Abstract
BACKGROUND
Pemphigus is an autoimmune blistering disease. According to a report, in areas of endemic pemphigus foliaceus (EPF) in Peru there are cases of pemphigus vulgaris with epidemiologic, clinical and histopathologic characteristics similar to those of "endemic pemphigus vulgaris" (EPV) in Brazil.
OBJECTIVES
To determine the clinical and epidemiologic characteristics of endemic pemphigus and the risk factors of patients for developing complications during treatment.
METHODS
A study was carried out from July 2003 to March 2008. The study population was 60 patients with EPF and 7 patients with EPV evaluated in hospitals and clinics in the Peruvian Amazon and Lima. A multivariate analysis was carried out using binary logistic regression.
RESULTS
The average age of EPF patients was 31.4 years; 55% were men; 60% presented the generalized clinical variant. Non-compliance with the treatment was seen in 57.1% of the patients. Thirty-five percent presented complications (e.g. pyodermitis and pyelonephritis) during treatment. The risk factors for developing complications during treatment were non-compliance with the treatment and having the generalized clinical form. In the EPV group, the average age was 21.7 years; 71.4% were men. All patients presented with the mucocutaneous clinical variant and the initial presentation consisted of oral mucosa lesions; 71.4% presented complications during treatment, pyodermitis being the most frequent.
CONCLUSIONS
Non-compliance with the treatment and the generalized clinical form are risk factors for the development of complications during treatment of patients with EPF. Peru indeed has EPV cases with epidemiologic characteristics similar to EPF. Living in a rural area may represent a risk factor for the development of complications during treatment of patients with EPV.
Keywords: Epidemiology, Pemphigus, Peru
Abstract
FUNDAMENTOS
O pênfigo é uma doença auto-imune bolhosa. Segundo um relatório, em áreas de pênfigo foliáceo endêmico no Peru há casos de pênfigo vulgar com características epidemiológicas, clínicas e histopatológicas semelhantes às do "pênfigo vulgar endêmico" no Brasil.
OBJETIVOS
Determinar as características clínicas e epidemiológicas do pênfigo endêmico e os fatores de risco para o desenvolvimento de complicações durante o tratamento.
MÉTODOS
Um estudo foi realizado de julho de 2003 a março de 2008. 60 doentes de pênfigo foliáceo endêmico e 7 de pênfigo vulgar endêmico foram avaliados em hospitais e clínicas na Amazônia peruana e em Lima. Uma análise multivariante foi feita usando regressão logística binária.
RESULTADOS
A idade média dos doentes de pênfigo foliáceo endêmico foi 31,4 anos; 55% eram homens, 60% apresentavam a forma clínica generalizada. 57,1% nao cumpriram o tratamento. 35% apresentaram complicações (por exemplo, piodermites e pielonefrite). Os fatores de risco foram não cumprir o tratamento e ter a forma clínica generalizada. No grupo pênfigo vulgar endêmico, a idade média foi 21,7 anos; 71,4% eram homens. Todos os pacientes apresentavam a variante clínica mucocutânea e a apresentação inicial consistia de lesões da mucosa bucal; 71,4% apresentaram complicações durante o tratamento, piodermites sendo a mais freqüente.
CONCLUSÕES
Não cumprir o tratamento e ter a forma clínica generalizada são fatores de risco para o desenvolvimento de complicações durante o tratamento de pênfigo foliáceo endêmico. Peru realmente tem casos de pênfigo vulgar endêmico com características epidemiológicas semelhantes às do pênfigo foliáceo endêmico. Viver numa área rural pode ser um fator de risco para o desenvolvimento de complicações.
INTRODUCTION
Endemic pemphigus foliaceus (EPF) is an autoimmune blistering disease that is histologically characterized by subcorneal acantholysis and, immunologically, by the presence of IgG4 circulating autoantibodies against desmoglein 1 of the squamous epidermal epithelium.1 The disease has been reported in Brazil, Colombia, El Salvador, Paraguay, Tunisia and Venezuela, with variants in each country. 2-9
EPF is endemic in Peru and it has been described since the beginning of the 1950s in the Peruvian Amazon by Muñoz and Weiss.2 The first report was published in the 70s (Heimgartner and De Heimgartner) and the second in the 90s (Castillo) but the main clinical and epidemiologic studies were carried out between 2001 and 2005 (Galarza; Díaz y De Amat; Cruz).10-14
Recently, Rocha-Alvárez et al. reported a variant of pemphigus vulgaris in Brazil which they called "endemic pemphigus vulgaris" (EPV). According to the report, the patients presented clinical findings of pemphigus vulgaris with the epidemiological characteristics of EPF.15 In Peru, a report by Galarza et al. (2004) showed that in areas of EPF there is an occurrence of cases of pemphigus vulgaris with epidemiologic, clinical and histopathologic characteristics similar to those reported in Brazil.16
It is known that subjects living in areas of EPF and EPV develop non-pathogenic IgG antibodies antidesmoglein 1 and 3 as an immune disorder before developing the disease itself. Ortega-Loayza and Ramos showed the presence of antidesmoglein 1 and 3 antibodies in healthy people in the community of Pueblo Libre as well as in relatives of EPF patients in Peru.17,18
There are studies that describe possible risk factors for the development of complications during treatment of patients with EPF, such as generalized clinical form, lesions on the scalp, dietary factors, and non-compliance with the treatment.3,4,19 There are very few reports on EPV, and is possible that it has similar risk factors to EPF.15
In Peru, most of the studies of EPF are retrospective and no published studies show the existence of EPV. The aim of this study was to determine the epidemiologic and clinical characteristics of EPF and EPV in the Peruvian Amazon, as well as the risk factors for complications during treatment of patients in the same area.
