Abstract
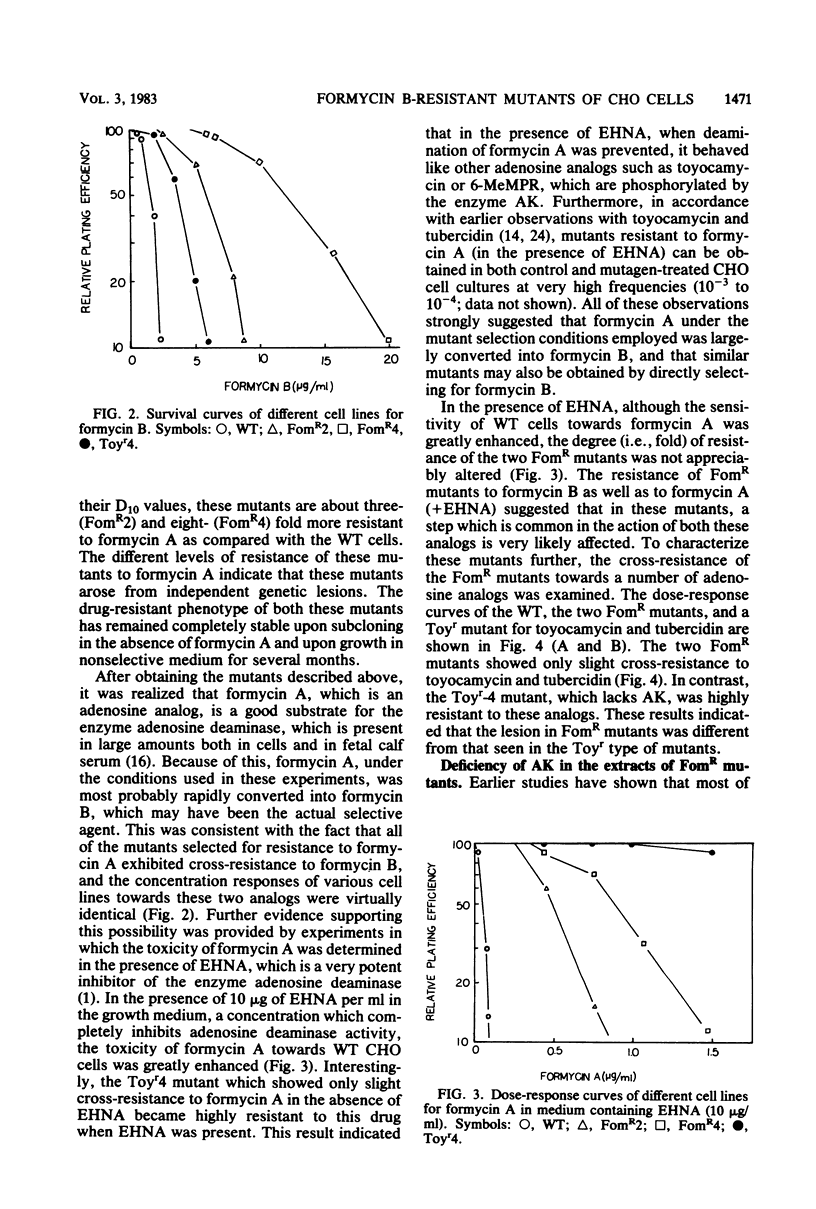
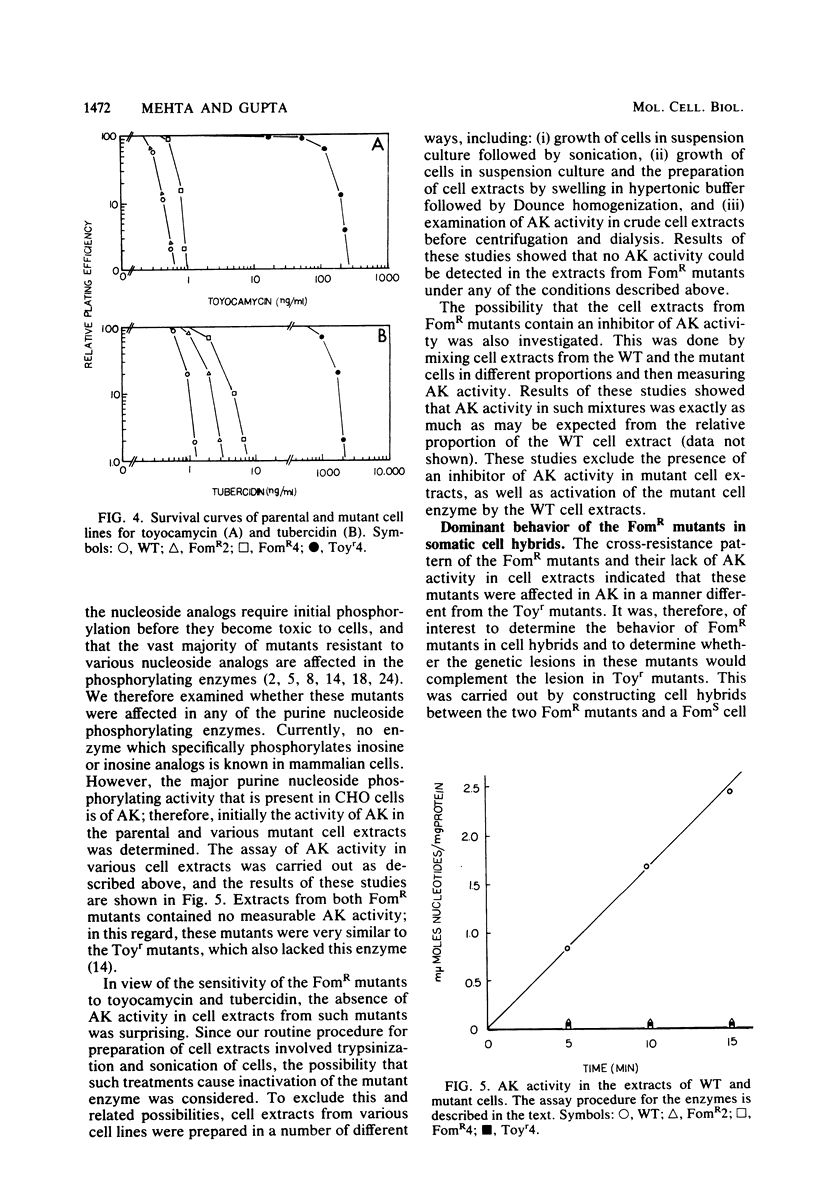
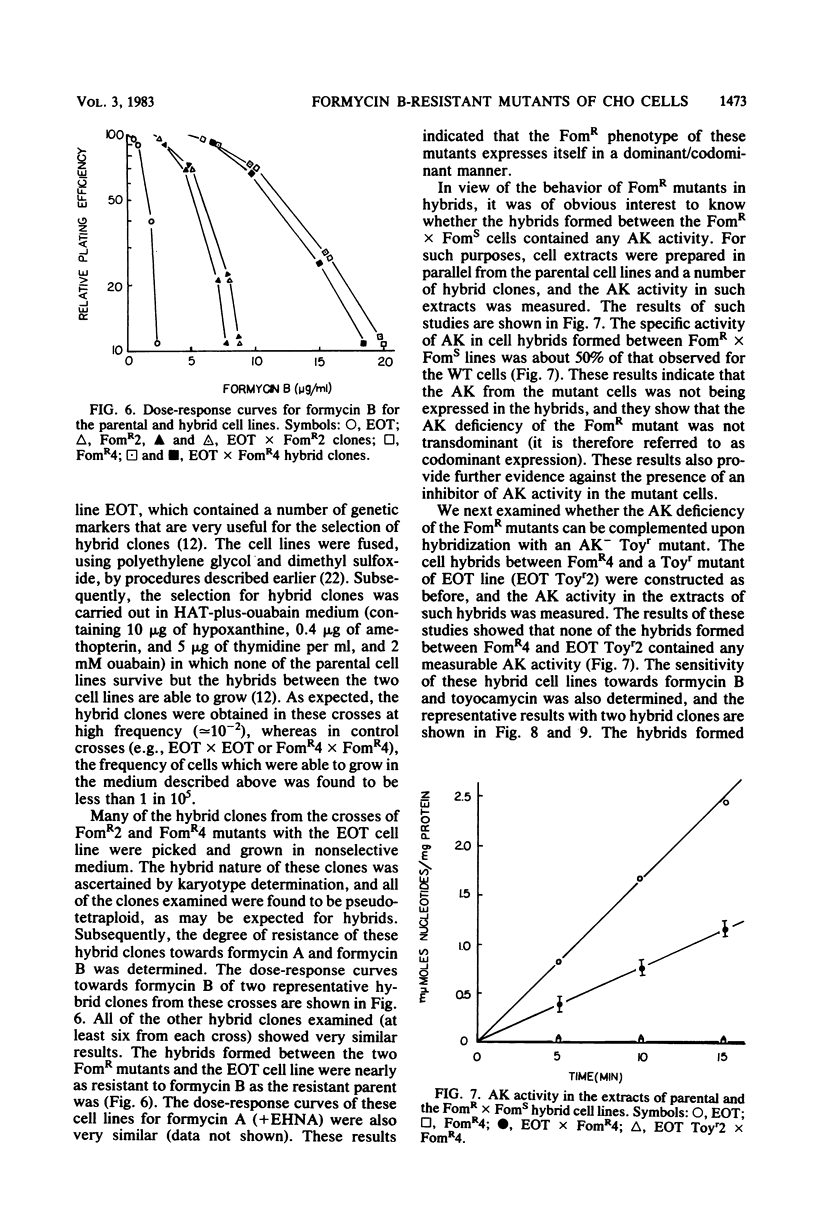
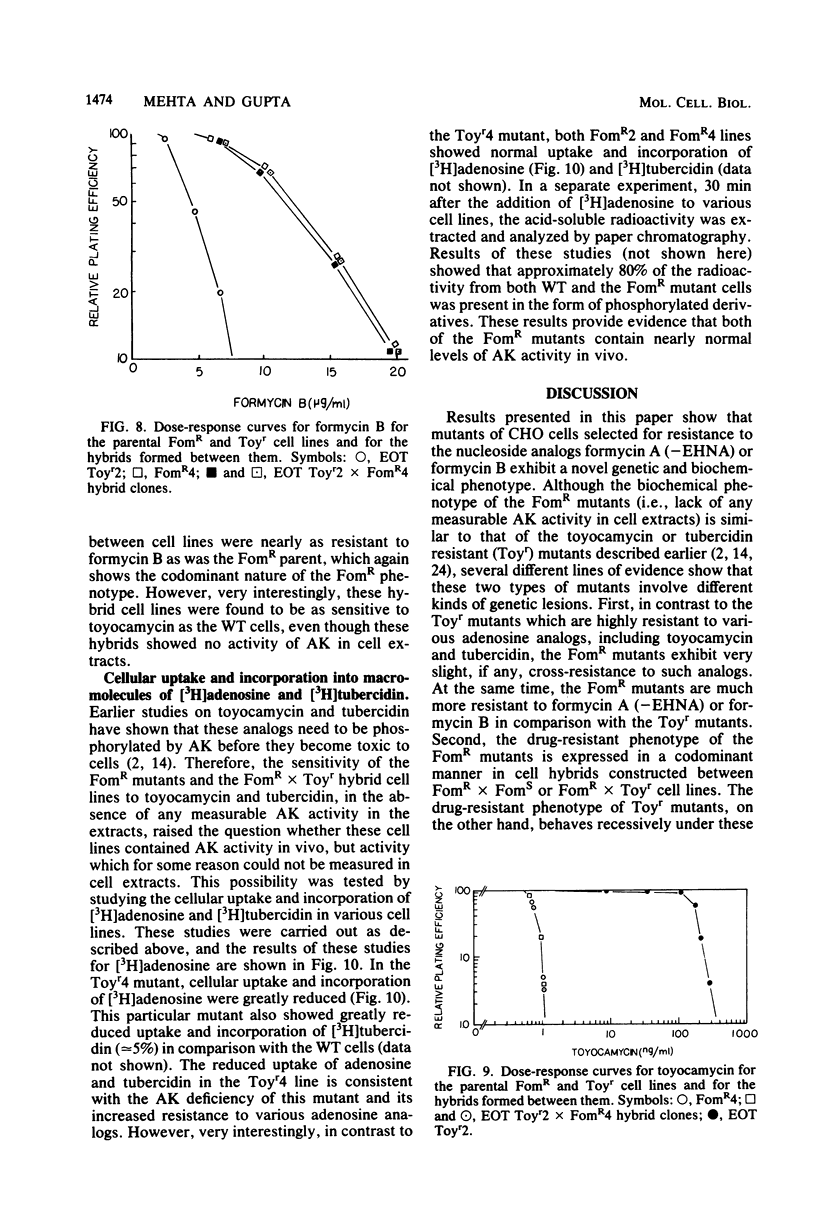
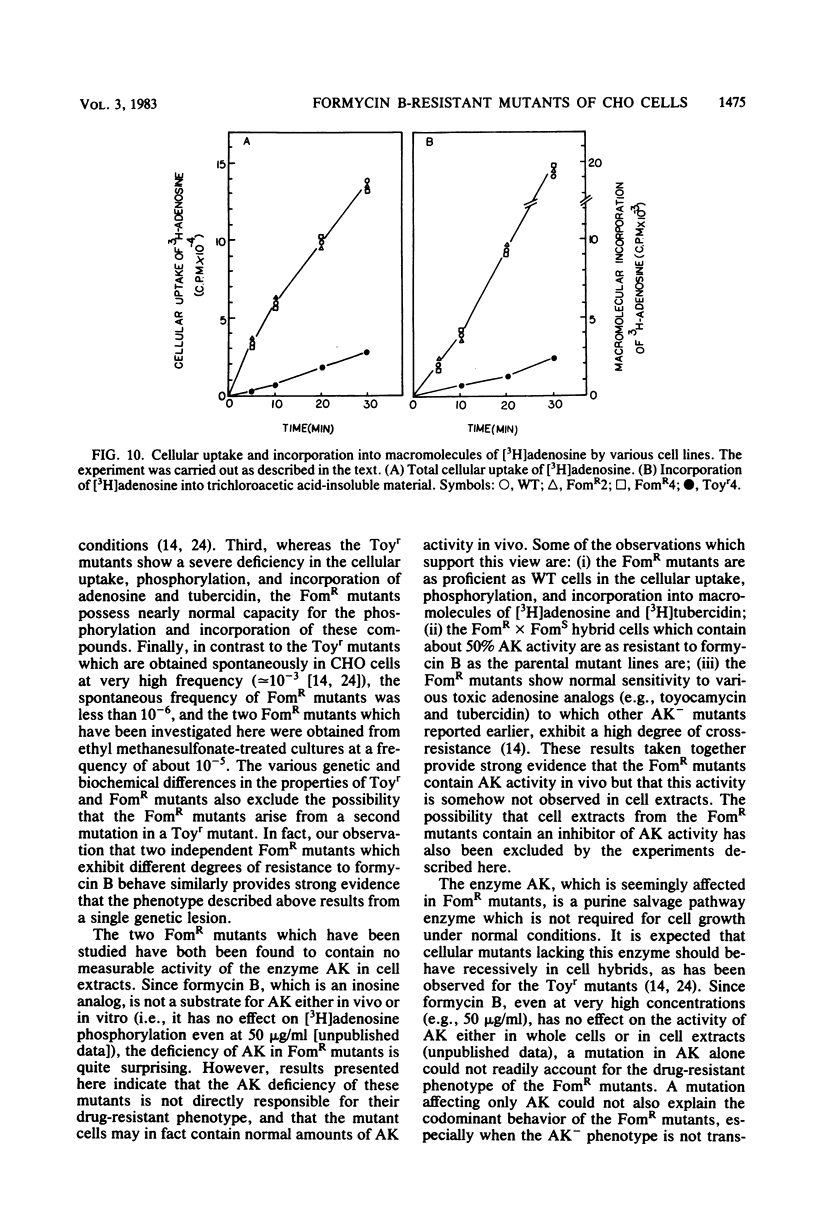
Stable mutants which are approximately three- and eightfold resistant to the pyrazolopyrimidine nucleosides formycin A and formycin B (FomR) have been selected in a single step from mutagenized Chinese hamster ovary cells. In cell extracts, the two FomR mutants which were examined were both found to contain no measurable activity of the enzyme adenosine kinase (AK). However, cross-resistance studies with other adenosine analogs such as toyocamycin and tubercidin show that these mutants are distinct from toyocamycin or tubercidin resistant (Toyr) mutants which also contain no measurable AK activity in cell extracts. Studies on the uptake and incorporation of [3H]adenosine and [3H]tubercidin by various mutants and parental cell lines show that unlike the Toyr mutants, which are severely deficient in the phosphorylation of these compounds, the FomR mutants possess nearly normal capacity to phosphorylate these compounds and incorporate them into cellular macromolecules. These results suggest that the FomR mutants contain normal levels of AK activity in vivo. In cell hybrids formed between FomR X FomS cells and FomR X Toyr cells, the formycin-resistant phenotype of both of the FomR mutants behaved codominantly. However, the extracts from these hybrid cells contained