Abstract
Aims
Heart valve tissue engineering aims to create a graft with improved durability compared to routinely used valve substitutes. This study presents the function and morphological changes of a tissue-engineered aortic valve (TEV) compared to the cryopreserved valve (CPV), aortic valve (AV) allografts in an orthotopic position in sheep.
Methods and Results
Ovine AV conduits (n=5) were decellularized with detergents. Autologous endothelial cells (ECs) were seeded onto the valve surface and cultured under physiological conditions using a high pulsatile flow. Grafts were implanted as a root with reimplantation of coronary ostia in sheep. Crystalloid cardioplegia and isogenic blood transfusions from previous sacrificed sheep were used. Only antiplatelet aggregation therapy was used postoperatively. CPVs (n=4) served as controls. The grafts were investigated for function (echocardiography, magnetic resonance investigation), morpho/histological appearance, graft rejection, and calcification at 3 months. Decellularization led to cell-free scaffolds with preserved extracellular matrices, including the basement membrane. TEVs were covered with ECs expressing typical endothelial markers. Neither dilatation, stenosis, reductions of cusp mobility nor a significant transvalvular gradient, were observed in the TEV group. Explanted valves exhibited normal morphology without signs of inflammation. An endothelial monolayer covered cusps and the valve sinus. In the CPV group, sporadic, macroscopic, calcified degeneration with mild AV insufficiency was noted. Histology revealed signs of rejection and incipient calcification of the tissue.
Conclusion
Tissue-engineered AV based on decellularized valve allografts satisfy short-term requirements of the systemic circulation in sheep. Although results of long-term experiments are pending, the lack of degenerative traits thus far, makes these grafts a promising alternative for future aortic heart valve surgery.
Introduction
Valve replacement is the common treatment in advanced aortic valve (AV) disease and biological and mechanical valve prostheses are the most used substitutes. Limited durability of biological valves and life-long anticoagulation therapy for mechanical prostheses are the major concerns in their clinical use.1,2 Accelerated degeneration of biological allo- and xenovalves is partially attributed to remaining cells within the valve tissue, cytotoxicity of chemical crosslinking agents as well as incomplete biocompatibility if referred to αGal epitopes masking.3,4 Preserved antigenicity induces a chronic inflammatory response with subsequent valve failure.5,6 In addition, all described grafts have a limited acceptance in patients that are still growing. Immunological responses are avoided by the use of the patient's own pulmonary valve in replacement of the diseased AV, as in the Ross operation. The pulmonary autograft is also advantageous, as it has been shown to grow along with the child, resulting in fewer reoperations. Factors contributing to a limited acceptance of this procedure include operation complexity and the replacement requirement of both aortic and pulmonary valves. Moreover, cryopreserved allografts undergo degenerative processes, the leading cause for reoperation in 20% of patients after 10 years.7,8 Subsequently, implantation of acellular or with patients' own cells reseeded heart valves may solve the immune response problems and facilitate in vivo graft remodeling.
Over the last decade, tissue engineering (TE) has become a promising strategy by which to obtain such valves. The use of pulmonary valve homografts, proceeded by methods of TE before implantation, showed excellent hemodynamic parameters in the pulmonary valve position, limited degeneration and capacity to grow in pediatric patients.9,10 At the aortic position, AV replacements with TE grafts are currently tested at the preclinical level. In sheep, a model considered to be the standard in predicting calcification of biological heart valves, the decellularized AV demonstrated superior durability as compared to unprocessed allografts in long-term experiments.11 The aim of this study was twofold. First, to create a tissue-engineered aortic valve (TEV) based on detergent decellularized ovine AV scaffolds reseeded with autologous endothelial cells (ECs) and second, to compare the TEVs with standard cryopreserved allograft valves (CPVs) after orthotopic implantation at three months.
Materials and Methods
All animal experiments and surgical procedures were performed in compliance with the Guide for the Care and Use of Laboratory Animals as published by the US National Institutes of Health (NIH Publication 85-23, revised 1996) and were approved by the local animal care committee (Experiment # 06/1186).
Aortic allografts
AV conduits were harvested from sheep (30–40 kg) under sterile conditions and were either immediately decellularized (n=5 for in vitro, n=5 for in vivo experiments) or cryopreserved (n=4).
Decellularization
Decellularization of AVs was performed as described.11
Cell source and culture
Isolation and cultivation of autologous ECs from ovine jugular veins were performed as previously described.12 In brief, jugular veins were harvested from future recipient juvenile sheep. ECs were removed from the vessel wall by digestion with 2% collagenase A and resuspended afterward in a culture medium (CM) composed from the Endothelial Cell Basal Medium-2 (Clonetics), supplemented with SingleQuot Kit (Clonetics), 10% FCS (Biochrom), 100 μg/mL P/S (Biochrom), and finally seeded into a culture flask. Cells from 2nd or 3rd passage were used for reseeding of the valve conduit. Prior seeding, cells were checked microscopically for cobblestone morphology and expression of endothelial markers as endothelial nitric oxide synthase (eNOS) and CD31 (Polyclonal Rabbit IgG,) by immunohistochemistry (NOS Type III, BD Bioscience; CD31, Santa Cruz).