MATERIALS AND METHODS
DESIGN
This is a multicenter nested case-control study carried out in groups of Peruvian patients with EPF and EPV. The study was carried out between January 2003 and March 2008. Patients were recruited in the regions of Ucayali (Hospital Regional de Pucallpa, Hospital Amazónico de Yarinacocha, and the health centers of Nueva Requena and Pueblo Libre), and Lima (patients referred to the Hospital Nacional Dos de Mayo).
POPULATION AND SAMPLE
We included patients who met the clinical and histopathological criteria for the diagnosis of EPF and EPV, who did not have complications at the beginning of the study, who agreed to participate voluntarily, and/or had the consent of parents in the case of minors.
During the treatment and follow-up of patients with EPF, those who developed complications were the cases group and those who did not develop complications at the end of the follow-up period (March 2008) were the control group. The small number of subjects with EPV did not allow a statistical analysis but the complications during treatment and possible risk factors were recorded.
TECHNIQUES AND METHODS
We performed a 2-mm punch biopsy on all the patients for histopathologic examination with hematoxylin and eosin staining. Whenever possible, we performed immunological studies. Unfortunately, immunological studies in Peru were very limited because of lack of monetary and laboratory resources.
For the detection of antidesmoglein 1 antibodies by ELISA, the samples were processed per protocol at the University of North Carolina at Chapel Hill, USA. For direct and indirect immunofluorescence studies, the samples were processed at the University of North Carolina at Chapel Hill (USA), the pathology institute of the Universidad Nacional Mayor de San Marcos and the Hospital Guillermo Almenara Irigoyen, both in Lima, Peru.
The treatment of choice was systemic corticosteroids: intravenous dexamethasone sodium phosphate in hospitalized patients and oral prednisone in outpatients. Once the formation of new skin lesions and pruritus stopped and/or the Nikolsky's sign became negative, the dose of prednisone was tapered gradually until reaching a maintenance dose of 5 mg/day. If the patients were recalcitrant to treatment with corticosteroids, adjuvant therapy with dapsone or azathioprine was added. When secondary infection occurred, empiric antibiotic therapy was used based on the sensitivity and culture results.
During the course of the patients' treatment we evaluated possible risk factors for the development complications such as age less than 15 years, generalized clinical form, lesions on the scalp, diet rich in tannins and/or thiols, and non-compliance with the treatment. A patient's failure to follow the prescribed therapy was categorized as non-compliant with the treatment.
STATISTICAL ANALYSIS
The statistical analysis was performed using SPSS version 20.0. A univariate analysis was performed to obtain frequencies, percentages, measures of central tendency and relative dispersion. The bivariate analysis was performed with the Pearson Chi-square test while for the multivariate analysis, we used a binary logistic regression model (forward and bootstrap method) being the dependent variable the occurrence of complication during the course of treatment. The analysis of the independent risk factors variables-age younger than 15 years, the generalized clinical form, non-adherence to treatment, presence of lesions on the scalp, and a diet rich in tannins, thiols and isothiocyanates, yielded unadjusted odds ratio and adjusted odds ratio. The calculations were performed with a confidence level of 95%.
RESULTS
Epidemiologic characteristics of endemic pemphigus foliaceus
Sixty cases of EPF were studied; 55.0% were men, their average age being 31.4 ± 18.2 years (median was 18 years); the most affected age group was between 10 and 39 years of age (Table 1).
Table 1.