either congruent to 50% (FomR X FomS) or no measurable (FomR X Toyr) AK activity, indicating that the lesion in these mutants neither suppresses the wild-type AK activity nor complements the AK deficiency of the Toyr mutants. The presence of AK activity in the FomR mutants in vivo, but not in their cell extracts, along with the codominant behavior of the mutants in hybrids, indicates that the lesions in the FomR mutant are of a novel nature. It is suggested that the genetic lesion in these mutants affects AK activity indirectly and that it may involve an essential cellular function which exists in a complex form with AK. Some implications of these results regarding the mechanism of action of formycin B are discussed.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Spector T., Parks R. E., Jr Tight-binding inhibitors--IV. Inhibition of adenosine deaminases by various inhibitors. Biochem Pharmacol. 1977 Mar 1;26(5):359–367. doi: 10.1016/0006-2952(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Bennett L. L., Jr, Schnebli H. P., Vail M. H., Allan P. W., Montgomery J. A. Purine ribonucleoside kinase activity and resistance to some analogs of adenosine. Mol Pharmacol. 1966 Sep;2(5):432–443. [PubMed] [Google Scholar]
- Carson D. A., Chang K. P. Phosphorylation and anti-leishmanial activity of formycin B. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1377–1383. doi: 10.1016/0006-291x(81)91976-8. [DOI] [PubMed] [Google Scholar]
