Abstract
Spontaneous differentiation of human embryonic stem cells (hESCs) is generally inefficient and leads to a heterogeneous population of differentiated and undifferentiated cells, limiting the potential use of hESCs for cell-based therapy and studies of specific differentiation programs. Here, we demonstrate biomaterial-dependent commitment of a mesenchymal cell population derived from hESCs toward the osteogenic lineage in vivo. In skeletal development, bone formation from condensing mesenchymal cells involves two distinct pathways: endochondral and intramembraneous bone formation. In this study, we demonstrate that the hESC-derived mesenchymal cells differentiate and regenerate in vivo bone tissues through two different pathways depending upon the local cues present in a scaffold microenvironment. Hydroxyapatite (HA) was incorporated into biodegradable poly(lactic-co-glycolic acid)/poly(l-lactic acid) (PLGA/PLLA) scaffolds to enhance bone formation. The HA microenvironment stabilized the β-catenin and upregulated Runx2, resulting in faster bone formation through intramembraneous ossification. hESC-derived mesenchymal cells seeded on the PLGA/PLLA scaffold without HA, however, showed minimal levels Runx2, and differentiated via endochondral ossification, as evidenced by formation of cartilaginous tissue, followed by calcification and increased blood vessel invasion. These results indicate that the ossification mechanisms of the hESC-derived mesenchymal stem cells can be regulated by the scaffold-mediated microenvironments, and bone tissue can be formed.
Introduction
Cell-based therapies and tissue engineering may provide a solution for craniofacial tissue reconstruction. However, limited proliferative capacity and donor-site morbidity due to isolation of chondrocytes, osteogenic cells, or bone marrow-derived mesenchymal stem cells (MSCs) pose a major challenge in providing adequate cell numbers for transplantation therapy.1–4 Human embryonic stem cells (hESCs) have been proposed as a promising cell source for cell-based applications as well as a powerful tool for investigating the fundamentals of human development.5,6 Previously, several groups have demonstrated the isolation of multipotent mesenchymal cells from differentiating embryoid bodies (EBs) via simple mechanical isolation or cell sorting.7–12 Further, our laboratory has demonstrated the multipotent differentiation potential of hESC-derived mesenchymal cells in vitro and their chondrocytic commitment via a chondrocyte-conditioned medium.12 Yet, in vivo osteogenic commitment mechanisms of these hESC-derived mesenchymal cells remain unexplored.
Controlling mesenchymal differentiation and engineering bone in vivo are challenging, as it often leads to heterotypic and inferior osseous tissues. Here we sought to investigate the biomaterial-dependent commitment of mesenchymal cells derived from hESCs toward the osteogenic lineage in vivo. Porous biodegradable polymer scaffolds are an attractive system to support ES cells and promote formation of complex three-dimensional (3D) tissues during differentiation.13 Biodegradable polyesters such as poly(lactic-co-glycolic acid), poly(l-lactic acid), and their copolymers have been widely used for preparation of the 3D scaffolds for bone tissue-engineering applications.14–16 Levenberg et al. produced complex structures with features of various committed embryonic tissues in vitro using ES cells in the poly(lactic-co-glycolic acid)–poly(l-lactic acid) (PLLA/PLGA) polymer scaffolds.13 In this study, we fabricated hydroxyapatite (HA)-based PLGA/PLLA biodegradable composite scaffolds to provide the necessary biochemical and biophysical cues to recreate a suitable niche for controlling cellular proliferation and differentiation of hESC-derived mesenchymal cells.17,18 HA is a bioactive, biocompatible, osteoinductive, and osteoconductive ceramic material having a similar chemical structure to that of the mineral phase of a native bone. Scaffolds based on HA are osteoinductive and promote direct osteogenic differentiation of mesenchymal cells.19–21 In the present study, we demonstrate that the mechanisms of bone formation from mesenchymal precursor cells are modulated by scaffold properties, indicating the importance of scaffold parameters on the modulation of hESC-derived mesenchymal cells in an ectopic bone regeneration model. In addition, we used hESC-derived mesenchymal cells seeded on to the scaffolds to successfully heal critical-size skull defects in mice. This MSC population derived from differentiating hESCs and EBs can be used in a range of different tissue engineering and cell therapy strategies. Further, employing the biomaterials to direct the differentiation commitment of hESC-derived mesenchymal cells will further elucidate functional capabilities of these cells in addition to building tissues.
Materials and Methods
Cell culture and mesenchymal cell derivation
The hESC line (Hues9) was cultured as previously reported22 (www.mcb.harvard.edu/melton/hues). To generate mesenchymal cell lines, hES cell cultures were dissociated into small clumps by incubating at 37°C for 30 min with 1 mg/mL collagenase IV (GIBCO) and cultured to form EBs for 10 days as previously reported.12 The EBs were transferred onto gelatin- (0.1% w/v) coated plates, and migrating cells were selectively isolated and subcultured at an initial cell density of 2×104 cells/cm2 in a mesenchymal stem cell growth medium (MSCGM) consisting of Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Hyclone), 2 mM l-glutamine (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco). For osteogenic differentiation, cells were cultured in an osteogenic differentiation medium (MSCGM; 50 μM ascorbic acid-2-phosphate, 10 mM β-glycerophosphate, and 100 nM dexamethasone) for 2 weeks.
Reverse transcriptase–PCR and PCR array
Total RNA was extracted with Trizol, and reverse-transcribed into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen). The polymerase chain reaction (PCR) primers are provided in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/tea). RT reactions were first denatured for 2 min at 95°C, followed by 35 cycles of 30-s denaturation at 95°C, -s annealing, and -min elongation at 72°C. PCR array analyses were performed using Osteogenesis PCR Arrays (SuperArray Biosciences) according to the manufacturer's instructions using the ABI Prism 7700 Sequence Detector System (Perkin Elmer/Applied Biosystems). Data were first normalized to a control gene, β-actin, and were further analyzed by comparing Day-1 control.