Reseeding and cultivation under pulsatile conditions
For recellularization of AVs, dynamic bioreactors were used as described.12
Rotation conditions
Decellularized AVs (DAV; n=10) were inserted into bioreactors and incubated in the CM for 24 h. The lumen of the conduits was filled with the CM supplemented with 500 ng of the recombinant human proangiogenic factor CCN1. ECs (0.5×107 cells/valve) were injected precisely into the valve lumen through specially designed cell-seeding inlets in two rounds. After the first seeding, the bioreactor remained in the vertical position for 6 h to allow the EC to attach in the valve sinuses. The second reseeding step was followed by a slow rotation of the bioreactor (0.05 rpm) for another 6 h, exposing the entire valve surface to achieve optimal attachment conditions.
Dynamic conditions
Following the reseeding, the bioreactors were attached to a pulsatile pump. The pulsatile circulation was started at 0.1 L/min. The flow rate was increased by 0.05 L/min, twice a day, until day 4. After day 4, a maximal flow of 1.0 L/min was achieved by repeatedly increasing the flow by 0.2 L/min/day until day 7. The mean system pressure was maintained at 80 mm/Hg during the entire duration of dynamic cultivation. The morphology of reseeded AVs (n=5) was analyzed. The remaining AVs (n=5) were transported to the operation room in closed, but disconnected bioreactors at 37°C where they were removed from the bioreactor and used for implantation.
Cryopreservation and thawing
After harvesting, the grafts were placed in the cryoprotectant solution containing 10% dimethyl sulfoxid and 20% FCS. AVs were cryopreserved at a controlled rate (−1°C/min) from 15°C to −120°C, and then rapidly cooled, and finally stored in the −196°C gas phase of liquid nitrogen (Kryo 10/Serie III; Messer Griesheim). Thawing was performed in two steps. In step 1, the CPVs were placed in a cooler containing dry ice without direct contact to the ice and warmed slowly to −100°C. In step 2, CPVs were placed in a 37°C water bath, to obtain rapid warming until all ice macroscopically disappeared. This type of valve preservation follows current clinical practice.13
Implantation of the AV conduits
TEVs and CPVs were implanted into young sheep (30–40 kg), obtained from the local breeder (Lower Saxony) in the orthotopic position as described.11 Through a left antero-lateral thoracotomy, a right atrial to the descending aorta cardiopulmonary bypass was established. Crystalloid cardioplegia was introduced directly into the ostia. The valve conduit was sutured using single 5.0 polypropylene sutures (Ethicon). Coronary vessels were reimplantated using 6.0 polypropylene sutures. Isogenic blood transfusions from previously sacrificed sheep were used. Daily aspirin (100 mg) was given orally to all animals.
Echocardiography
Transesophageal echocardiography was performed during the explantation operation and valve function was assessed as previously described.11
Magnetic resonance investigation
Cardiac magnetic resonance imaging was performed before explantation under general anesthesia. Examinations were completed in a standard 1.5 Tesla scanner (GE CV/I, General Electric) with retrospective peripheral pulse gating. Analysis was performed using dedicated software (Qmass MR7.1; Medis).
Explantation of the AV conduits
The animals were euthanized by intravenous pentobarbital (1 mL/kg body weight; WDT) following heparinization. The grafts were excised, examined macroscopically, and prepared for histological analysis.
Histology and immunohistochemistry
Formalin-fixed and paraffin-embedded tissue sections (6 μm thick) were stained by standard hematoxylin and eosin, Movat pentachrome, and von Kossa stains and visualized in a bright field using an Olympus BX40 microscope. Immunohistochemistry was performed as described.11,14 In brief, frozen tissue sections were investigated for extracellular matrix (ECM) integrity: collagen IV (clone CI22; Dako); the presence of ECs: the von Willebrand factor (vWF) (polyclonal rabbit IgG; Dako), eNOS (clone 3/eNOS/NOS Type III; BD Bioscience), CD31 (Polyclonal Rabbit IgG; Santa Cruz); the presence of smooth muscle cells and myofibroblasts: alpha-smooth muscle actin (α-SMA) (clone 1A4; Dako); and for inflammatory cells, CD45 (clone 1.11.32; Serotec). Furthermore, the stain against procollagen I (Developmental Studies Hybridoma Bank) stands for newly synthetized collagen I and therefore for matrix remodeling. Phalloidin stain was used to show the subcellular, cytoskeletal actin filaments of the cells. Tissue samples were fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton, and subsequently labeled with Alexa 488 phalloidin (Molecular Probes) as described.15 Frozen sections of ovine AVs served as positive controls.
Statistics
All data are reported as the mean±SD. The unpaired student t-test was used to test for differences among groups. A p-value of less than 0.05 was considered significant. The SPSS statistical software package 14.0 for Windows (SPSS) was used for all statistical analyses.