Distribution of patients with EPF and EPV in Peru according to age group
| Age Group (years) | EPF | % | EPV | % |
| 0 - 9 | 2 | 3.3 | 1 | 14.3 |
| 10 - 19 | 16 | 26.7 | 3 | 42.8 |
| 20 - 29 | 13 | 21.7 | 2 | 28.6 |
| 30 - 39 | 14 | 23.3 | 1 | 14.3 |
| 40 - 49 | 5 | 8.3 | 0 | 0.0 |
| 50 - 59 | 2 | 3.3 | 0 | 0.0 |
| 60 - 69 | 6 | 10.0 | 0 | 0.0 |
| 70 - 79 | 2 | 3.3 | 0 | 0.0 |
| Total | 60 | 100.0 | 7 | 100.0 |
The main occupations were farmer (45.0%), housewife (33.3%), student (16.7%) and sheep rancher (5.0%). The main origins of the patients were: Campo Verde (15.0%), Calleria (13.3%) and Nueva Requena (10.0%) %) in the region of Ucayali; Tingo Maria (5.0%) in the region of Huanuco; and Satipo (5.0%) the region of Junin. Less frequent cases came from the Amazonas (Bagua, Tuemal) and San Martin (Moyabamba) Regions. We observed pemphigus cases in the same family in only 8.3% of the patients.
The characteristics of the patients' houses (Figure 1) were: 65.0% had wooden walls, 53.3% had straw roofs and 31.7% used a thin sheet metal called calamina. The floor was usually dirt (71.6% of the cases) or wood (20.0%). We found that 88.3% of the households were not using drinkable water, using instead water from the well in Pueblo Libre and the riverditch in Nueva Requena.
FIGURE 1.

Housing of a patient with EPF. In the area of Sheshea-Nueva Requena, in the region of Ucayali
Clinical characteristics of EPF
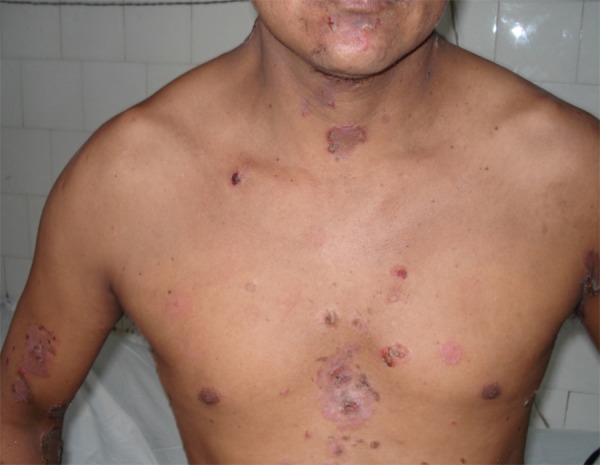
The average disease duration was 2.1 ± 1.9 years (median 1.3 years), 60.0% of patients presented the generalized clinical form and 40.0% the localized form (Table 2 and Figure 2). The lesions started on the facial region (35.0%), chest (26.7%), scalp (15.0%) and on other locations (8.7%). In the physical exam, the most predominant findings were erythema (96.7%) and desquamative lesions (95.0%). Nikolsky's sign was present in 83.3% and scalp lesions were seen in 70.0% of the cases.
Table 2.
Clinical stages in patients with EPF in Peru
| Clinical stages | Male | Female | Total | % |
| Chronic active | 25 | 18 | 43 | 71.7 |
| Recurrent | 4 | 2 | 6 | 10.0 |
| Remission | 2 | 4 | 6 | 10.0 |
| Cure | 1 | 2 | 3 | 5.0 |
| Death | 1 | 1 | 2 | 3.3 |
| Total | 33 | 27 | 60 | 100.0 |
FIGURE 2.
Localized Clinical Form of EPF. In a 38-year-old patient from San Alejandro, in the region of Ucayali
The main symptoms were pruritus (98.3%), burning sensation (38.3%) and photosensitivity (51.6%). We found that 81.7% presented a diet rich in tannins, phenols and thiols, and 18.3% reported that their disease worsened when consuming food with high contents of these compounds.
Subcorneal acantholysis was found in the histopathologic exam in all the patients; we performed immunologic studies in 22 patients and 20 had positive results (16 with ELISA, 2 with direct immunofluorescence and 2 with indirect immunofluorescence).
Viral and autoimmune diseases
The frequency of viral diseases was 3.3%(2 cases). The presence of viral infections did not significantly influence the immunologic characteristics of the disease. The indirect immunofluorescence titers in both patients with viral diseases were 1/160 and 1/640 respectively, no higher than in the patients without viral diseases. A 15-year-old patient developed a generalized cutaneous herpes simplex infection, which healed without complications, and another developed Kaposi's varicelliform eruption.