Cell staining, histology, and immunostaining
Calcium deposition was detected by Alizarin Red S (Sigma) following the manufacturer's instructions. Alkaline phosphatase was stained using Sigma kit #85 following the manufacturers' instructions. For histological analysis, tissue samples were fixed in 4% paraformaldehyde, dehydrated in serial ethanol dilutions, and paraffin-embedded. The tissue constructs were cut into 5-μm sections and stained with hematoxylin and eosin, Safranin-O/fast green, Alcian blue, or Alizarin Red-S. For immunostaining, the sections were blocked with 5% normal goat serum in PBS for 30 min, and incubated with rabbit polyclonal antibodies against type I, II, and X collagen (RDI), and osteocalcin (Biogenesis) with 1:100, 1:100, 1:40, 1:100 dilutions, respectively. the sections were incubated with either FITC- or Texas Red-conjugated goat anti-rabbit secondary antibody (all 1:100 dilutions) for 1 h (Jackson ImmunoResearch laboratory). The nuclei were counterstained with DAPI (Chemicon) for 10 min, and images were collected with a Zeiss LSM Metal Confocal microscope. Immunohistochemistry was performed with the Histostain-SP kit (Zymed Laboratories) with rabbit anti-Runx2 (Zymed) and rabbit anti-β-catenin (Cell Signaling) primary antibodies with 1:50 and 1:100 dilutions, respectively.
Polymer scaffold preparation and scanning electron microscopy
Three-dimensional porous HA composite scaffolds composed of PLLA and PLGA were fabricated as previously described with slight modifications.13 Briefly, PLLA+PLLA (1:1 ratio) were dissolved in chloroform to yield a solution of 5% (wt/vol) polymer with or without HA particles (1% or 5% wt/vol). The polymer solution (0.25 mL) was loaded into molds packed with 0.4 g of sodium chloride particles. The solvent was allowed to evaporate overnight, and the sponges were subsequently immersed for 12 h in distilled water (changed every 2 h) to leach the salt and create pore structures. The sponges were soaked in 75% (vol/vol) ethyl alcohol overnight, washed three times with PBS, and coated with fibronectin (10 ng/mL) for 3 h before cell seeding. For SEM photomicrographs, scaffolds were prepared, and sponges were cut with a razor blade. The cross-sections were coated with platinum using a sputter coater. The samples were observed with a scanning electron microscope (JEOL 6700F).
Osteocalcin ELISA assay
The osteocalcin ELISA assay was performed with the Metra Osteocalcin EIA Kits (Cat 8002; Quidel) following the manufacturer's instruction. For assaying the osteocalcin in the constructs, a serum free medium was used 48 h before osteocalcin harvest. Samples for each group were analyzed in triplicate for secreted soluble osteocalcin.
Transmission electron microscopy
Implants were retrieved, fixed for 1 hr in 2.5% (wt/vol) glutaraldehyde, 3% (wt/vol) paraformaldehyde, and 2.5% (wt/vol) sucrose in 0.1 M sodium cacodylate buffer (pH 7.4) and then postfixed in 1% (wt/vol) OsO4 in veronal acetate buffer for 1 h. The cells were stained en block overnight with Kellenberger uranyl acetate (pH 6.0), dehydrated, and embedded in an Epon resin. The sections were cut on a Leica ULTRACUT UCT ultramicrotome, poststained in uranyl acetate, and observed in a Philips EM420 transmission electron microscope.
In vivo subcutaneous transplantation and harvesting
hESC-derived mesenchymal cells were expanded (P9), seeded (3×106) onto the polymer sponges, and cultured for 10 days in osteogenic conditions. The cell-seeded scaffolds were implanted subcutaneously into the dorsal region of 6-week-old athymic nude mice (Charles River Laboratories; n=6). After predifferentiation in a differentiation medium for 10 days, the cell–scaffolds were implanted, and the skin was closed with a nylon suture. Constructs were harvested after 4 and 8 weeks and processed for histology or electron microscopy.
Statistical analysis
Data are expressed as mean±standard deviation (SD). Statistical significance was determined by analysis of variance (ANOVA single-factor) with p<0.05 or p<0.01.
Results
Osteogenic differentiation of hESC-derived mesenchymal cells
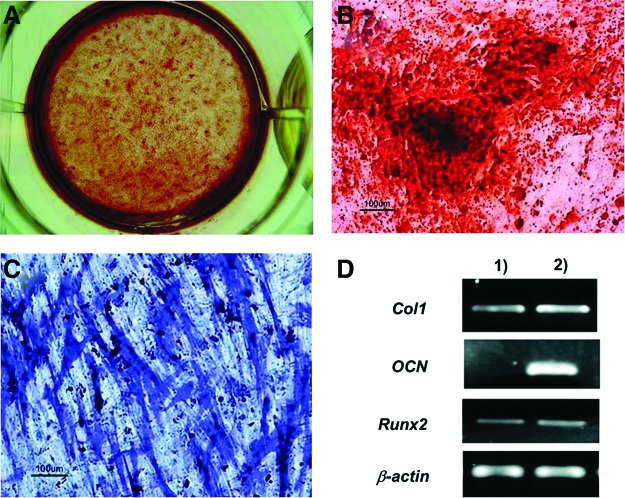
We have previously demonstrated that the homogenous population of mesenchymal cells can be derived from hESCs with differentiation potentials and surface markers characteristic of adult MSCs.12 In the current study, we have further evaluated that the differentiation potential of hESC-derived mesenchymal cells into an osteogenic lineage was first evaluated in vitro and in vivo. In vitro osteogenic differentiation of hESC-derived mesenchymal cells was evaluated by seeding the cells at 5000 cells/cm2 on gelatin-coated plates where cells were exposed to osteogenic conditions for 14 days. After osteogenic induction, hESC-derived mesenchymal cells produced areas of intense ALP staining (Fig. 1C). Moreover, calcium accumulated around the hESC-derived cells, indicating efficient osteogenic differentiation of hESC-derived mesenchymal cells (Fig. 1A, B). To confirm osteogenic differentiation, we performed reverse transcriptase–PCR on 2-week cultures to evaluate the molecular markers of the osteoblast lineage (Fig. 1D). Osteocalcin, a late marker for bone, was not expressed by undifferentiated hESC-derived mesenchymal cells. However, upon induction with an osteogenic differentiation medium, osteocalcin gene expression increased markedly at day 14. Undifferentiated hESC-derived mesenchymal cells expressed basal levels of Runx2 and type I collagen genes, the early markers of osteogenic differentiation.
