the T helper-2 lymphocyte phenotype and diminished interleukin-12 production associated with decreased resistance to infection. Ann Surg. 1995;222(4):482–490. doi: 10.1097/00000658-199522240-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spolarics Z, Siddiqi M, Siegel JH, Garcia ZC, Stein DS, Denny T, et al. Depressed interleukin-12-producing activity by monocytes correlates with adverse clinical course and a shift toward Th2-type lymphocyte pattern in severely injured male trauma patients. Crit Care Med. 2003;31(6):1722–1729. doi: 10.1097/01.CCM.0000063579.43470.AA. [DOI] [PubMed] [Google Scholar]
- 17.Huynh T, Lemasters JJ, Bracey LW, Baker CC. Proinflammatory Kupffer cell alterations after femur fracture trauma and sepsis in rats. Shock. 2000;14(5):555–560. doi: 10.1097/00024382-200014050-00010. [DOI] [PubMed] [Google Scholar]
- 18.Schirmer WJ, Schirmer JM, Townsend MC, Fry DE. Femur fracture with associated soft-tissue injury produces hepatic ischemia. Arch Surg. 1988;123:412–415. doi: 10.1001/archsurg.1988.01400280018002. [DOI] [PubMed] [Google Scholar]
- 19.Marriott I, Gray DL, Tranguch SL, Fowler VG, Jr, Stryjewski M, Scott Levin L, et al. Osteoblasts express the inflammatory cytokine interleukin-6 in a murine model of Staphylococcus aureus osteomyelitis and infected human bone tissue. Am J Pathol. 2004;164(4):1399–1406. doi: 10.1016/S0002-9440(10)63226-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 21.Kalicke T, Schierholz J, Schlegel U, Frangen TM, Koller M, Printzen G, et al. Effect on infection resistance of a local antiseptic and antibiotic coating on osteosynthesis implants: an in vitro and in vivo study. J Orthop Res. 2006;24(8):1622–1640. doi: 10.1002/jor.20193. [DOI] [PubMed] [Google Scholar]
- 22.Petty W, Spanier S, Shuster JJ, Silverthorne C. The influence of skeletal implants on incidence of infection. J Bone Joint Surg Am. 1985;67(8):1236–1244. [PubMed] [Google Scholar]
- 23.Chehimi J, Trinchieri G. Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J Clin Immunol. 1994;14(3):149–161. doi: 10.1007/BF01533364. [DOI] [PubMed] [Google Scholar]
- 24.Trinchieri G, Scott P. The role of interleukin 12 in the immune response, disease and therapy. Immunol Today. 1994;15(10):460–463. doi: 10.1016/0167-5699(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh CS, Macatonia SE, Tripp CS, Wolf SF, O'Garra A, Murphy KM. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 26.Scott P. IL-12: initiation cytokine for cell-mediated immunity. Science. 1993;260(5107):496–497. doi: 10.1126/science.8097337. [DOI] [PubMed] [Google Scholar]
- 27.Orange JS, Wolf SF, Biron CA. Effects of IL-12 on the response and susceptibility to experimental viral infections. J Immunol. 1994;152(3):1253–1264. [PubMed] [Google Scholar]
- 28.Heinzel FP, Schoenhaut DS, Rerko RM, Rosser LE, Gately MK. Recombinant interleukin 12 cures mice infected with Leishmania major. J Exp Med. 1993;177(5):1505–1509. doi: 10.1084/jem.177.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sypek JP, Chung CL, Mayor SE, Subramanyam JM, Goldman SJ, Sieburth DS, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. J Exp Med. 1993;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egilmez NK, Jong YS, Sabel MS, Jacob JS, Mathiowitz E, Bankert RB. In situ tumor vaccination with interleukin-12-encapsulated biodegradable microspheres: induction of tumor regression and potent antitumor immunity. Cancer Res. 2000;60(14):3832–3837. [PubMed] [Google Scholar]
- 31.Kuriakose MA, Chen FA, Egilmez NK, Jong YS, Mathiowitz E, DeLacure MD, et al. Interleukin-12 delivered by biodegradable microspheres promotes the antitumor activity of human peripheral blood lymphocytes in a human head and neck tumor xenograft/SCID mouse model. Head Neck. 2000;22(1):57–63. doi: 10.1002/(sici)1097-0347(200001)22:1<57::aid-hed9>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 32.Shih IL, Van YT, Shen MH. Biomedical applications of chemically and microbiologically synthesized poly(glutamic acid) and poly(lysine) Mini Rev Med Chem. 2004;4(2):179–188. doi: 10.2174/1389557043487420. [DOI] [PubMed] [Google Scholar]
- 33.Jiang B, Li B. Tunable drug loading and release from polypeptide multilayer nanofilms. Int J Nanomedicine. doi: 10.2147/ijn.s4970. (submitted). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bostman OM, Pihlajamaki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop Relat Res. 2000;371:216–227. [PubMed] [Google Scholar]
- 35.Bajetta E, Del Vecchio M, Mortarini R, Nadeau R, Rakhit A, Rimassa L, et al. Pilot study of subcutaneous recombinant human interleukin 12 in metastatic melanoma. Clin Cancer Res. 1998;4(1):75–85. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.