There were three cases with associated autoimmune disorders (5.0%). The first case presented a localized non-segmental vitiligo which had a stable clinical course during four years until the time of diagnosis and low titers of indirect immunofluorescence. The second case was a woman who presented psoriasis vulgaris one year previous to the onset of EPF, and had positive anti-IgG antibodies by indirect immunofluorescence. Her ELISA index values for antidesmoglein 1 were situated in the undetermined zone (between 14 and 20) and did not express antidesmoglein 3 antibodies. The third case had a 7-year history of the generalized clinical form, and later presented psoriasis vulgaris. The patient expressed antidesmoglein 1 and 3 antibodies and negative indirect immunofluorescence.
The presence of autoimmune diseases did not influence the immunologic characteristics of the disease. The indirect immunofluorescence titers in the two patients with psoriasis were 1/160, whereas the values indexes of the ELISA IgG antidesmoglein 1 were 16 and 42 respectively.
Complications during treatment
Forty-eight percent of the patients were non-compliant with the treatment and 35.0% presented some complications during treatment: pyodermatitis, acute pyelonephritis and sepsis (Table 3). The mortality rate was 3.3%. One of the patients died of a hospital-acquired pneumonia and the other one died of adrenal insufficiency due to abrupt discontinuation of corticosteroid therapy.
Table 3.
Complications during treatment of patients with EPF and EPV
| Complications | EPF | % | EPV | % |
| Pyodermitis | 8 | 38.1 | 2 | 40.0 |
| Acute pyelonephritis | 3 | 14.3 | 0 | 0.0 |
| Sepsis | 3 | 14.3 | 0 | 0.0 |
| Conjuntivitis | 2 | 9.5 | 1 | 20.0 |
| Pneumonia | 2 | 9.5 | 0 | 0.0 |
| Aseptic necrosis of the hip | 1 | 4.8 | 1 | 20.0 |
| Adrenal insufficiency | 1 | 4.8 | 0 | 0.0 |
| Acute otitis media | 1 | 4.8 | 0 | 0.0 |
| Cervical abcess | 0 | 0.0 | 1 | 20.0 |
| Total | 21 | 100.0 | 5 | 100.0 |
The multivariate analysis showed that the risk factors for developing complications during the course of treatment were the generalized clinical form (p=0.009; OR=9.4; CI 95%: 1.756-50.377) and non-compliance with the treatment (p=0.019; OR=7.4; CI 95%: 1.877-29.573). The presence of lesions on the scalp may represent a potential risk factor to develop treatment complications (p=0.01; OR=6.6; CI: NS). The logistic regression with the boopstrap method confirmed the generalized clinical variant (p=0.003) and the non-adherence to treatment in patients with EPF (p=0.002) as risk factors for developing treatment complications. We did not find any statistically significant difference for age, gender, occupation, rural residence, presence of Nikolsky sign, or dietary factors.
Please see table 4 for the distribution and multivariate analysis of potential risk factors for complications during treatment.
Table 4.
Distribution and multivariate analysis of potential risk factors for complications during treatment
| Factor | Frequency of cases | Frequency in controls | Odds ratio unadjusted | I.c 95% odds ratio unadjusted | Odds ratio adjusted | I.c 95% odds ratio adjusted |
| Age under 15 | 6/21 | 6/37 | NS | NS | NS | NS |
| Diet rich in tannins, thiols and / or isothiocyanates | 17/21 | 32/39 | NS | NS | NS | NS |
| Generalized clinical form | 19/21 | 17/39 | 12.294 | 2.511 -60.200 | 9.406 | 1.756 -50.377 |
| Lesions on the scalp | 19/21 | 23/39 | 6.609 | 1.347 -32.427 | NS | NS NS |
| Adherence to treatment | 17/21 | 12/39 | 9.563 | 2.648 -34.532 | 7.451 | 1.877 -29.573 |
ENDEMIC PEMPHIGUS VULGARIS
Epidemiologic characteristics
During our study, seven cases of pemphigus vulgaris met the criteria of EPV. Seventy-one percent were men, the average age of the patients was 21.7 ± 13.9 years (median of 13 years) and the most affected age group was between 10 and 19 years (Table 1).
The main occupations were farmer, student and housewife (28.6% each). The patients came from Nueva Requena, Neshuya, Nueva Bellavista and El Progreso (Ucayali), Tingo Maria (Huanuco) and Trompeteros (Loreto), which are also endemic areas for EPF.
Regarding the characteristics of the housing of the patients, 57.1% had wooden walls and roof of straw, and 28.6% used calamina. The floor was dirt in 71.4% of the houses. We found that 85.7% of the households used non-drinkable water obtained from a well, river or ditch.
