Abstract
The diaphragm muscle (DIAm) is responsible for breathing and determines the ability to generate both ventilatory and non-ventilatory behaviors. Size limitations of the mouse make transdiaphragmatic pressure (Pdi) measurement using a dual balloon system untenable. Adult C57BL/6J mice (n=8) and C57BL/6 × 129 (n=9), underwent Pdi measurements using solid-state pressure catheters spanning the thoracic and abdominal surfaces of the DIAm. Measurements were conducted during eupnea, hypoxia (10% O2)–hypercapnia (5% CO2), chemical airway stimulation (i.e., sneezing), spontaneously occurring deep breaths, sustained tracheal occlusion, and bilateral phrenic nerve stimulation. There was a difference in the Pdi generated across the range of ventilatory and non-ventilatory behaviors (p=0.001). No difference in Pdi across behaviors was evident between mouse strains (p=0.161). This study establishes a novel method to determine Pdi across a range of DIAm behaviors in mice that may be useful in evaluating conditions associated with reduced ability to perform expulsive, non-ventilatory behaviors.
Keywords: diaphragm muscle, mouse strain, phrenic nerve, respiratory compliance
1. Introduction
The diaphragm muscle (DIAm) is the primary muscle of inspiration and is necessary to sustain ventilation throughout the lifespan. The DIAm is also essential in generating expulsive, non-ventilatory behaviors necessary for airway clearance. Transdiaphragmatic pressure (Pdi) measurements have been a useful tool used in a number of species to determine the DIAm force during both ventilatory and non-ventilatory behaviors. Such measurements are commonly performed using a dual balloon catheter system (ATS/ERS Statement on respiratory muscle testing, 2002), however the size of the mouse makes measuring Pdi difficult with this method. Recently the availability of solid-state pressure transducers has made this size limitation of the mouse less of an obstacle and thus Pdi measurements may be possible in mice.
To date Pdi has been used clinically as well as in pigs, cats, hamsters, and rats as a surrogate for DIAm force (Mantilla et al., 2010; Sieck, 1991; Watchko et al., 1986). Pdi measurements can be conducted across a range of DIAm behaviors, from eupneic breathing to maximal DIAm activation induced by sneezing (Mantilla et al., 2010) and gagging (Sieck et al., 1989). Indeed, Pdi is significantly correlated to the peak root mean squared EMG amplitude across a range of ventilatory and non-ventilatory DIAm behaviors in rats (Mantilla et al., 2010). Substantial reserve capacity for generating maximal behaviors beyond quiet ventilation is evident in force, root mean squared EMG and Pdi. In humans, cats, rats and hamsters, quiet breathing can be accomplished by generating 10, 12, 21 and 27% of maximal Pdi (Mantilla et al., 2010; Sieck, 1994). The relationship between Pdi and ventilation is influenced by mechanical components of the respiratory system. Thus, in this study respiratory system, chest, and lung mechanics were measured in mice to aid in interpretation of Pdi results.
Characterizing the function of respiratory muscles and specifically Pdi in mice is important in determining how DIAm function is impaired by injury or disease. The purpose of this study was to develop a novel method to measure Pdi in mice across ventilatory and non-ventilatory behaviors. Two commonly used mouse models, C57BL/6J and C57BL/6 × 129 mice, were evaluated. Many studies use 129 mice to isolate embryonic stem cells for genetic manipulation, which are backcrossed and maintained on a C57BL/6 × 129 mixed background.
2. Methods
2.1. Animals
Adult (6 month old) male mice were used for all experimental groups. The comparison of strains consisted of C57BL/6J (C57, Jax stock # 000664; n=8) purchased from Jackson Laboratories (Bar Harbor, ME) and C57BL/6 × 129 mice (C57×129, n=9) bred and maintained at the Mayo Clinic. Mice were group housed by genotype, maintained on a 12-hour light cycle with free access to food and water. At the terminal experiment, mice were weighed (average body mass: 32.0±0.8 g, p=0.451), anesthetized by an intramuscular injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) and euthanized by exsanguination. All protocols were approved by the Institutional Animal Care and Use Committee at the Mayo Clinic, in compliance with National Institute of Health guidelines.
2.2. Transdiaphragmatic pressure measurements
Methodology for Pdi measurements was adapted from previous studies (Mantilla et al., 2010; Sieck, 1994). Briefly, the abdomen was tightly bound and the trachea cannulated (19 G) while mice maintained spontaneous ventilation. Two 3.5 French Millar solid-state pressure catheters (SPR-524; Millar Instruments, Houston TX) were then inserted through the mouth into the esophagus and stomach, spanning the thoracic and abdominal surfaces of the DIAm, respectively. Correct catheter position was determined based on the signal deflection and postmortem analysis.