Results
Morphology of decellularized and re-endothelialized AV conduits
After detergent treatment of the AV followed by DNase I digestion, samples showed <5% of residual DNA as compared with native tissue samples. Histology and immunohistochemistry revealed a preserved threedimensional scaffold with preserved collagens, elastic fibers, and glycosaminoglycans. Complete maintenance of the basement membrane along the inner surface of the aortic wall and on both sides of the leaflet was demonstrated by the presence of Collagen IV.11
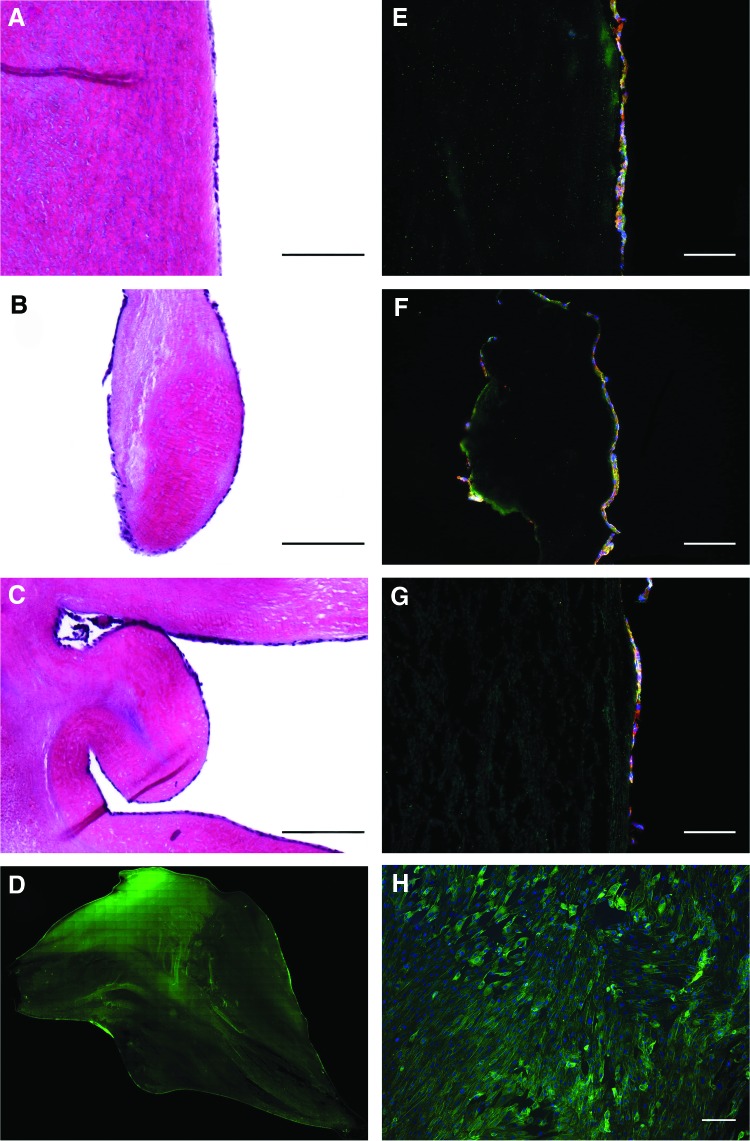
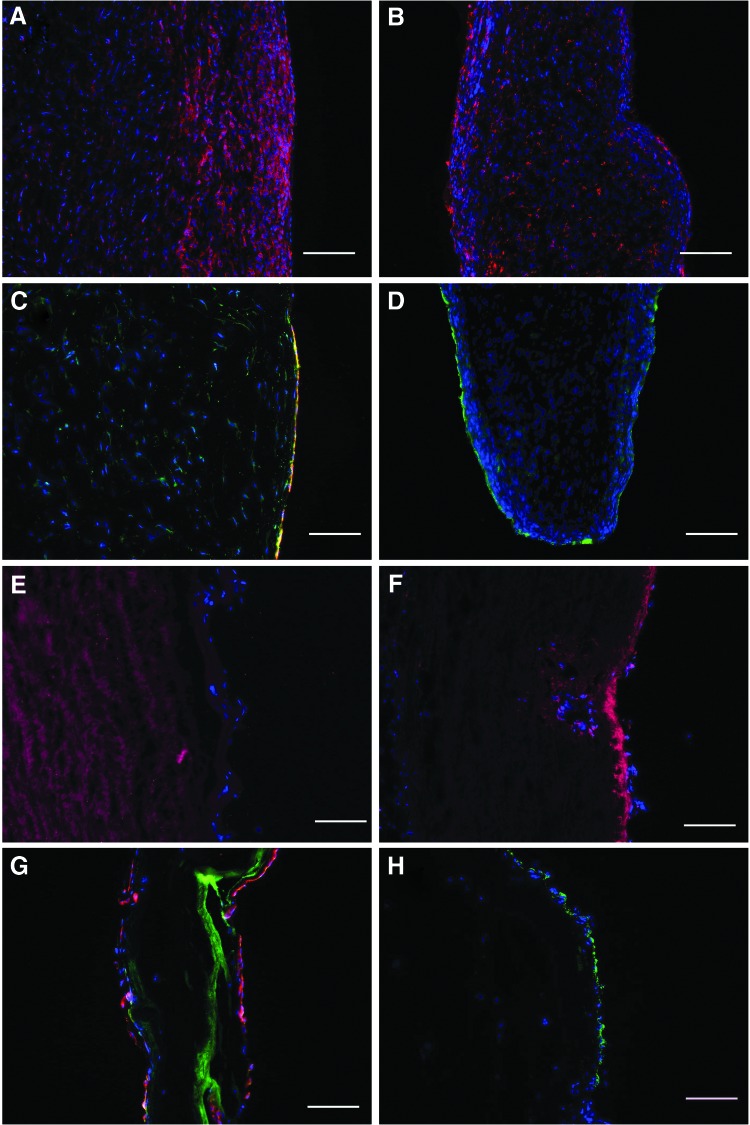
After re-endothelialization, the luminal surface of the DAV was covered with a confluent cell monolayer (Fig. 1A–C). Cobblestone-like morphology with phalloidin staining (Fig. 1D, H), expression of CD31 and eNOS (Fig. 1E–G) demonstrated an endothelial origin. No interstitial cells were detected on the conduit surface or inside the scaffold (data not shown).