Clinical characteristics
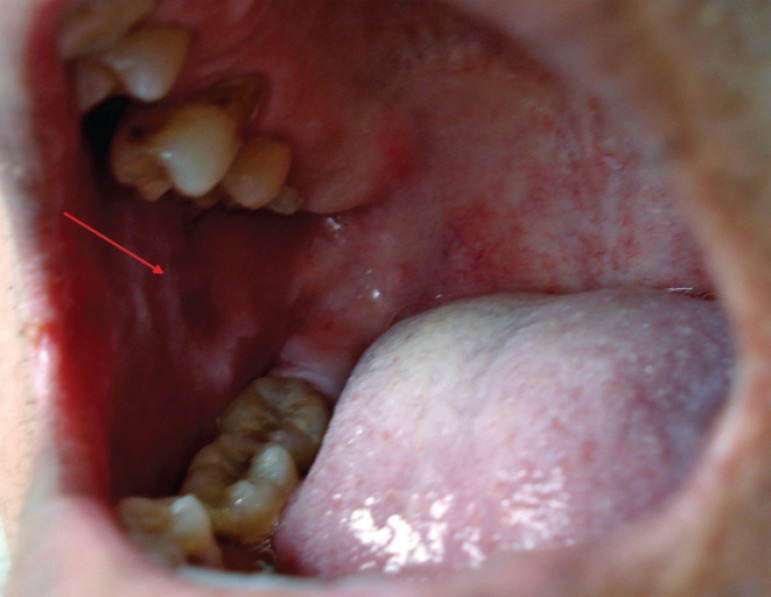
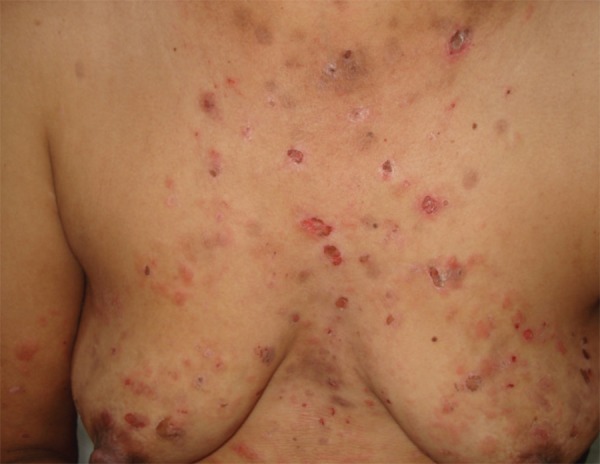
The average time of the present illness was 13.6 ± 11.0 months (the median was 12 months). All had the mucocutaneous clinical form and their initial presentation was with oral mucosa lesions (Figures 3 and 4). The skin sites most commonly affected initially were the face (42.9%) and chest (28.6%).
FIGURE 3.
EPV Skin Lesions of a patient with EPV
FIGURE 4.
Lesion on the right side of the oral mucosa a patient with EPV
On physical examination, erythema and erosive lesions were present in all the patients; the Nikolsky sign was positive in 85.7% of the cases and 71.4% presented lesions on the scalp. The main symptoms were pruritus (100%) and burning sensation (42.9%). We also documented that 85.7% of the patients ingested a diet rich in tanins, thiols and phenols; 28.6% attributed the worsening of the disease to consuming any of these foods. We found that 71.4% of the patients required hospitalization for an average of 8.2 ± 3.5 days. Systemic corticosteroids produced rapid clinical response in the patients, so adjuvant therapy was not necessary.
All the patients showed histologic features consistent with pemphigus vulgaris. One patient had positive direct immunofluorescence and three patients had positive indirect immunofluorescence.
Complications during treatment
Non-compliance with the treatment was seen in 57.1% of patients, and 71.4% presented at least one complication during the course of treatment (Table 3). The most frequent complication was pyodermitis (40%). None of the patients died from the complications of the medical management. The multivariate analysis of the risk factors for complications was not completed due to the low frequency of cases.
We followed three patients who moved from the rural zones of the Peruvian Amazon to the city of Lima (a non-endemic area). It is important to highlight that their skin lesions improved rapidly with the conventional treatment after they moved out from the rural areas. An environmental factor present in rural areas might therefore represent a risk factor for the development of complications.