Measurements were collected during the following conditions: 1) breathing of room air (eupnea) for 5 min, 2) exposure to hypoxia (10% O2)-hypercapnia (5% CO2) for 5 min, 3) sustained tracheal occlusion for 15 s, 4) bilateral phrenic nerve stimulation (0.5 ms duration pulses at 75 or 150 Hz in 300 ms trains repeated each s) using straight bipolar electrodes (FHC, Bowdoin ME), and 5) stimulation of the nasal airway (i.e., sneezing) induced chemically by intranasal infusion of 10 μl of 30 μM capsaicin. Mice were allowed ~5 min intervals between interventions to allow for Pdi amplitude to return to eupneic values.
Intra -thoracic and -abdominal pressures were measured independently and recorded with a PowerLab 8/35 data acquisition system with an integrated amplifier following the manufacturer recommended calibration procedure. Data was analyzed using LabChart (Millar Instrumentation), band-pass filtered (0.3 – 30 Hz), and sampled at 100 Hz. Data from LabChart was exported to MATLAB for custom-designed automated analyses of peak amplitude and corresponding baseline. Baseline values were determined for each peak from the average of all inflection points in the segment preceding each peak, such that peak amplitude was the difference between the two values. Data was analyzed and average across behaviors for 1 minute of eupnea, 1 minute of hypoxia-hypercapnia, the 5 maximal breaths during occlusion, all spontaneous deep breaths, all sneezes, and the maximal value obtained during stimulation (representative tracings in Fig. 1). Deep breaths were defined as spontaneously occurring inspiratory event that were ~2x eupneic Pdi amplitude (Mantilla et al., 2010). Breathing frequency during both eupnea and hypoxia-hypercapnia was calculated.
Figure 1.
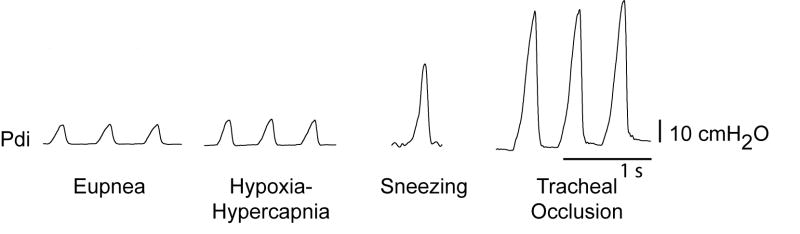
Representative transdiaphragmatic pressure (Pdi) tracings from adult 6 month old mice during eupnea (breathing room air), hypoxia (10% O2)-hypercapnia (5% CO2), sneezing (induced by intranasal capsaicin), and sustained tracheal occlusion (15 s).
2.3. Respiratory system mechanics
Following Pdi measurements, mice were connected via tracheal cannula to the flexiVent computer controlled ventilator system (SCIREQ; Montreal Canada). Analyses of respiratory system compliance were conducted following manufacturer recommendations, while mechanically ventilated (10 ml/kg). Measurements were collected with the chest wall intact and subsequently in the isolated lung following a midline sternotomy. Thus, respiratory system, chest, and lung mechanics were obtained. Briefly, mechanics were assessed during a 1.2-second, 2.5 Hz forced oscillation maneuver (Snapshot-150 v5.2) followed by a 3-second low frequency forced oscillation containing mutually prime frequencies between 1–20.5 Hz (QuickPrime-3 v5.2). Data were fit with a constant phase model, and only included if a coefficient of determination > 0.95 was achieved.
2.4. Statistical analysis
Data were analyzed by repeated-measures two-way ANOVA with Tukey-Kramer honestly significant difference post hoc tests, when appropriate, based on mouse strain and motor behavior (Pdi) and one-way ANOVA for mechanical measurement across strains. Correlation between eupneic Pdi and respiratory system resistances was assessed using Spearman’s rank order test. Statistical analyses were conducted using JMP (Version 8.0; SAS Institute, Cary NC); data are presented as mean ± standard error (SE). Significance was accepted at the α < 0.05 level.