FIG. 1.
Complete re-endothelialization of the aortic valve scaffold after cultivation in a dynamic bioreactor. Hematoxylin and eosin and immunohistochemical images show complete coverage by cells expressing eNOS (red) and CD31 (green) on the surface of the aortic wall (A, E), the edge of the leaflet (B, F), and the sinus of the valve (C, G). Cell nuclei positive for DAPI. (Bars 200 μm) Phalloidin stain shows homogenous cell distribution over the whole leaflet surface (D, H). (Scale bars 50 μm). eNOS, endothelial nitric oxide synthase. Color images available online at www.liebertpub.com/tea
Operative results
Both, TEVs and CPVs represent a stable tissue that enables to perform appropriate surgical anastomosis. The cross-clamp time was 98±27 min. All animals survived the operative procedure. In both groups, no signs of neurological deficits were observed.
Echocardiography and magnetic resonance investigation
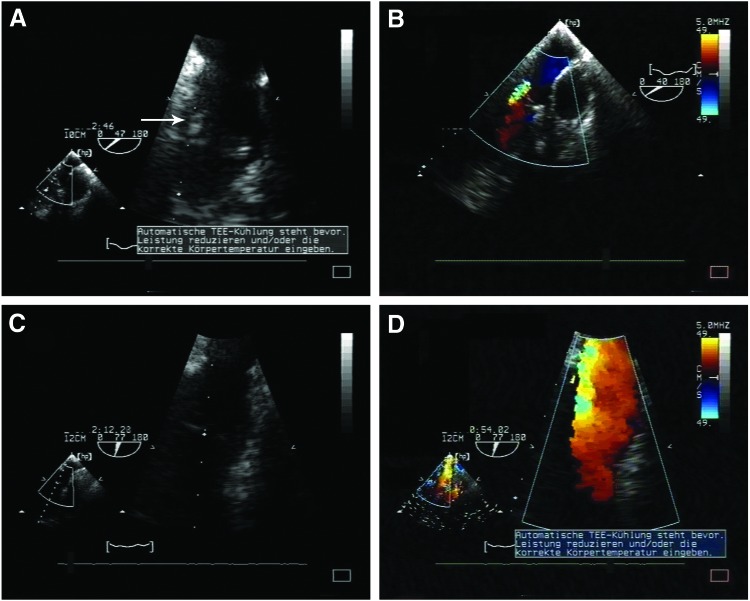
Echocardiography showed a comparable mean diameter and an effective orifice area. Although the pressure gradient was low in both groups, it was significantly higher in CPVs (Table 1). In the CPV group, evident leaflet thickening and trivial to mild insufficiency was observed (Fig. 2A, B). In contrast, TEVs appeared normal with no valvular insufficiency or stenosis (Fig. 2C, D). No signs of cusp thickening or reduction of cusp's mobility were observed. This data correlate with magnetic resonance investigation results where no aortic regurgitation was noticed on flow measurements in the aorta. The volumetric analysis showed regular end-diastolic and end-systolic volumes of the left ventricles as well as adequate ejection fractions. There were no signs of left ventricular hypertrophy. Stroke volumes by volumetry matched stroke volume analysis by phase-contrast flow measurements, thereby, ruling out significant mitral regurgitation (Fig. 3).
Table 1.