FIG. 1.
Osteogenic potential of human embryonic stem cell (hESC)-derived mesenchymal cells in vitro. Cells were plated at 5000 cells/cm2 and differentiated in an osteogenic medium for 14 days. Mineralized deposits were evident with the alizarin red staining after 2 weeks of osteoinductive conditions (A, B), and bone-specific alkaline phosphatase (ALP) was also detected after 14 days (C). (D) Osteogenic differentiation was confirmed with reverse transcriptase–polymerase chain reaction (RT-PCR) for bone markers. (1) Undifferentiated hESC-derived mesenchymal cells; (2) differentiated hESC-derived mesenchymal cells. Color images available online at www.liebertpub.com/tea
Effects of HA on osteogenic differentiation of hESC-derived mesenchymal cells in polyester scaffolds
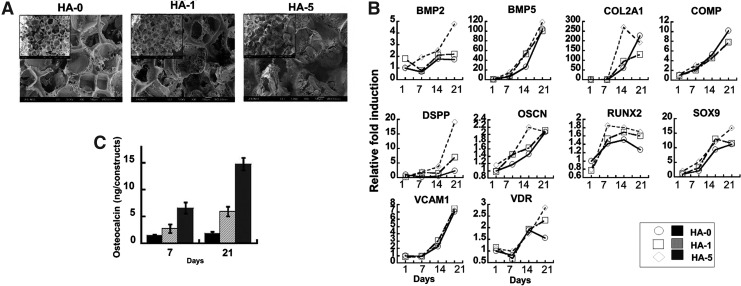
To induce hESC-derived mesenchymal cell differentiation with 3D tissue organization, biodegradable PLGA/PLLA scaffolds with varying amounts of HA were fabricated with a pore size of 200–400 μm (Fig. 2A). SEM observation showed that the PLGA/PLLA (HA-0) scaffolds were highly porous, whereas the addition of HA particles markedly changed the surface characteristics of the scaffolds (HA-1 and HA-5), creating a nanoscale surface topology (Fig. 2A). Scaffolds were seeded with 1×106 cells/construct, and all the scaffolds demonstrated similar adhesion percentages. Introduction of HA greatly increased the osteogenic response of hESC-derived mesenchymal cells in vitro (Fig. 2B). HA incorporation resulted in a dose-dependent upregulation of osteogenic gene markers such as BMP-2, DSPP, VDR, OSCN, and RUNX2, suggesting that osteogenic stimulation may result from the direct contact of seeded cells with the HA particles exposed on the scaffold surface. Interestingly, other bone-related markers such as BMP-5 and VCAM1 showed HA-independent expression. After 3 weeks of in vitro culture, cells seeded on the scaffolds without HA showed minimal accumulation during the culture periods in vitro, whereas the osteocalcin accumulation in the HA-containing scaffolds displayed significant changes during the 3-week culture period (Fig. 2C).
FIG. 2.
Biomaterial-directed osteogenic differentiation of hESC-derived mesenchymal cells in vitro. (A) The pore structures and surface topography of the scaffolds were examined by scanning electron microscopy (SEM). SEM photomicrographs of a cross-section of poly(lactic-co-glycolic acid)/poly(l-lactic acid) (PLGA/PLLA) scaffolds with or without hydroxyapatite (HA) (1% or 5% w/v) at original magnification×80. The inset shows the original magnification×35. HA-0: PLGA/PLLA scaffold, HA-1: PLGA/PLLA scaffold with 1% w/v HA; HA-5: PLGA/PLLA scaffold with 5% w/v HA. (B) In vitro osteogenic potential of hESC-derived mesenchymal cells in polymeric scaffolds was evaluated by seeding hESC-derived mesenchymal cells on PLGA/PLLA scaffolds or HA composite PLGA/PLLA scaffolds for 3 weeks for Osteogenesis PCR Arrays. (C) Addition of HA to scaffolds increased osteocalcin accumulation in a dose-dependent manner.
Subcutaneous implantation of hESC-derived mesenchymal cells and scaffolds
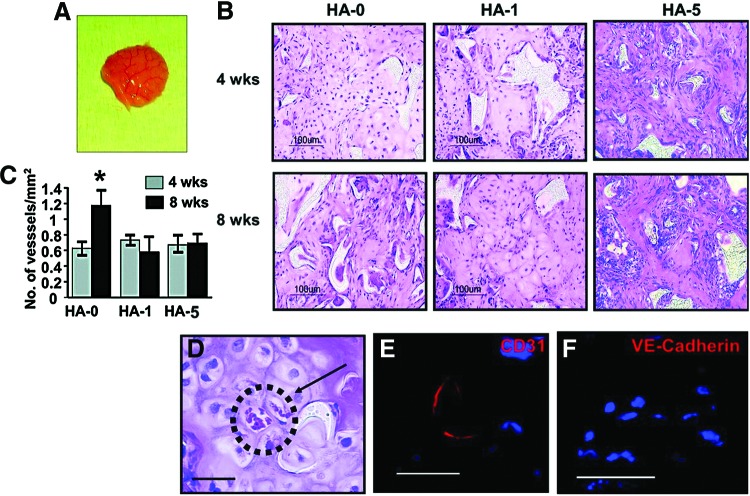
Our previous report demonstrated that hESC-derived mesenchymal cells display enhanced osteogenic potential compared to hMSCs, with upregulated gene expression for Runx2, type I collagen, alkaline phosphatase, and osteonectin.12 The hESC-derived cells continued to display robust osteogenic differentiation even after significant proliferation, expressing higher levels of osteoblast makers. We further investigated the in vivo response of hESC-derived cells in biomaterials. Scaffolds seeded with cells (Passage 9) were incubated in an osteogenic medium for 10 days. The cell-laden scaffolds were then implanted subcutaneously in the dorsal region of the 6-week-old athymic nude mice and analyzed after 4 and 8 weeks (Fig. 3A). Histological examination of the implants revealed formation of connective tissues within the scaffold (Fig. 3B). At the 4-week time point, the extent of osseous tissue formation was HA concentration-dependent. The cells on HA-5 composite scaffolds produced the greatest osseous tissue area, followed by HA-1 composite scaffolds. In contrast, cross-sections of the HA-0 scaffolds pictured largely cartilaginous tissue, with minimal bone tissue formation. After 8 weeks, histological examination of the explanted grafts demonstrated that mature bone was formed in which osteocytes were embedded in the mineralizing extracellular matrix (Fig. 3B). No evidence of teratoma or marked inflammation around the site of implant was observed in any of the scaffolds with varying HA concentrations.