DISCUSSION
Our results show that the generalized clinical form and non-compliance with the treatment are risk factors for the development of complications during the treatment of patients with EPF. Our results are similar to those previously reported by Castillo and Maguiña (1993), Galarza (2002), De Amat and Díaz (2004)13 and Cruz (2005) who found that the disease affects mainly by adolescents and young adults, with a predominance of the generalized clinical form. 11-14
A distinct pattern of EPF in Peru is the low frequency of familial cases; the cases were distributed in small conglomerates in the endemic areas but did not have any direct family link. It is then probable that environmental factors are more relevant than familial ones, which is compatible with the finding of the development of antidesmoglein 1 antibodies in healthy individuals according to reports by Warren,1 Ortega-Loayza and Ramos. 17,18 Moreover, it is possible that relatives of Peruvian patients in the endemic areas might have antibodies but do not develop the disease18 because they lack immunogenetic susceptibility. Therefore, their immunologic response would be similar to that of healthy subjects in the Peruvian endemic areas. For instance, in a five-year cohort of 21 patients positive for antidesmoglein 1, none of them developed EPF.20 Another difference is the ethnic background. In Peru the predominant race is the Amazonian mixed race, while in Brazil the predominant races are white and mulatto.19,21
The most frequent occupations of patients with EPF were farmer and sheep rancher in men, and housewife in women. It is important to highlight that in the case of the men, both occupations involve intense exposure to environmental factors including hematophagus insects, and for the women the occupation of housewife involves exposure to a group of indoor insects. Studies from other countries have shown the relationship between EPF and hematophagus insects such as simulids, phlebotomus (outdoor) and triatomines (indoor), particularly the vectors of parasitic diseases.22-24
There are very few studies that describe possible risk factors for the development of complications during treatment of patients with endemic pemphigus. In this investigation, factors such as age, clinical form, and lesions involving the scalp, dietary factors, and non-compliance with corticosteroid treatments were studied. 19 The multivariate analysis showed that the only risk factors for complications during treatment were non-compliance with the treatment and the generalized clinical form (9 and 7 times more likely respectively). The presence of lesions on the scalp might be a potential risk factor to develop complications as well.
We observed a high frequency of non-compliance with the treatment among the patients, who came in to the hospital only when the situation was severe. Part of the problem in this group of patients was the low economic resources, since typically in their families the father works as a farmer or a sheep rancher and the mother performs the housework. The father's monthly income (which generally constitutes the family income) is in average 250-350 New Soles (equivalent to 100-150 USD), less than the minimum salary in Peru (approximately 250 USD). The patients cannot afford medical treatment and transportation from the rural areas to the cities where the hospitals are located. Although it is true that diet can have a role as a triggering and exacerbating factor for EPF (many foods rich in tanins and thiols such as fish, mango, yucca, onions and garlic have been reported as inductors of acantholysis in vitro), the exact statistical analysis did not confirm it as a risk factor for the development of complications. 25-30
The present study shows that infectious complications are frequent and the generalized clinical form is a risk factor for this. Of the two patients who died, one of them died of an infectious complication (hospital-acquired pneumonia). In patients with EPF and EPV, pyodermitis was the main infectious complication, and in one case it became the source for sepsis. The cutaneous lesions constitute an entrance for germs to the body. The lack of drinkable water in rural areas negatively affects the hygiene of the patients. In addition, there is a lack of antibiotics and access to medical centers in many rural areas. Immunosuppressive state secondary to glucocorticoids is a well-known factor that predisposes people to infections as well.
Our results highlight the importance of educating patients about the need of compliance with the treatment. Due to the nature of the disease and the low resources of the patients, who come from areas of extreme poverty in the Amazon, treatment could be covered by the Peruvian state through Integral Health Security (the Seguro Integral de Salud or SIS) or Universal Health Security (Aseguramiento Universal en Salud or AUS), which would ensure the provision of medications and reduce non-compliance with the therapy. Nevertheless, our results will remind physicians about caring for patients with the generalized clinical form to take the necessary precautions to prevent complications. The precautions include diet, hygiene, early treatment and close follow-up especially at the beginning of the treatment, in order to verify the clinical response as well as to diagnose and treat complications as soon as possible.
Taking into consideration the triggering of the disease has been associated with environmental factors, in particular exposure to hematophagous insects, 22-24 and that some people might later develop endemic pemphigus, it might be helpful to suggest to the patients to temporarily move to an urban area, where the environment does not play a negative role in the control of the disease and the development of complications. Other possible factors for complications in patients with EPV were the mucocutaneous clinical form and non-compliance with the treatment (as observed in EPF). Nevertheless, the small number of patients prevented a statistically suitable analysis.
In our study, it is clear that in Peru there are patients who meet the criteria proposed by Rocha-Álvarez for EPV. The patients come from endemic areas of EPF and present an age group distribution similar to EPF. It seems that the course of the disease is more benign than the non-endemic clinical form, with fast response to the systemic corticosteroids and without association to any other autoimmune disease. Brazilian researchers have reported the occurrence of one case of EPV for each 12 to 72 cases of EPF in endemic areas.15 We found seven cases of EPV for 60 cases of EPF, a 1 to 9 ratio close to that reported in Brazil.
CONCLUSION
In conclusion, non-compliance with the treatment and the generalized clinical form are risk factors for the development of complications during treatment of patients with EPF. The presence of lesions on the scalp might be a potential risk factor to develop complications as well. We demonstrate the existence of patients EPV in Peru with epidemiologic characteristics similar to patients EPF. Living in a rural area may represent a risk factor for the development of complications during the treatment of treatment of patients with EPV.