Functional Analysis of Implanted Tissue-Engineered Valves and Cryopreserved Allograft Valves
| TEVs | CPVs | p | |
|---|---|---|---|
| Orifice area (cm2) | 2.0±0.2 | 2.1±0.6 | n.s. |
| Mean transvalvular systolic pressure gradient (mm/Hg) | 0.5±0.5 | 2.1±0.6 | <0.006 |
| Max. transvalvular systolic pressure gradient (mm/Hg) | 1.1±0.4 | 4.2±0.7 | <0.001 |
| AV Insufficiency | 0.2±0.4 | 1.5±0.6 | <0.001 |
| Mean AV diameter (mm) | 19.0±2.0 | 19.8±1.7 | n.s. |
| Ejection fraction (%) | 52.0±10.4 | 54.0±16.7 | n.s. |
AV, aortic valve; TEVs, tissue-engineered aortic valves; CPVs, cryopreserved allograft valves; n.s., not significant.
FIG. 2.
Representative echocardiography results for tissue-engineered aortic valves (TEVs) and cryopreserved allograft valves (CPVs) at 3 months. Degenerated aortic valve cusps of CPV with vegetation (arrow) (A) and valve insufficiency (B). Thin aortic valve cusps of the TEV without signs of degeneration (C) or insufficiency (D). Color images available online at www.liebertpub.com/tea
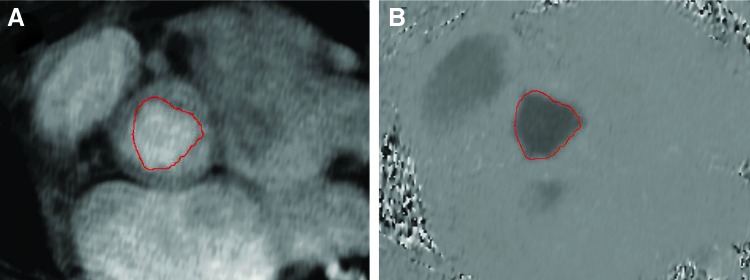
FIG. 3.
Direct planimetric analysis of the aortic valve orifice area (red line) using a modulus (A) and phase-contrast images (B), showing symmetric opening of the valve. Color images available online at www.liebertpub.com/tea
Characterization of the explanted grafts
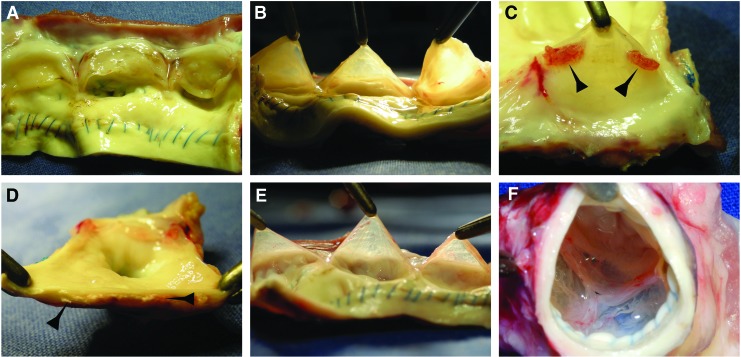
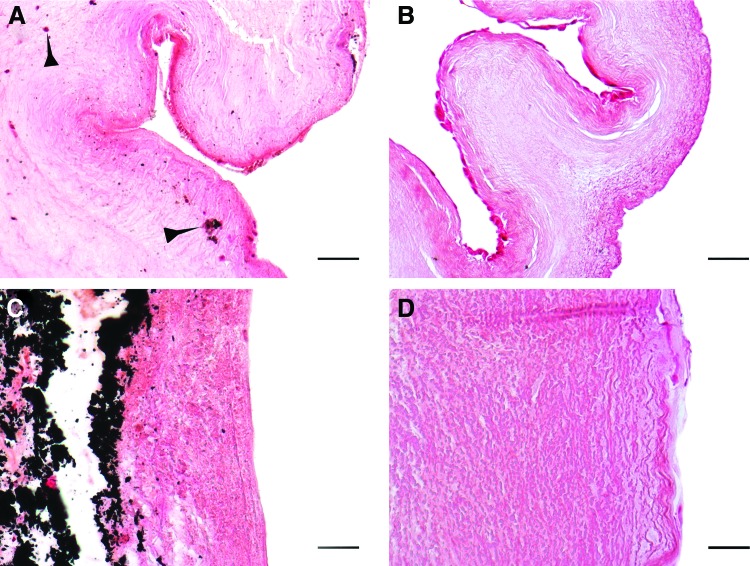
None of the explanted conduits exhibited signs of rupture, aneurysmatic dilatation, or infection. By explantation, there was a sizeable difference in the adhesion reaction with surrounding tissues. The CPVs showed more adhesions due to inflammation and the cusps were visibly thickened and shrunken (Fig. 4A, B). Vegetation spots, as well extensive macroscopic calcification were observed in the wall and annulus of explanted CPVs (Fig. 4C, D). In contrast, the TEVs had a smooth aortic wall surface. The leaflets remained tender, translucent, and without vegetations, calcification, or signs of degeneration. (Fig. 4E, F). Some small hematoma spots were observed on the cusps surface of both types of grafts.