FIG. 3.
Developmental potential of hESC-derived mesenchymal cells in vivo. hESC-derived mesenchymal cells were seeded on the PLGA/PLLA scaffolds or HA-composite PLGA/PLLA scaffolds and a predifferentiated in osteogenic condition for 10 days in vitro before implantation. (A) Gross image of in vivo engineered tissues after 8 weeks of implantation. (B) H&E staining of the implanted scaffolds after 4 and 8 weeks. (C) Quantification of blood vessels normalized to the unit area after 4 and 8 weeks of implantation. (D) Blood vessel networks were evident throughout the engineered tissue in the HA-0 scaffold. Arrow denotes red blood cells. Immunostaining using human specific anti-CD31 (E) and anti-VE-Cadherin (F) taken from the HA-0 scaffold. HA-0: PLGA/PLLA scaffold, HA-1: PLGA/PLLA scaffold with 1% w/v HA; HA-5: PLGA/PLLA scaffold with 5% w/v HA. Scale bar, 50 μm. *p<0.05. Color images available online at www.liebertpub.com/tea
Constructs harvested after 8 weeks of implantation confirmed that the implanted cells survived and maintained vascular connection with host tissue. The constructs were permeated with host blood vessels with intraluminal red blood cells (Fig. 3C, D). Host vessel recruitment with increasing implantation time was observed in the HA-0 scaffolds, while no significant increase was observed in the HA-1 and HA-5 composite scaffolds (Fig. 3C). Histological evaluation suggested that the majority of blood vessels were host derived; however, immunostaining for human specific anti-CD31 and anti-VE-Cadherin confirmed contribution of hESC-derived cells toward the blood vessel network formation (Fig. 3E, F).
Predifferentiation condition and duration determine the outcome of in vivo tissue-engineered constructs
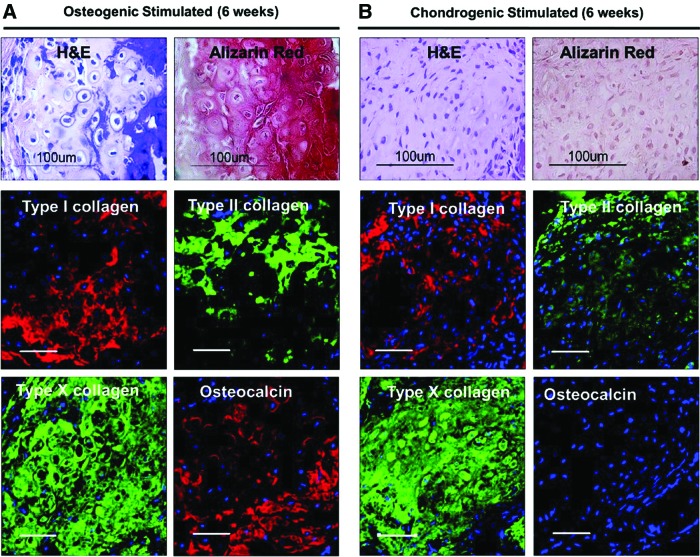
Previous studies have shown that in vitro differentiation patterns continue to progress in vivo.13,23 We predifferentiated hESC-derived mesenchymal cells on the biomaterials in a dexamethasone-containing osteogenic medium for 10 days before transplantation into athymic nude mice. This preconditioning time was sufficient to observe distinct bone formation in vivo. However, heterotypic osseous tissue with high levels of calcium accumulation was evident in vivo after 3 weeks in vitro osteogenic stimulation without HA (Fig. 4). In addition, predifferentiating the cells seeded in the PLGA/PLLA scaffolds with transforming growth factor (TGF)-β1 for 3-week culture resulted in the development of full cartilaginous tissues with minimal ossification after 6 weeks in vivo, indicating that TGF-β1 treatment of the cells may have delayed or prevented the calcification process.
FIG. 4.