Footnotes
Conflict of interest: None
Financial funding: None
* Work performed at the Universidad Nacional Mayor de San Marcos - Lima, Peru.
REFERENCES
- 1.Warren S, Lin MS, Giudice G, Hoffman R, Hans-Filho G, Aoki V, et al. The Prevalence of antibodies against Desmoglein 1 in Endemic Pemphigus Foliaceus in Brazil. N Eng J Med. 2000;343:23–30. doi: 10.1056/NEJM200007063430104. [DOI] [PubMed] [Google Scholar]
- 2.Aranha-Campos J. Invasao do Pénfigo Foliaceo na América do Sul. Arq Dermat Sif. 1952;14:12–20. [Google Scholar]
- 3.Hans-Filho G, Aoki V, Rivitti EA, Eaton DP, Lin MS, Diaz LA. Endemic Pemphigus Foliaceus (Fogo Selvagem)-1998. Clin Dermatol. 1999;17:225–235. doi: 10.1016/s0738-081x(99)00014-0. [DOI] [PubMed] [Google Scholar]
- 4.Campbell I, Reis V, Aoki V, Cunha P, Hans-Filho G, Alves G, et al. Pênfigo foliáceo endêmico/Fogo selvagem. An Bras Dermatol. 2001;76:13–31. [Google Scholar]
- 5.Abreu AM. Pénfigo Foliáceo Endémico. Situación en Colombia. Acta Med Colomb. 1996;21:27–34. [Google Scholar]
- 6.Hernández-Pérez E. Pemphigus in El Salvador. An eight-year study (1970-1977) Int J Dermatol. 1979;18:645–648. doi: 10.1111/j.1365-4362.1979.tb04683.x. [DOI] [PubMed] [Google Scholar]
- 7.Aldama A. Pénfigo foliáceo endémico en Paraguay [disertación]; XV Congreso Ibero-Latinoamericano de Dermatología; 2003.Buenos Aires: [Google Scholar]
- 8.Kallel Sellami M, Ben Ayed M, Mouquet H, Drouot L, Zitouni M, Mokni M, et al. Antidesmoglein 1 antibodies in Tunisian healthy subjects: arguments for the role of environmental factors in the occurrence of Tunisian pemphigus foliaceus. Clin Exp Immunol. 2004;137:195–200. doi: 10.1111/j.1365-2249.2004.02493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzáles F, Sáenz AM, Cirocco A, Tacaronte IM, Fajardo JE, Calebotta A. Endemic Pemphigus Foliaceus in Venezuela: Report of Two Children. Pediatric Dermatol. 2006;23:132–135. doi: 10.1111/j.1525-1470.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- 10.Heimgartner E, De Heimgartner V. Experiencias en enfermedades dermatológicas endémicas en la selva peruana: Leishmaniasis y Pénfigo Foliáceo Endémico. Med Cutan Ibero Lat Am. 1976;4:1–6. [PubMed] [Google Scholar]
- 11.Castillo A, Maguiña C. Pénfigo Foliáceo variedad fuego salvaje en la selva peruana. Provincias de Requena y Ucayali. Bol Soc Per Med Int. 1993;6:65–67. [Google Scholar]
- 12.Galarza C, Ronceros G, Mendoza D, Sánchez G, Vilcarromero M, Ráez E. Pénfigo foliáceo endémico en el departamento de Ucayali - Perú. Reporte de 16 casos. An Fac Med (Lima) 2002;63:19–24. [Google Scholar]
- 13.De Amat F, Diaz J. Pénfigo foliáceo endémico en las comunidades de Vista Alegre y San Francisco (Ucayali, Perú) Octubre 2000 - septiembre 2001. [tesis]. Lima: Universidad Nacional Mayor de San Marcos; 2001. [Google Scholar]
- 14.Cruz A. Estudio clínico epidemiológico del pénfigo foliáceo endémico en pacientes del Hospital de Yarinacocha-Pucallpa, 1995-2002 [tesis]. Lima: Universidad Nacional de San Marcos; 2005. [Google Scholar]
- 15.Rocha-Álvarez R, Ortega-Loayza AG, Friedman H, Campbell I, Aoki V, Rivitti EA, et al. Endemic pemphigus vulgaris. Arch Dermatol. 2007;143:895–899. doi: 10.1001/archderm.143.7.895. [DOI] [PubMed] [Google Scholar]
- 16.Galarza C, Ortega-Loayza AG, Ramos W, Hurtado J, Lindo G, Ávila J, et al. Pénfigo foliáceo endémico y pénfigo vulgar en pacientes de edad pediátrica en Ucayali. Dermatol Peru. 2004;14:99–103. [Google Scholar]