FIG. 4.
Macroscopic morphology of explanted valves. Fibrous degeneration and thickening (A, B), as well as cusp vegetation (arrows) on the leaflet surface of the CPV (C). Macroscopic calcification (arrows) of the CPV wall (D). Translucent leaflets (E) and complete compliance of the valvular apparatus (F) of the TEV. Color images available online at www.liebertpub.com/tea
Histology and immunohistochemistry
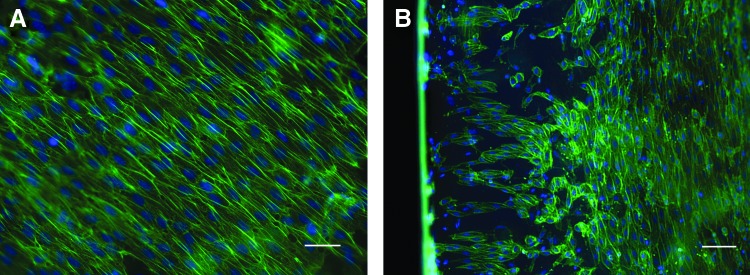
Explanted grafts had the preserved, typical layered organization of the leaflets and dense scaffold organization of the arterial wall. CPVs showed massive inflammation and leukocyte infiltration into the entire graft tissue (Fig. 5A, B). Over the luminal surface of CPVs, CD31- and vWF-positive cells could be identified (Fig. 5C, D). The explanted TEVs revealed neither signs of rejection nor inflammation (Fig. 5E, F). The luminal surface of the aorta, sinus, and cusps remained covered by the endothelium. These cells expressed eNOS, CD31 (Fig. 5G), and vWF (Fig. 5H), supporting an endothelial character. The EC covered the luminal surface up to the free margin of the leaflet as a monolayer (Fig. 6). The area around all suture lines exhibited a typical subendothelial neointima formation (panus). The degree of panus was relatively equal in both groups. Cells expressing α-SMA and procollagen I repopulated approximately one third of the TEV adventitial site (data not shown).
FIG. 5.
Immunhistochemical micrographs from explants. Massive leukocyte infiltration of the CPV wall (A) and leaflet (B) with CD45 cells (red). The preserved endothelial cell layer over the CPV wall (C) positive for CD31/eNOS (green/red) and cusp (D)-positive for vWF (green). The TEV wall (E) and leaflet (F) without leukocyte infiltration (red—classified as background staining in the absence of concomitant staining of cell nuclei in blue). The TEV cusp (G) covered by CD31/eNOS (green/red)-positive cells. Expression of vWF by cells over the TEV wall (H). (Scale bars 200 μm). vWF, von Willebrand factor. Color images available online at www.liebertpub.com/tea
FIG. 6.
Phalloidin stain of the TEV leaflet. Homogenous cells cover the cusp surface (A) and free edge (B). (Scale bars 100 μm). Color images available online at www.liebertpub.com/tea
Von Kossa staining showed signs of leaflet calcification and massive deposits of calcium in the wall of the CPVs (Fig. 7A, C) In contrast, no histological signs of calcification were observed inside the explanted TEV cusps or the arterial wall (Fig. 7B, D).
FIG. 7.
Von Kossa stain shows calcium spots (arrows) within the CPV cusp (A) and massive CPV wall (C) calcification. In contrast, no signs of calcification were observed in TEV tissue (B, D). (Scale bars 100 μm). Color images available online at www.liebertpub.com/tea
Discussion
In this study, we show for the first time, the results of an orthotopic implantation of TE aortic allografts. Our concept is based on the re-endothelialization of detergent DAV conduits with the vascular EC. We demonstrated in our previous study that the DAVs, possess excellent hemodynamic parameters and are able to withstand systemic pressures without suffering structural dilatation or deterioration for as long as 9 months.11 However, the process of in vivo remodeling that occurs after implantation required a longer period of time for complete re-endothelialization of the graft lumen. In the pulmonary position, in vitro cultivation of the EC seeded onto decellularized pulmonary valve scaffolds using a pulsatile bioreactor system improves graft compatibility and shows the maintenance of an EC monolayer after implantation.14 Subsequent to these data, we used the same principle to obtain a TE aortic graft. We used the sheep model as considered to be ideal to test heart valve durability, as these animals exhibit increased calcium metabolism and reach full size by 2 years of age. Consequently, estimation of cardiovascular implant durability is possible in a shorter period of time.16
Autologous venous ECs were used to have cells with a stable and unvariable phenotype for reseeding procedures. Because of the invasiveness, this method of cell acquisition is not ideal, since the TE grafts should be primarily used in pediatric patients. To this point, several groups have demonstrated the use of human marrow stromal cells, amniotic fluid, and blood-derived progenitor cells for heart valve TE17,18; however, the use of these cells in the sheep model remains to be determined. Currently, method development for the isolation and stable cultivation of ovine blood-derived ECs for TE purposes is underway.