Predifferentiation condition and duration determine the outcome of in vivo tissue-engineered constructs. hESC-derived mesenchymal cells were seeded on the PLGA/PLLA scaffolds and predifferentiated in vitro for 3 weeks with (A) an osteogenic differentiation medium containing β-glycerophosphate or (B) a chondrogenic differentiation medium containing transforming growth factor-β1 (10 ng/mL) before subcutaneous implantation. Constructs were retrieved after 6 weeks and processed for histology and immunostaining as described in the Materials and Method section. Scale bar=50 μm. Color images available online at www.liebertpub.com/tea
Intramembraneous versus endochondral bone formation, implication of WNT/β-catenin signaling
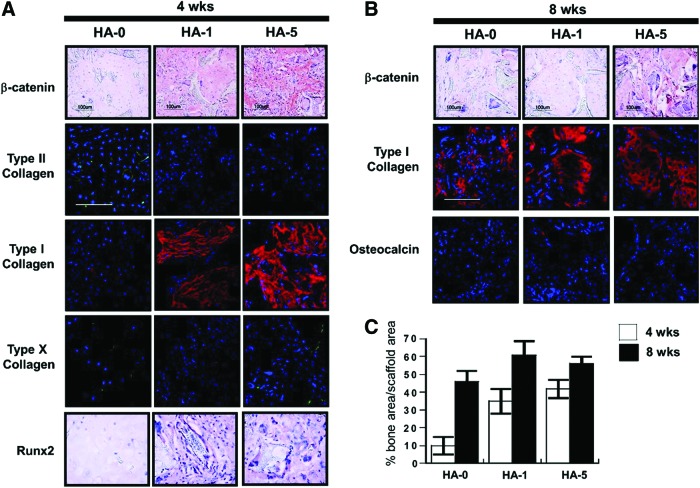
Bone formation of hESC-derived cells after 4 weeks indicated two distinct processes of differentiation depending on the scaffold composition (Fig. 5). In particular, a positive cartilage-like structure was observed in addition to lacuna-like structures in the cell-laden HA-0 scaffolds at 4 weeks, which further calcified to form bone-like tissues at 8 weeks. In contrast, cells seeded on the HA-5 composite scaffolds showed minimal cartilaginous tissue and significant osseous tissue at both time points, suggesting that the bone formation occurred through intramembraneous ossification. The capacity to develop a mineralized matrix and bone formation has been implicated in β-catenin activity and the transcription factor, Runx2. Immunostaining results confirmed minimal levels of β-catenin and Runx2 in the HA-0 scaffold, while the presence of HA in the scaffold produced an HA concentration-dependent stabilization of β-catenin and Runx2 transcription activity (Fig. 6A). After 4 weeks, cells seeded on the HA–PLGA/PLLA composite scaffolds (HA-1 and HA-5) produced predominantly type I collagen, while a mixture of type II and I collagen was evident in the HA-0 scaffold. Type X collagen, a marker for chondrocyte hypertrophy, was not observed in any of the implants after 4 weeks. Type I collagen and osteocalcin were detected in all constructs after 8 weeks, further supporting bone formation (Fig. 6B). Semiquantitative analysis of bone formation by hESC-derived mesenchymal cells on the scaffolds cultured in vivo confirmed that the HA-0 scaffold formed small areas of bone at 4 week, while bone formation was significantly greater in the HA-containing scaffolds (Fig. 6C).
FIG. 5.
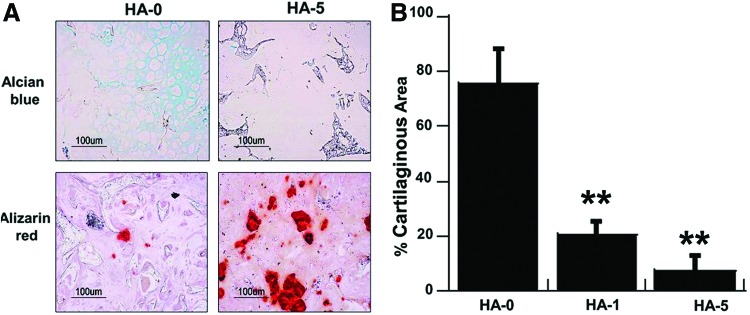
Biomaterial-directed osteogenic differentiations of hESC-derived mesenchymal cells in vivo. (A) Cells seeded on the PLGA/PLLA scaffold show positive for Alcian blue staining for negatively charged proteoglycans, and while 5% HA–PLGA/PLLA composite scaffolds show direct bone formation by Alizarin red mineral staining after 4 weeks of implantation. (B) Quantification of cartilaginous area. Scale bar, 100 μm. **p<0.01. Color images available online at www.liebertpub.com/tea
FIG. 6.
Microenvironment-mediated intramembraneous vs. endochondral ossification. (A) HA concentration-dependent β-catenin and Runx2 activity indicates that the HA microenvironment may play a role in regulating the two distinct ossification processes. Cells seeded on the PLGA/PLLA scaffolds stained for type II collagen and weakly for type I collagen. The intensity of staining for type I collagen increased in the HA-PLGA/PLLA composite scaffolds (1% and 5%). (B) Stronger type I collagen staining was observed after 8 weeks in all implants along with a detectable amount of osteocalcin. (C) Quantitative analysis of bone formation by hESC-derived mesenchymal cells in the recovered implants. HA-0: PLGA/PLLA scaffold, HA-1: PLGA/PLLA scaffold with 1% w/v HA; HA-5: PLGA/PLLA scaffold with 5% w/v HA. Scale bar=100 μm. Color images available online at www.liebertpub.com/tea
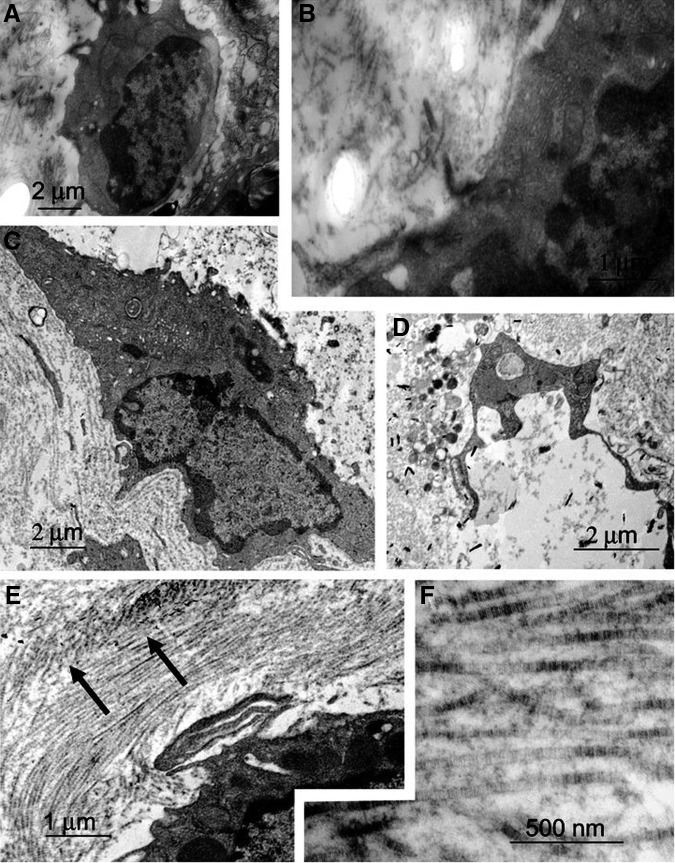
Electron micrographs demonstrated biomaterial-dependent differences in the cell size, shape, and organization of the extracellular matrix (Fig. 7). Cells in HA-0 maintained a round, chondrocytic morphology with the collagen matrix sparsely assembled in the pericellular region (Fig. 7A, B). Flattened cellular morphology reminiscent of osteoblastic cells was observed in the HA-1 composite scaffolds (Fig. 7C). In HA-5 composite scaffolds, cells were elongated with assembly of organized collagen fibers, indicative of a bone extracellular matrix (Fig. 7D–F).