- Chan T. S., Ishii K., Long C., Green H. Purine excretion by mammalian cells deficient in adenosine kinase. J Cell Physiol. 1973 Jun;81(3):315–322. doi: 10.1002/jcp.1040810304. [DOI] [PubMed] [Google Scholar]
- Chan V. L., Juranka P. Isolation and preliminary characterization of 9-beta-d-arabinofuranosyladenine-resistant mutants of baby hamster cells. Somatic Cell Genet. 1981 Mar;7(2):147–160. doi: 10.1007/BF01567654. [DOI] [PubMed] [Google Scholar]
- Cowan M. J., Cashman D., Ammann A. J. Effects of formycin B on human lymphocyte Deoxyribonucleic acid synthesis. Optimization of cell culture conditions. Biochem Pharmacol. 1981 Oct 1;30(19):2651–2656. doi: 10.1016/0006-2952(81)90533-5. [DOI] [PubMed] [Google Scholar]
- Daves G. D., Jr, Cheng C. C. The chemistry and biochemistry of C-nucleosides. Prog Med Chem. 1976;13:303–349. doi: 10.1016/s0079-6468(08)70141-3. [DOI] [PubMed] [Google Scholar]
- Debatisse M., Buttin G. The control of cell proliferation by preformed purines: a genetic study. I. Isolation and preliminary characterization of Chinese hamster lines with single or multiple defects in purine "salvage" pathways. Somatic Cell Genet. 1977 Sep;3(5):497–511. doi: 10.1007/BF01539121. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Chan D. Y., Siminovitch L. Evidence for functional hemizygosity at the Emtr locus in CHO cells through segregation analysis. Cell. 1978 Aug;14(4):1007–1013. doi: 10.1016/0092-8674(78)90354-9. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Ho T. K., Moffat M. R., Gupta R. Podophyllotoxin-resistant mutants of Chinese hamster ovary cells. Alteration in a microtubule-associated protein. J Biol Chem. 1982 Jan 25;257(2):1071–1078. [PubMed] [Google Scholar]