To avoid the wash-off of the reseeded cells from the luminal surface, we slowly increase the flow in the pulsatile bioreactor system trying to adapt ECs to higher shear stress. The final protocol, used in this study, resulted in the maintenance of a confluent EC layer at flows up to 1.0 L/min. In our previous studies, we demonstrated persistence of the EC layer after transportation in the operating room as well as operative stress for 24 h (data not shown).
Since Dr. Ross implanted the first aortic allograft in 1961,19 the methods of valve procurement and graft preservation have changed substantially. Maintenance of the graft viability appears to have a major importance in long-term functionality of cryopreserved or homovital allografts.20 In this study, cryopreserved allografts were used as controls. Although functional investigations did not reveal major differences in hemodynamic parameters between the grafts in both groups, considerable signs of degeneration, both macro- and microscopically, in the CAVs were observed as early as 3 months following implantation. These data confirm that the presence of allogenic cells and disorganization of ECM components as a result of storage or mechanical stress can predict tissue degeneration and secondary graft failure. Lack of calcification in TEV grafts, can be explained in part by an effective decellularization process and scaffold preservation. Previously, we noted that mechanical stress, at the level of leaflet coaptation, did not permit complete re-endothelialization of cusp edges in unseeded grafts.11 This observation may be correct as the leaflets of TEVs in this study, showed ECs covering the entire cusp surface. Lack of thrombotic formation in grafts of both groups, highlights the importance of a functional endothelial layer before implantation. This is in line with our findings with implanted TE pulmonary valves.14 Coverage of the matrix with cells demonstrates once again that the use of detergents for decellularization of allografts is feasible, even supposed that traces remain likely entrapped in the matrix. Intimal hyperplasia observed predominantly in coronary sinuses and in close proximity to anastomoses was in a decreasing thickness from the suture lines. This occurred in both types of grafts and was interpreted as a physiological panus, secondary to surgical trauma.
This study has a limitation. Implantation for three months in the ovine model provided considerable immunological-related data and was sufficient to identify calcification trends in different valvular grafts. However, susceptibility of TE AVs to growth was not determined in this study. Long-term implantations in lambs are pending. Here the in vivo repopulation of the grafts by interstitial cells might have an essential role in graft remodeling.
In conclusion, the protocol used for TE of the pulmonary valve was successfully used in this study for engineering of AVs based on allograft scaffolds reseeded with venous ECs. Despite comparable short-term functionality, cryopreserved allografts revealed considerable degeneration, compared to the tissue-engineered grafts. The lack of degenerative signs makes the TE AV a promising alternative for use in future AV surgery.
Acknowledgments
We are grateful to Rosalinde Katt, Astrid Diers-Ketterkat, Karin Peschel, Slavica Schümann, Petra Zieme, and Jost Dörr for their technical assistance. We give special thanks to Doreen Unger for her excellent and careful completion of histological and immunohistochemical staining. This study was supported, in part, by the Cortiss Foundation.
Disclosure Statement
No competing financial interests exist.
References
- 1.Carrier M. Pellerin M. Perrault L.P. Page P. Hebert Y. Cartier R. Dyrda I. Pelletier L.C. Aortic valve replacement with mechanical and biologic prosthesis in middle-aged patients. Ann Thorac Surg. 2001;71:253. doi: 10.1016/s0003-4975(01)02512-7. [DOI] [PubMed] [Google Scholar]
- 2.van Geldorp M.W. Eric Jamieson W.R. Kappetein A.P. Ye J. Fradet G.J. Eijkemans M.J. Grunkemeier G.L. Bogers A.J. Takkenberg J.J. Patient outcome after aortic valve replacement with a mechanical or biological prosthesis: Weighing lifetime anticoagulant-related event risk against reoperation risk. J Thorac Cardiovasc Surg. 2009;137:881. doi: 10.1016/j.jtcvs.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Konakci K.Z. Bohle B. Blumer R. Hoetzenecker W. Roth G. Moser B. Boltz-Nitulescu G. Gorlitzer M. Klepetko W. Wolner E. Ankersmit H.J. Alpha-gal on bioprostheses: Xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35:17. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 4.Kasimir M.T. Rieder E. Seebacher G. Nigisch A. Dekan B. Wolner E. Weigel G. Simon P. Decellularization does not eliminate thrombogenicity and inflammatory stimulation in tissue-engineered porcine heart valves. J Heart Valve Dis. 2006;15:278. [PubMed] [Google Scholar]