FIG. 7.
Electron micrograph of hESC-derived mesenchymal cells in the PLGA/PLLA polymer scaffold, 1% HA-PLGA/PLLA composite scaffold, and 5% HA-PLGA/PLLA composite scaffold. Cells in PLGA/PLLA maintained a round chondrocytic morphology (A) with the collagen matrix in the pericellular region (B). Note the meshwork of the cell-associated matrix. (C) Cells seeded on 1% HA-PLGA/PLLA composite scaffolds exhibit elongated morphology. Note the assembly of the matrix consisting of fibrous elements around the cell. Cells seeded on the 5% HA-PLGA/PLLA composite scaffolds showed organized collagen fibers (E, arrow) on the periphery of the cells (D–F).
Discussion
Recently, the osteogenic potential of hESCs has been demonstrated, providing the exciting potential to treat patients with bone defects.24,25 However, the application of hESCs in bone regenerative medicine requires the development of efficient methodologies for differentiating hESCs into an osteogenically committed lineage in vitro and production of functional bone formation in vivo. Osteogenic commitment and stem cell-based bone tissue engineering may be augmented through systemic administration of growth/bioactive factors or by scaffold-mediated delivery of these factors. Even though there a number of studies evaluating the osteogenic differentiation of hESCs, there is little information describing biomaterial-directed bone formation in vivo, which led us to investigate whether an HA microenvironment can enhance in vivo bone formation of hESC-derived mesenchymal cells.26,27 In addition to providing structural stability for developing tissues, scaffolds with desirable biochemical and biophysical cues can direct cellular function and differentiation commitment.28,29 Scaffolds frequently support the incorporation of biological signals that aim to mimic the natural extracellular matrix and its ability to regulate complex morphogenetic processes in tissue formation and regeneration.30 In this study, HA, with a similar composition and structure to the natural bone mineral, was incorporated into biodegradable scaffolds to enhance bone formation in vitro and in vivo.31,32 A recent study by Siddappa et al. showed that ectopic transplantation of hMSCs resulted in tissue with only 5% of bone area.23 In addition, they showed that activation of cAMP/PKA signaling increased the total bone area to 15%–20% in an ectopic transplantation model.23 The approach that we developed enables microenvironment-dependent commitment and efficient in vivo tissue formation with up to 60% of boney areas from hESC-derived mesenchymal cells when utilized with the HA composite scaffolds. In addition, unlike conventional hESC differentiation protocols requiring growth factor, coculture, or genetic manipulation, our methodology utilizes novel culture techniques to derive a mesenchymal cell population from hESCs.
In skeletal development, bone formation from condensing mesenchymal cells involves two distinct pathways: endochondral and intramembraneous ossification. The endochondral ossification process is marked by mesenchymal cell condensation leading to chondrocyte differentiation and cartilage tissue formation, which serves as a template for future bone. The cartilage matrix calcifies, followed by blood vessel invasion, leading to eventual bone formation. During intramembraneous ossification, progenitor cells differentiate to osteoblasts directly within a condensed mesenchyme. Recent studies have shown that β-catenin activity plays a crucial role in regulating the pathways of endochondral versus intramembraneous bone formation.33–36 Elevated β-catenin signaling induces Runx2, resulting in osteoblast differentiation, while reduced β-catenin signaling has the opposite effects on gene expression, inducing chondrogenesis.34,37 We demonstrate that the hESC-derived mesenchymal cells differentiated toward an osteogenic phenotype and produced in vivo bone tissues through two different pathways depending upon the local cues present in their microenvironment. Our ectopic bone formation model with subcutaneous transplantation of hESC-derived mesenchymal cells demonstrated that the HA microenvironment upregulated Runx2 and resulted in bone formation through an intramembraneous ossification pathway. hESC-derived mesenchymal cells seeded on PLGA/PLLA alone, however, produced minimal levels of Runx2. These cells differentiated via endochondral ossification, as evidenced by the formation of cartilaginous tissue, followed by calcification and increased blood vessel invasion. This clearly demonstrates that the osteogenic potential of hESC-derived mesenchymal cells is highly dependent on the physical and chemical architecture and surface properties of the polymeric scaffolds. Molecular mechanisms and signaling pathways that regulate the osteogenic differentiation and bone formation on scaffolds and in vivo have not been clearly elucidated.38 However, our data indicate that two distinct mechanisms of bone formation via mesenchymal precursor cells from hESCs can be mediated by scaffold properties, demonstrating the importance of scaffold characteristics on the modulation of hESC-derived mesenchymal cells to engineer organized in vivo bone tissue.