- Gupta R. S. Podophyllotoxin resistance: a codominant selection system for quantitative mutagenesis studies in mammalian cells. Mutat Res. 1981 Sep;83(2):261–270. doi: 10.1016/0027-5107(81)90010-5. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. Random segretation of multiple genetic markers from CHO-CHO hybrids: evidence for random distribution of functional hemizygosity in the genome. Somatic Cell Genet. 1980 Jan;6(1):115–125. doi: 10.1007/BF01538700. [DOI] [PubMed] [Google Scholar]
- Gupta R. S., Siminovitch L. Genetic and biochemical studies with the adenosine analogs toyocamycin and tubercidin: mutation at the adenosine kinase locus in Chinese hamster cells. Somatic Cell Genet. 1978 Nov;4(6):715–735. doi: 10.1007/BF01543160. [DOI] [PubMed] [Google Scholar]
- Gupta R. S. Taxol resistant mutants of Chinese hamster ovary cells: genetic biochemical, and cross-resistance studies. J Cell Physiol. 1983 Jan;114(1):137–144. doi: 10.1002/jcp.1041140122. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Paterson A. R., Caldwell I. C., Hori M. Biochemical effects of formycin, an adenosine analog. Cancer Res. 1967 Apr;27(4):715–719. [PubMed] [Google Scholar]