- 17.Ortega-Loayza A, Ramos W, Elgart G, Bouman P, Jiménez G, Ávila J, et al. Antibodies against desmoglein 1 in healtly subjects in endemic and nonendemic areas of pemphigus foliaceus (fogo selvagem) in Peru. Int J Dermatol. 2006;45:538–542. doi: 10.1111/j.1365-4632.2006.02823.x. [DOI] [PubMed] [Google Scholar]
- 18.Ramos W, Ortega-Loayza AG, Hancco J, Gutierrez E, Hurtado J, Jiménez G, et al. Inmunopatología de sujetos sanos de un área endémica para pénfigo foliáceo en Perú: estudio comparativo con familiares. Acta Med Per. 2007;24:153–158. [Google Scholar]
- 19.Ribeiro AM, Alvarez RR, Friedman H, Campbell I, Cooperative Group osn Fogo Selvagem Research The profile of fogo selvagem (endemic pemphigus foliaceus) at the University Hospital of Brasilia-Brazil. Epidemiological and clinical considerations. Int J Dermatol. 2005;44:293–298. doi: 10.1111/j.1365-4632.2004.01739.x. [DOI] [PubMed] [Google Scholar]
- 20.Ramos W, Galarza C, Gutierrez EL, Jiménez G, Rojas I, Hancco J, et al. Características epidemiológicas e inmunopatológicas de una cohorte de sujetos sanos positivos para anticuerpos anti desmogleína 1 procedentes de áreas endémicas de pénfigo foliáceo y vulgar del Perú. Dermatol Peru. 2009;19:12–20. [Google Scholar]
- 21.Chiossi MP, Rosselino AM. Endemic pemphigus foliaceus ("fogo selvagem"): a series from the northeastern Region of the state of São Paulo, Brazil, 1973-1998. Rev Inst Med Trop S Paulo. 2001;43:59–62. doi: 10.1590/s0036-46652001000200001. [DOI] [PubMed] [Google Scholar]
- 22.Aoki V, Millikan RC, Rivitti EA, Hans-Filho G, Eaton DP, Warren S, et al. Environmental Risk Factors in endemic pemphigus foliaceus (fogo selvagem) J Investig Dermatol Symp Proc. 2004;9:34–40. doi: 10.1111/j.1087-0024.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 23.Eaton DP, Diaz LA, Hans-Filho G, dos Santos VD, Aoki V, Friedman H, et al. Comparison of black fly species (Diptera: Simullidae) on an Amerindian reservation with a high prevalence of Fogo Selvagem to neighboring disease of free sites in the States of Mato Grosso do Sul, Brazil. The Cooperative Group on Fogo Selvagem Research. J Med Entomol. 1998;35:120–131. doi: 10.1093/jmedent/35.2.120. [DOI] [PubMed] [Google Scholar]
- 24.Diaz LA, Arteaga LA, Hilario-Vargas J, Valenzuela JG, Li N, Warren S, et al. AntiDesmoglein-1 Antibodies in Onchocerciasis, Leismaniasis and Chagas Disease Suggest a Possible etiological Link to Fogo Selvagem. J Invest Dermatol. 2004;123:1045–1051. doi: 10.1111/j.0022-202X.2004.23438.x. [DOI] [PubMed] [Google Scholar]
- 25.Tur E, Brener S. Diet and Penphigus. In pursuit of exogenus factors in phemphigus and fogo selvagem. Arch Dermatol. 1998;143:1406–1410. doi: 10.1001/archderm.134.11.1406. [DOI] [PubMed] [Google Scholar]
- 26.Tur E, Brener S. Contribuiting exogenous factors in pemphigus. Int J Dermatol. 1997;36:888–893. doi: 10.1046/j.1365-4362.1997.00334.x. [DOI] [PubMed] [Google Scholar]
- 27.Brener S, Wolf R. Possible nutritional factors in induced pemphigus. Dermatology. 1994;189:337–339. doi: 10.1159/000246874. [DOI] [PubMed] [Google Scholar]
- 28.Chorzelski TO, Hashimoto T, Jablonska S. Can pemphigus vulgaris be induced by nutritional factor? Eur J Dermatol. 1996;6:284–286. [Google Scholar]
- 29.Brener S, Roucco V, Wolf R, de Angelis E, Lombardi ML. Pemphigus and dietary factors. Dermatology. 1995;190:197–202. doi: 10.1159/000246684. [DOI] [PubMed] [Google Scholar]
- 30.Ruocco V, Brenner S, Lombardi ML. A case of diet-related pemphigus. Dermatology. 1996;192:373–374. doi: 10.1159/000246417. [DOI] [PubMed] [Google Scholar]