Angiogenesis plays an important role during the development and maturation of bone.39 Induction of angiogenesis by biochemical stimulation or coculture with endothelial cells has been shown to induce better tissue formation as well as survival of engineered tissues.40–42 Recent studies emphasize the need for prevascularization of engineered tissues through endothelial coculture to optimize angiogenesis.43 However, our data indicate that hESC-derived mesenchymal cells, in addition to organizing 3D bone tissues, contributed to in vivo vascularization of the engineered tissues. Most of the capillary-like network structures originated from host tissue, but donor cell-derived blood vessel networks were evident in the engineered cartilage tissue undergoing endochondral ossification. Studies by Kaigler et al. demonstrated the endothelial differentiation of marrow-derived MSCs.41 Therefore, the hESC-derived mesenchymal cells may have contributed to vessel formation by direct endothelial cell differentiation. On the other hand, hESC-derived mesenchymal cells may also contain a subpopulation of endothelial progenitor cells, as evident by the presence of a population of cells expressing CD146.12
In the present work, we demonstrated the biomaterial-directed in vivo tissue formation of hESC-derived mesenchymal cells. Further, we observed that the duration of predifferentiation and components of medium influenced the outcome of in vivo tissue formation by hESC-derived mesenchymal cells. These hESC-derived cells, which have a significant proliferative and differentiation capacity, have the potential to significantly improve bone regeneration. In addition, these cells may provide a tool for elucidating the mechanism of lineage commitment specification of embryonic-derived cells in combination with the biomaterials.
Supplementary Material
Acknowledgments
This work was supported by funding from the NIDCR R01DE016887 and the Basic Science Research Program through the National Research Foundation of Korea (Grant Number 0458-20120013). We also gratefully acknowledge J.M. McCaffery and E. Perkins of the Integrated Imaging Facility (JHU Department of Biology) for help with electron microscopy.
Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Fehrer C. Lepperdinger G. Mesenchymal stem cell aging. Exp Gerontol. 2005;40:926. doi: 10.1016/j.exger.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Schnabel M. Marlovits S. Eckhoff G. Fichtel I. Gotzen L. Vecsei V., et al. Dedifferentiation-associated changes in morphology and gene expression in primary human articular chondrocytes in cell culture. Osteoarthritis Cartilage. 2002;10:62. doi: 10.1053/joca.2001.0482. [DOI] [PubMed] [Google Scholar]
- 3.D'Ippolito G. Schiller P.C. Ricordi C. Roos B.A. Howard G.A. Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J Bone Miner Res. 1999;14:1115. doi: 10.1359/jbmr.1999.14.7.1115. [DOI] [PubMed] [Google Scholar]
- 4.Sharma B. Elisseeff J.H. Engineering structurally organized cartilage and bone tissues. Ann Biomed Eng. 2004;32:148. doi: 10.1023/b:abme.0000007799.60142.78. [DOI] [PubMed] [Google Scholar]
- 5.Lerou P.H. Daley G.Q. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Thomson J.A. Itskovitz-Eldor J. Shapiro S.S. Waknitz M.A. Swiergiel J.J. Marshall V.S., et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 7.Lee E.J. Lee H.N. Kang H.J. Kim K.H. Hur J. Cho H.J., et al. Novel embryoid body-based method to derive mesenchymal stem cells from human embryonic stem cells. Tissue Eng Part A. 2010;16:705. doi: 10.1089/ten.tea.2008.0596. [DOI] [PubMed] [Google Scholar]
- 8.Barberi T. Willis L.M. Socci N.D. Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. doi: 10.1371/journal.pmed.0020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivier E.N. Rybicki A.C. Bouhassira E.E. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914. doi: 10.1634/stemcells.2005-0648. [DOI] [PubMed] [Google Scholar]
- 10.Lian Q. Lye E. Suan Yeo K. Khia Way Tan E. Salto-Tellez M. Liu T.M., et al. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425. doi: 10.1634/stemcells.2006-0420. [DOI] [PubMed] [Google Scholar]
- 11.Brown S.E. Tong W. Krebsbach P.H. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2009;189:256. doi: 10.1159/000151746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang N.S. Varghese S. Lee H.J. Zhang Z. Ye Z. Bae J., et al. In vivo commitment and functional tissue regeneration using human embryonic stem cell-derived mesenchymal cells. Proc Natl Acad Sci U S A. 2008;105:20641. doi: 10.1073/pnas.0809680106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levenberg S. Huang N.F. Lavik E. Rogers A.B. Itskovitz-Eldor J. Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci U S A. 2003;100:12741. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren J. Ren T. Zhao P. Huang Y. Pan K. Repair of mandibular defects using MSCs-seeded biodegradable polyester porous scaffolds. J Biomater Sci Polym Ed. 2007;18:505. doi: 10.1163/156856207780852578. [DOI] [PubMed] [Google Scholar]
- 15.Ishaug S.L. Yaszemski M.J. Bizios R. Mikos A.G. Osteoblast function on synthetic biodegradable polymers. J Biomed Mater Res. 1994;28:1445. doi: 10.1002/jbm.820281210. [DOI] [PubMed] [Google Scholar]
- 16.Holland T.A. Mikos A.G. Biodegradable polymeric scaffolds. Improvements in bone tissue engineering through controlled drug delivery. Adv Biochem Eng Biotechnol. 2006;102:161. doi: 10.1007/b137205. [DOI] [PubMed] [Google Scholar]
- 17.Luklinska Z.B. Schluckwerder H. In vivo response to HA-polyhydroxybutyrate/polyhydroxyvalerate composite. J Microsc. 2003;211:121. doi: 10.1046/j.1365-2818.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y.X. Ren J. Chen C. Ren T.B. Zhou X.Y. Preparation and properties of poly(lactide-co-glycolide) (PLGA)/nano-hydroxyapatite (NHA) scaffolds by thermally induced phase separation and rabbit MSCs culture on scaffolds. J Biomater Appl. 2008;22:409. doi: 10.1177/0885328207077632. [DOI] [PubMed] [Google Scholar]