- Ishii K., Green H. Lethality of adenosine for cultured mammalian cells by interference with pyrimidine biosynthesis. J Cell Sci. 1973 Sep;13(2):429–439. doi: 10.1242/jcs.13.2.429. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McBurney M. W., Whimore G. F. Mutants of chinese hamster cells resistant to adenosine. J Cell Physiol. 1975 Feb;85(1):87–99. doi: 10.1002/jcp.1040850110. [DOI] [PubMed] [Google Scholar]
- McCairns E., Fahey D., Sauer D., Rowe P. B. De novo purine synthesis in human lymphocytes. Partial co-purification of the enzymes and some properties of the pathway. J Biol Chem. 1983 Feb 10;258(3):1851–1856. [PubMed] [Google Scholar]
- Müller W. E., Rohde H. J., Steffen R., Maidhof A., Lachmann M., Zahn R. K., Umezawa H. Influence of formycin B on polyadenosine diphosphoribose synthesis in vitro and in vivo. Cancer Res. 1975 Dec;35(12):3673–3681. [PubMed] [Google Scholar]
- Nelson D. J., Lafon S. W., Jones T. E., Spector T., Berens R. L., Marr J. J. The metabolism of formycin B in Leishmania donovani. Biochem Biophys Res Commun. 1982 Sep 16;108(1):349–354. doi: 10.1016/0006-291x(82)91873-3. [DOI] [PubMed] [Google Scholar]
- Norwood T. H., Zeigler C. J., Martin G. M. Dimethyl sulfoxide enhances polyethylene glycol-mediated somatic cell fusion. Somatic Cell Genet. 1976 May;2(3):263–270. doi: 10.1007/BF01538964. [DOI] [PubMed] [Google Scholar]
- Osborne W. R., Sullivan J. L., Scott C. R. Formycin B, purine nucleoside phosphorylase and lymphocyte function. Immunol Commun. 1980;9(3):257–267. doi: 10.3109/08820138009065998. [DOI] [PubMed] [Google Scholar]
