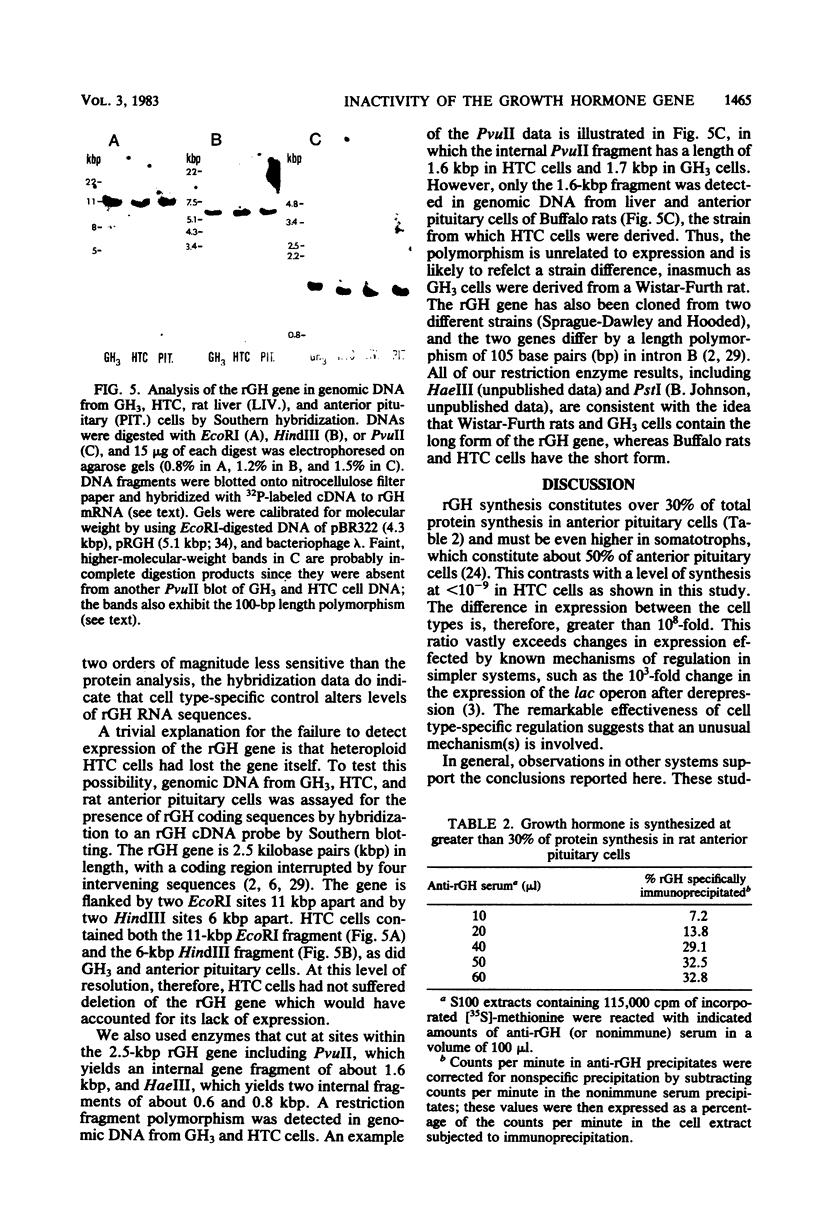
Abstract
Rat liver hepatoma cells (HTC) which express liver-specific gene products were assayed for the expression of the rat growth hormone (rGH) gene, which is normally expressed in anterior pituitary somatotrophs. The combination of immunoprecipitation and two-dimensional gel electrophoresis provided a highly sensitive assay for rGH synthesis at levels as low as one part in 10(9) of cell protein synthesis (or four molecules of rGH per cell). No rGH expression was detected at this level. The lack of expression in HTC cells did not derive from a deletion of the rGH gene, as shown by Southern hybridization analysis of genomic DNA. Because the gene is expressed at greater than 30% of anterior pituitary protein synthesis, differentiation regulated rGH expression by over 10(8)-fold between the two cell types. Additionally, DNA-excess solution hybridization was used to measure the level of rGH mRNA sequences. A novel and general method for preparing single-strand probes from recombinant plasmids was developed. Hybridization analyses with a sensitivity of detection of 1 part in 10(8) failed to detect any rGH RNA sequences in either the nucleus or cytoplasm of HTC cells. It is concluded, therefore, that the restriction in rGH expression in the liver tumor cells is likely to occur at the level of the transcription of the gene, and that for all practical purposes, the rGH gene is completely shut off in the hepatoma cells.