- 5.Hawkins J.A. Hillman N.D. Lambert L.M. Jones J. Di Russo G.B. Profaizer T. Fuller T.C. Minich L.L. Williams R.V. Shaddy R.E. Immunogenicity of decellularized cryopreserved allografts in pediatric cardiac surgery: comparison with standard cryopreserved allografts. J Thorac Cardiovasc Surg. 2003;126:247. doi: 10.1016/s0022-5223(03)00116-8. [DOI] [PubMed] [Google Scholar]
- 6.Shaddy R.E. Hunter D.D. Osborn K.A. Lambert L.M. Minich L.L. Hawkins J.A. McGough E.C. Fuller T.C. Prospective analysis of hla immunogenicity of cryopreserved valved allografts used in pediatric heart surgery. Circulation. 1996;94:1063. doi: 10.1161/01.cir.94.5.1063. [DOI] [PubMed] [Google Scholar]
- 7.Horer J. Hanke T. Stierle U. Takkenberg J.J. Bogers A.J. Hemmer W. Rein J.G. Hetzer R. Hubler M. Robinson D.R. Sievers H.H. Lange R. Homograft performance in children after the ross operation. Ann Thorac Surg. 2009;88:609. doi: 10.1016/j.athoracsur.2009.04.100. [DOI] [PubMed] [Google Scholar]
- 8.Boethig D. Goerler H. Westhoff-Bleck M. Ono M. Daiber A. Haverich A. Breymann T. Evaluation of 188 consecutive homografts implanted in pulmonary position after 20 years. Eur J Cardiothorac Surg. 2007;32:133. doi: 10.1016/j.ejcts.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Cebotari S. Tudorache I. Ciubotaru A. Boethig D. Sarikouch S. Goerler A. Lichtenberg A. Cheptanaru E. Barnaciuc S. Cazacu A. Maliga O. Repin O. Maniuc L. Breymann T. Haverich A. Use of fresh decellularized allografts for pulmonary valve replacement may reduce the reoperation rate in children and young adults: early report. Circulation. 2011;124:115. doi: 10.1161/CIRCULATIONAHA.110.012161. [DOI] [PubMed] [Google Scholar]
- 10.Dohmen P.M. Lembcke A. Holinski S. Pruss A. Konertz W. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg. 2011;92:1308. doi: 10.1016/j.athoracsur.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Baraki H. Tudorache I. Braun M. Hoffler K. Gorler A. Lichtenberg A. Bara C. Calistru A. Brandes G. Hewicker-Trautwein M. Hilfiker A. Haverich A. Cebotari S. Orthotopic replacement of the aortic valve with decellularized allograft in a sheep model. Biomaterials. 2009;30:6240. doi: 10.1016/j.biomaterials.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 12.Lichtenberg A. Cebotari S. Tudorache I. Sturz G. Winterhalter M. Hilfiker A. Haverich A. Flow-dependent re-endothelialization of tissue-engineered heart valves. J Heart Valve Dis. 2006;15:287. [PubMed] [Google Scholar]
- 13.Fischlein T. Schutz A. Uhlig A. Frey R. Krupa W. Babic R. Thiery J. Reichart B. Integrity and viability of homograft valves. Eur J Cardiothorac Surg. 1994;8:425. doi: 10.1016/1010-7940(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenberg A. Tudorache I. Cebotari S. Suprunov M. Tudorache G. Goerler H. Park J.K. Hilfiker-Kleiner D. Ringes-Lichtenberg S. Karck M. Brandes G. Hilfiker A. Haverich A. Preclinical testing of tissue-engineered heart valves re-endothelialized under simulated physiological conditions. Circulation. 2006;114:559. doi: 10.1161/CIRCULATIONAHA.105.001206. [DOI] [PubMed] [Google Scholar]
- 15.Small J. Rottner K. Hahne P. Anderson K.I. Visualising the actin cytoskeleton. Microsc Res Tech. 1999;47:3. doi: 10.1002/(SICI)1097-0029(19991001)47:1<3::AID-JEMT2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Ali M.L. Kumar S.P. Bjornstad K. Duran C.M. The sheep as an animal model for heart valve research. Cardiovasc Surg. 1996;4:543. doi: 10.1016/0967-2109(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 17.Hoerstrup S.P. Kadner A. Melnitchouk S. Trojan A. Eid K. Tracy J. Sodian R. Visjager J.F. Kolb S.A. Grunenfelder J. Zund G. Turina M.I. Tissue engineering of functional trileaflet heart valves from human marrow stromal cells. Circulation. 2002;106:143. [PubMed] [Google Scholar]
- 18.Schmidt D. Achermann J. Odermatt B. Genoni M. Zund G. Hoerstrup S.P. Cryopreserved amniotic fluid-derived cells: a lifelong autologous fetal stem cell source for heart valve tissue engineering. J Heart Valve Dis. 2008;17:446. [PubMed] [Google Scholar]
- 19.Ross D.N. Homograft replacement of the aortic valve. Lancet. 1962;2:487. doi: 10.1016/s0140-6736(62)90345-8. [DOI] [PubMed] [Google Scholar]
- 20.Yacoub M. Rasmi N.R. Sundt T.M. Lund O. Boyland E. Radley-Smith R. Khaghani A. Mitchell A. Fourteen-year experience with homovital homografts for aortic valve replacement. J Thorac Cardiovasc Surg. 1995;110:186. doi: 10.1016/S0022-5223(05)80025-X. [DOI] [PubMed] [Google Scholar]