- Rabin M. S., Gottesman M. M. High frequency of mutation to tubercidin resistance in CHO cells. Somatic Cell Genet. 1979 Sep;5(5):571–583. doi: 10.1007/BF01542695. [DOI] [PubMed] [Google Scholar]
- Rainey P., Santi D. V. Metabolism and mechanism of action of formycin B in Leishmania. Proc Natl Acad Sci U S A. 1983 Jan;80(1):288–292. doi: 10.1073/pnas.80.1.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen M. R., Kim B. K., Parks R. E., Jr Purine nucleoside phosphorylase from human erythrocytes. 3. Inhibition by the inosine analog formycin B of the isolated enzyme and of nucleoside metabolism in intact erythrocytes and sarcoma 180 cells. Mol Pharmacol. 1968 May;4(3):293–299. [PubMed] [Google Scholar]
- Stoeckler J. D., Cambor C., Kuhns V., Chu S. H., Parks R. E., Jr Inhibitors of purine nucleoside phosphorylase, C(8) and C(5') substitutions. Biochem Pharmacol. 1982 Jan 15;31(2):163–171. doi: 10.1016/0006-2952(82)90206-4. [DOI] [PubMed] [Google Scholar]
- Ullman B., Clift S. M., Cohen A., Gudas L. J., Levinson B. B., Wormsted M. A., Martin D. W., Jr Abnormal regulation of de novo purine synthesis and purine salvage in a cultured mouse T-cell lymphoma mutant partially deficient in adenylosuccinate synthetase. J Cell Physiol. 1979 Apr;99(1):139–151. doi: 10.1002/jcp.1040990115. [DOI] [PubMed] [Google Scholar]
- Umezawa H., Sawa T., Fukagawa Y., Homma I., Ishizuka M. Studies on formycin and formycin B in cells of Ehrlich carcinoma and E. coli. J Antibiot (Tokyo) 1967 Nov;20(6):308–316. [PubMed] [Google Scholar]