Full text
PDF

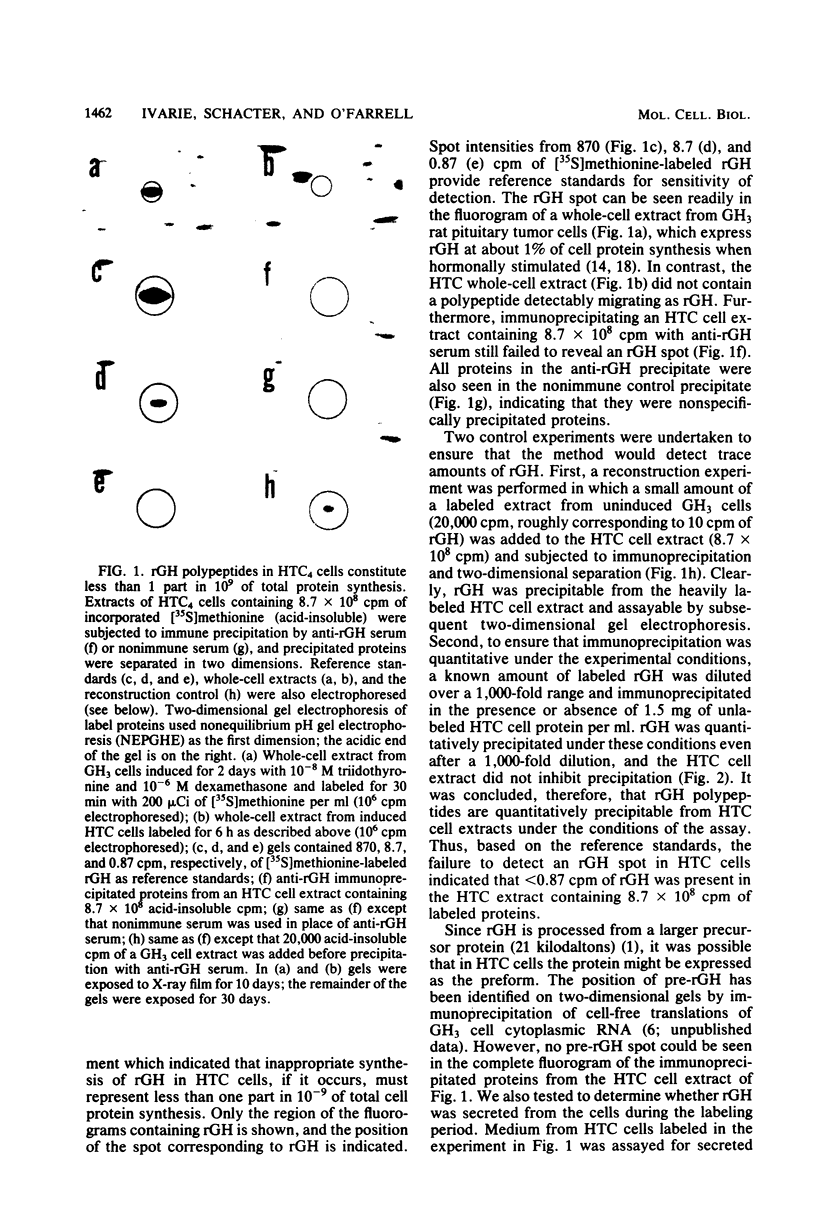
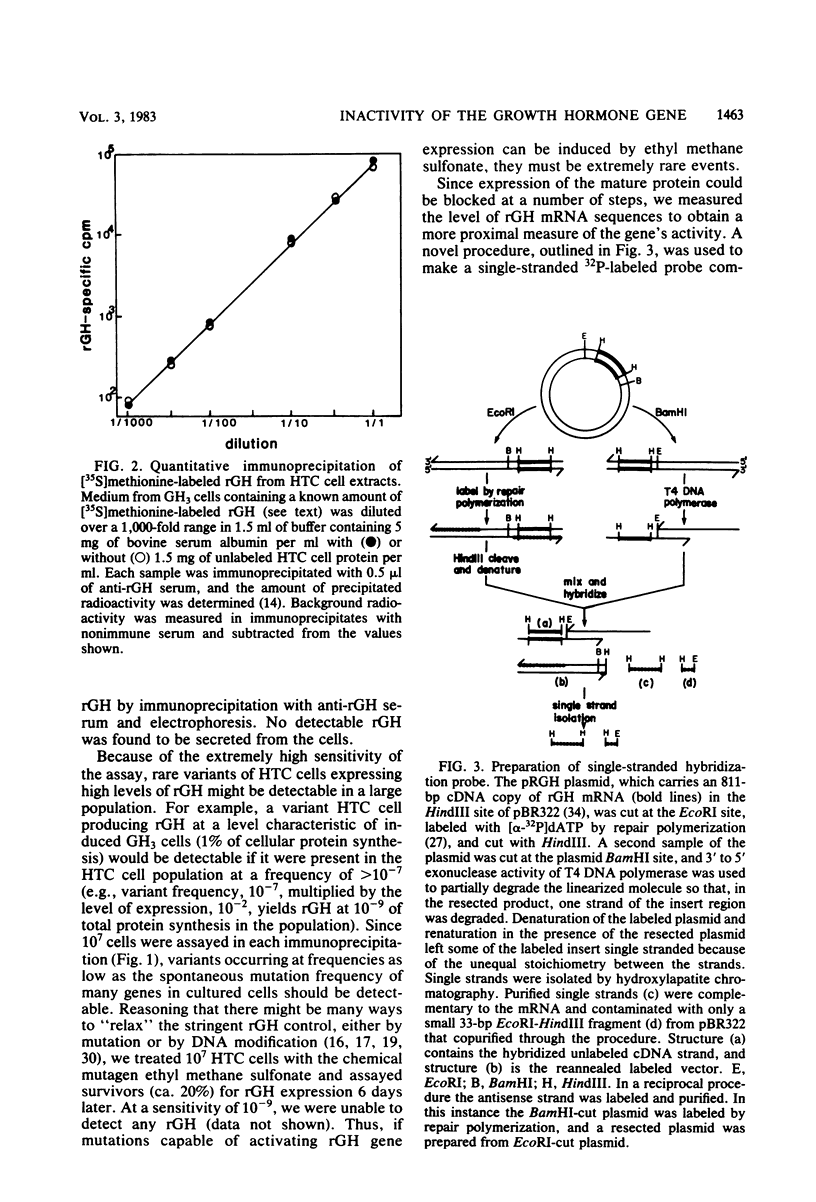
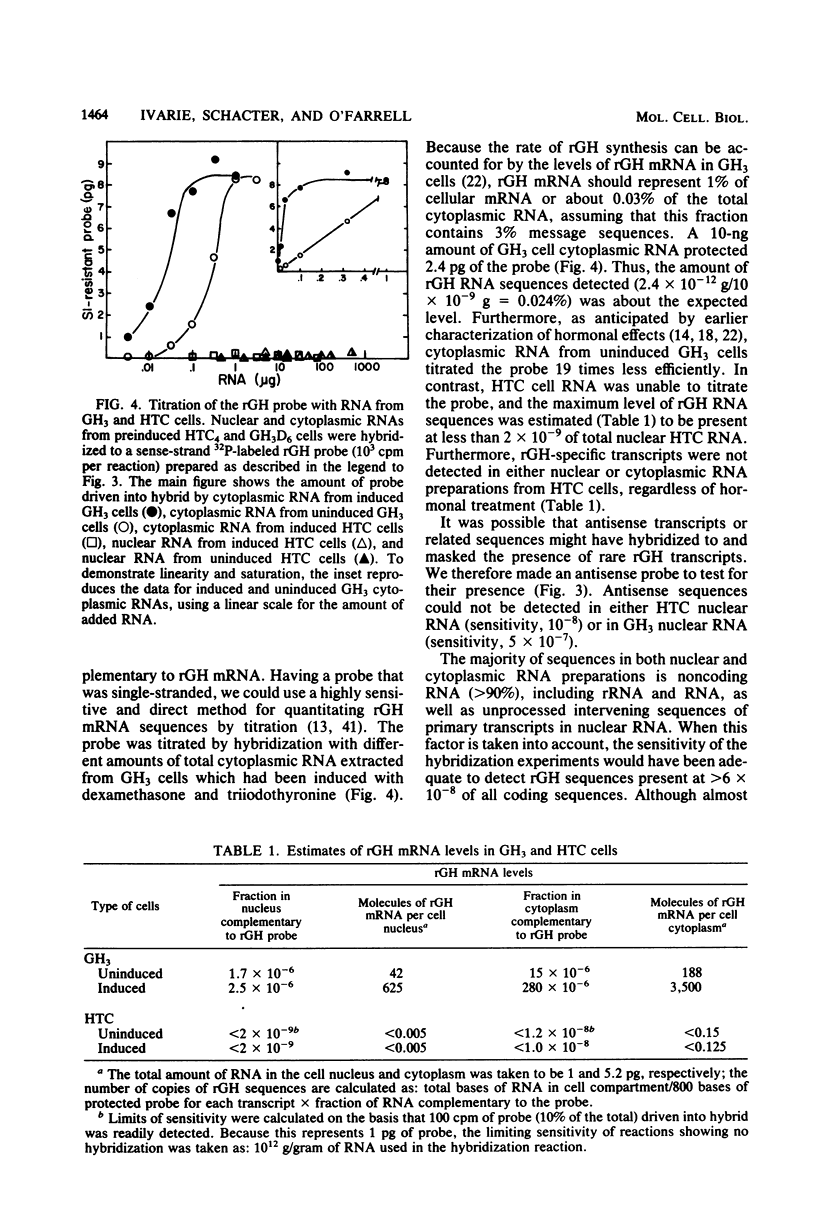


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft F. C., Wu G. J., Zubay G. Cell-free synthesis of rat growth hormone. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3646–3649. doi: 10.1073/pnas.70.12.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta A., Richards R. I., Baxter J. D., Shine J. Primary structure and evolution of rat growth hormone gene. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4867–4871. doi: 10.1073/pnas.78.8.4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Caplan A. I., Ordahl C. P. Irreversible gene repression model for control of development. Science. 1978 Jul 14;201(4351):120–130. doi: 10.1126/science.351805. [DOI] [PubMed] [Google Scholar]
- Chien Y. H., Thompson E. B. Genomic organization of rat prolactin and growth hormone genes. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4583–4587. doi: 10.1073/pnas.77.8.4583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel A., Axel R. Selective digestion of transcriptionally active ovalbumin genes from oviduct nuclei. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3966–3970. doi: 10.1073/pnas.73.11.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Eisenman R., Weintraub H. Chromatin structure of endogenous retroviral genes and activation by an inhibitor of DNA methylation. Nature. 1981 Jul 23;292(5821):311–317. doi: 10.1038/292311a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of cellular genes by avian RNA tumor viruses. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5351–5354. doi: 10.1073/pnas.77.9.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Rous sarcoma virus activates embryonic globin genes in chicken fibroblasts. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4464–4468. doi: 10.1073/pnas.72.11.4464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J. D., MacDonald R. J., Przybyla A. E., Chirgwin J. M., Pictet R. L., Rutter W. J. Changes in the frequency of specific transcripts during development of the pancreas. J Biol Chem. 1977 Oct 25;252(20):7391–7397. [PubMed] [Google Scholar]
- Humphries S., Windass J., Williamson R. Mouse globin gene expression in erythroid and non-erythroid tissues. Cell. 1976 Feb;7(2):267–277. doi: 10.1016/0092-8674(76)90026-x. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Baxter J. D., Morris J. A. Interaction of thyroid and glucocorticoid hormones in rat pituitary tumor cells. Specificity and diversity of the responses analyzed by two-dimensional gel electrophoresis. J Biol Chem. 1981 May 10;256(9):4520–4528. [PubMed] [Google Scholar]
- Ivarie R. D., Jones P. P. A rapid sensitive assay for specific protein synthesis in cells and in cell-free translations: use of Staphylococcus aureus as an adsorbent for immune complexes. Anal Biochem. 1979 Aug;97(1):24–35. doi: 10.1016/0003-2697(79)90322-1. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Morris J. A., Eberhardt N. L. Hormonal domains of response: actions of glucocorticoid and thyroid hormones in regulating pleiotropic responses in cultured cells. Recent Prog Horm Res. 1980;36:195–239. doi: 10.1016/b978-0-12-571136-4.50012-7. [DOI] [PubMed] [Google Scholar]
- Ivarie R. D., Morris J. A. Induction of prolactin-deficient variants of GH3 rat pituitary tumor cells by ethyl methanesulfonate: reversion by 5-azacytidine, a DNA methylation inhibitor. Proc Natl Acad Sci U S A. 1982 May;79(9):2967–2970. doi: 10.1073/pnas.79.9.