- 19.Cowan C.M. Shi Y.Y. Aalami O.O. Chou Y.F. Mari C. Thomas R., et al. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560. doi: 10.1038/nbt958. [DOI] [PubMed] [Google Scholar]
- 20.Toquet J. Rohanizadeh R. Guicheux J. Couillaud S. Passuti N. Daculsi G., et al. Osteogenic potential in vitro of human bone marrow cells cultured on macroporous biphasic calcium phosphate ceramic. J Biomed Mater Res. 1999;44:98. doi: 10.1002/(sici)1097-4636(199901)44:1<98::aid-jbm11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 21.Mankani M.H. Kuznetsov S.A. Marshall G.W. Robey P.G. Creation of new bone by the percutaneous injection of human bone marrow stromal cell and HA/TCP suspensions. Tissue Eng Part A. 2008;14:1949. doi: 10.1089/ten.tea.2007.0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowan C.A. Klimanskaya I. McMahon J. Atienza J. Witmyer J. Zucker J.P., et al. Derivation of embryonic stem-cell lines from human blastocysts. N Engl J Med. 2004;350:1353. doi: 10.1056/NEJMsr040330. [DOI] [PubMed] [Google Scholar]
- 23.Siddappa R. Martens A. Doorn J. Leusink A. Olivo C. Licht R., et al. cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc Natl Acad Sci U S A. 2008;105:7281. doi: 10.1073/pnas.0711190105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamashita A. Nishikawa S. Rancourt D.E. Microenvironment modulates osteogenic cell lineage commitment in differentiated embryonic stem cells. PLoS One. 5:e9663. doi: 10.1371/journal.pone.0009663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen W. Zhou H. Weir M.D. Tang M. Bao C. Xu H. Human embryonic stem cell-derived mesenchymal stem cell seeding on calcium phosphate cement-chitosan-RGD scaffold for bone repair. Tissue Eng Part A. 2013;19:915. doi: 10.1089/ten.tea.2012.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jukes J.M. Both S.K. Leusink A. Sterk L.M. van Blitterswijk C.A. de Boer J. Endochondral bone tissue engineering using embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:6840. doi: 10.1073/pnas.0711662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Domev H. Amit M. Laevsky I. Dar A. Itskovitz-Eldor J. Efficient engineering of vascularized ectopic bone from human embryonic stem cell-derived mesenchymal stem cells. Tissue Eng Part A. 2012;18:2290. doi: 10.1089/ten.TEA.2011.0371. [DOI] [PubMed] [Google Scholar]
- 28.Elisseeff J. Ferran A. Hwang S. Varghese S. Zhang Z. The role of biomaterials in stem cell differentiation: applications in the musculoskeletal system. Stem Cells Dev. 2006;15:295. doi: 10.1089/scd.2006.15.295. [DOI] [PubMed] [Google Scholar]
- 29.Kim H.N. Jiao A. Hwang N.S. Kim M.S. Kang D.H. Kim D.H., et al. Nanotopography-guided tissue engineering, regenerative medicine. http://dx.doi.org/10.1016/j.addr.2012.07.014. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2012.07.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin H. Jo S. Mikos A.G. Biomimetic materials for tissue engineering. Biomaterials. 2003;24:4353. doi: 10.1016/s0142-9612(03)00339-9. [DOI] [PubMed] [Google Scholar]
- 31.Ambrosio A.M. Sahota J.S. Khan Y. Laurencin C.T. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J Biomed Mater Res. 2001;58:295. doi: 10.1002/1097-4636(2001)58:3<295::aid-jbm1020>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 32.Marra K.G. Szem J.W. Kumta P.N. DiMilla P.A. Weiss L.E. In vitro analysis of biodegradable polymer blend/hydroxyapatite composites for bone tissue engineering. J Biomed Mater Res. 1999;47:324. doi: 10.1002/(sici)1097-4636(19991205)47:3<324::aid-jbm6>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 33.Kolpakova E. Olsen B.R. Wnt/beta-catenin—a canonical tale of cell-fate choice in the vertebrate skeleton. Dev Cell. 2005;8:626. doi: 10.1016/j.devcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 34.Day T.F. Guo X. Garrett-Beal L. Yang Y. Wnt/beta-catenin signaling in mesenchymal progenitors controls osteoblast and chondrocyte differentiation during vertebrate skeletogenesis. Dev Cell. 2005;8:739. doi: 10.1016/j.devcel.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 35.Hill T.P. Spater D. Taketo M.M. Birchmeier W. Hartmann C. Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes. Dev Cell. 2005;8:727. doi: 10.1016/j.devcel.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 36.Tamamura Y. Otani T. Kanatani N. Koyama E. Kitagaki J. Komori T., et al. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem. 2005;280:19185. doi: 10.1074/jbc.M414275200. [DOI] [PubMed] [Google Scholar]
- 37.Gaur T. Lengner C.J. Hovhannisyan H. Bhat R.A. Bodine P.V. Komm B.S., et al. Canonical WNT signaling promotes osteogenesis by directly stimulating Runx2 gene expression. J Biol Chem. 2005;280:33132. doi: 10.1074/jbc.M500608200. [DOI] [PubMed] [Google Scholar]
- 38.Heng B.C. Cao T. Stanton L.W. Robson P. Olsen B. Strategies for directing the differentiation of stem cells into the osteogenic lineage in vitro. J Bone Miner Res. 2004;19:1379. doi: 10.1359/JBMR.040714. [DOI] [PubMed] [Google Scholar]
- 39.Gerber H.P. Vu T.H. Ryan A.M. Kowalski J. Werb Z. Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999;5:623. doi: 10.1038/9467. [DOI] [PubMed] [Google Scholar]
- 40.Murphy W.L. Peters M.C. Kohn D.H. Mooney D.J. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 41.Kaigler D. Krebsbach P.H. West E.R. Horger K. Huang Y.C. Mooney D.J. Endothelial cell modulation of bone marrow stromal cell osteogenic potential. Faseb J. 2005;19:665. doi: 10.1096/fj.04-2529fje. [DOI] [PubMed] [Google Scholar]
- 42.Kaigler D. Wang Z. Horger K. Mooney D.J. Krebsbach P.H. VEGF scaffolds enhance angiogenesis and bone regeneration in irradiated osseous defects. J Bone Miner Res. 2006;21:735. doi: 10.1359/jbmr.060120. [DOI] [PubMed] [Google Scholar]
- 43.Levenberg S. Rouwkema J. Macdonald M. Garfein E.S. Kohane D.S. Darland D.C., et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.