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., Morris J. A., Martial J. A. Prolactin-deficient variants of GH3 rat pituitary tumor cells: linked expression of prolactin and another hormonally responsive protein in GH3 cells. Mol Cell Biol. 1982 Feb;2(2):179–189. doi: 10.1128/mcb.2.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivarie R. D., O'Farrell P. H. The glucocorticoid domain: steroid-mediated changes in the rate of synthesis of rat hepatoma proteins. Cell. 1978 Jan;13(1):41–55. doi: 10.1016/0092-8674(78)90136-8. [DOI] [PubMed] [Google Scholar]
- Ivarie R., Morris J. Phenotypic switching in GH3 rat pituitary tumor cells: linked expression of growth hormone and another hormonally responsive protein. DNA. 1983;2(2):113–120. doi: 10.1089/dna.1983.2.113. [DOI] [PubMed] [Google Scholar]
- Kemp D. J., Harris A. W., Cory S., Adams J. M. Expression of the immunoglobulin C mu gene in mouse T and B lymphoid and myeloid cell lines. Proc Natl Acad Sci U S A. 1980 May;77(5):2876–2880. doi: 10.1073/pnas.77.5.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martial J. A., Baxter J. D., Goodman H. M., Seeburg P. H. Regulation of growth hormone messenger RNA by thyroid and glucocorticoid hormones. Proc Natl Acad Sci U S A. 1977 May;74(5):1816–1820. doi: 10.1073/pnas.74.5.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather E. L., Perry R. P. Transcriptional regulation of immunoglobulin V genes. Nucleic Acids Res. 1981 Dec 21;9(24):6855–6867. doi: 10.1093/nar/9.24.6855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane P. K. Classifications of anterior pituitary cell types with immunoenzyme histochemistry. J Histochem Cytochem. 1970 Jan;18(1):9–20. doi: 10.1177/18.1.9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H., O'Farrell P. Z. Two-dimensional polyacrylamide gel electrophoretic fractionation. Methods Cell Biol. 1977;16:407–420. doi: 10.1016/s0091-679x(08)60116-8. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Page G. S., Smith S., Goodman H. M. DNA sequence of the rat growth hormone gene: location of the 5' terminus of the growth hormone mRNA and identification of an internal transposon-like element. Nucleic Acids Res. 1981 May 11;9(9):2087–2104. doi: 10.1093/nar/9.9.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Kemp J. D., Bradshaw W. S., Clark W. R., Ronzio R. A., Sanders T. G. Regulation of specific protein synthesis in cytodifferentiation. J Cell Physiol. 1968 Oct;72(2 Suppl):1–18. doi: 10.1002/jcp.1040720403. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Baxter J. D., Goodman H. M. Nucleotide sequence and amplification in bacteria of structural gene for rat growth hormone. Nature. 1977 Dec 8;270(5637):486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Tsai M. J., Lin C. T., O'Malley B. W. Effect of estrogen on ovalbumin gene expression in differentiated nontarget tissues. Biochemistry. 1979 Dec 11;18(25):5726–5731. doi: 10.1021/bi00592a032. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Groudine M. Chromosomal subunits in active genes have an altered conformation. Science. 1976 Sep 3;193(4256):848–856. doi: 10.1126/science.948749. [DOI] [PubMed] [Google Scholar]
- Weisbrod S., Groudine M., Weintraub H. Interaction of HMG 14 and 17 with actively transcribed genes. Cell. 1980 Jan;19(1):289–301. doi: 10.1016/0092-8674(80)90410-9. [DOI] [PubMed] [Google Scholar]
- Young B. D., Harrison P. R., Gilmour R. S., Birnie G. D., Hell A., Humphries S., Paul J. Kinetic studies of gene frequency. II. Complexity of globin complementary DNA and its hybridization characteristics. J Mol Biol. 1974 Apr 25;84(4):555–568. doi: 10.1016/0022-2836(74)90116-8. [DOI] [PubMed] [Google Scholar]