3. Results
3.1. Transdiaphragmatic pressure
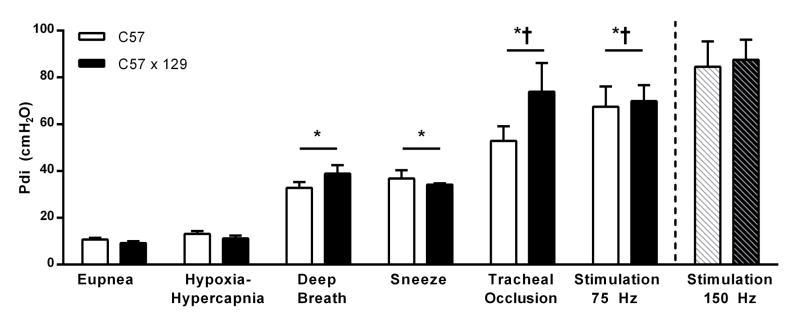
There was no difference in the Pdi generated between mouse strains (two-way ANOVA, strain × behavior; interaction p=0.297, strain p=0.161; Fig. 2). However, there was a difference in the Pdi generated across behaviors (main effect p=0.001; Fig. 2). During ventilatory behaviors the average (across mouse stains) Pdi generated was 9.9±0.6 and 12.2±0.9 cmH2O for eupnea and hypoxia-hypercapnia, respectively although there was no difference between these two ventilatory behaviors. Of note, there was a trend for an increase in breathing frequency between eupnea and hypoxia-hypercapnia from 155±7 to 165±8 min−1, with no differences across strains (two-way ANOVA, strain × behavior; interaction p=0.969, strain p=0.824, behavior p=0.344).
Figure 2.
The transdiaphragmatic pressure (Pdi) generated during ventilatory behaviors: eupnea, hypoxia (10% O2)-hypercapnia (5% CO2), spontaneous deep breaths, and non-ventilatory behaviors: sneezing, tracheal occlusion behaviors for C57BL/6J (C57; n=8) and C57BL/6 × 129 (C57×129; n=9) mice. Additionally, Pdi generated during bilateral phrenic nerve stimulation at 75 Hz (n=7 for each strain) and the estimated maximal stimulation at 150 Hz (noted with dashed bars; n=3). Data analyzed by repeated-measures two-way ANOVA (strain × behavior) interaction p=0.910, strain p=0.158; behavior p=0.001, presented as mean ± SE. *Significantly different than eupnea and hypoxia-hypercapnia; †significantly different than deep breaths and sneezing.
Naturally occurring deep breaths occurred in all mice except two C57 mice; on average spontaneous deep breaths generate 37.3±2.5 cmH2O. On average sneezing generated 35.9±2.2 cmH2O, chemical stimulation to induce sneezing was only achieved in 11 of the 17 mice tested. There was no significant difference in the Pdi generated between deep breaths and sneezing, but the Pdi generated in these behaviors was significantly greater than that generated during eupnea and hypoxia-hypercapnia.
On average, tracheal occlusion generated 63.5±7.0 cmH2O and bilateral phrenic nerve stimulation at 75 Hz generated 71.0±5.7 cmH2O. Consistent and repeatable phrenic nerve stimulation could be accomplished at frequencies up to 75 Hz, but stimulation at higher frequencies in vivo was less reliable. Of note, maximum isometric force was elicited at 150 Hz stimulation in ex vivo DIAm strips tested in a force-frequency protocol of 1-s trains of stimuli between 5–175 Hz at 37°C (n=4 mice). The difference in force between 75 and 150 Hz was found to be 23.4% in these muscle strips. In a subset of mice (n=3), bilateral phrenic nerve stimulation was conducted at both 75 and 150 Hz and indeed Pdi was 26.9% greater at 150 Hz vs. 75 Hz. The estimated Pdi with maximal bilateral phrenic nerve stimulation at 150 Hz was 89.0±7.1 cmH2O (Fig. 2).
3.2. Respiratory system mechanics
There were no differences in respiratory system resistances or compliances between mouse strains (Table 1). The isolated lung comprised ~80% of the resistance of the entire respiratory system and the chest wall comprised ~20%. There was no significant correlation between respiratory system resistance and Pdi generated during eupnea in adult mice (rs=0.017; p=0.948). Furthermore, there was not a significant relationship between the isolated lung resistance and Pdi (rs = −0.200; p=0.493). The compliance of both the respiratory system and lung was not different between mouse strains. There was no significant correlation between Pdi generated during eupnea in adult mice and respiratory system compliance (rs=0.096; p=0.715) or lung compliance (rs =0.191; p=0.513).
Table 1.
Respiratory system mechanics in adult mice
| C57 | C57Bx129 | ||
|---|---|---|---|
| Resistance (cmH2O s•ml−1) | p-value | ||
| Respiratory System | 2.0 ± 0.2 | 1.6 ± 0.1 | 0.207 |
| Isolated Lung | 1.5 ± 0.2 | 1.2 ± 0.2 | 0.403 |
| Chest Wall | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.671 |
| Compliance (ml/cmH2O) | p-value | ||
| Respiratory System | 0.021 ± 0.001 | 0.019 ± 0.002 | 0.180 |
| Isolated Lung | 0.024 ± 0.001 | 0.023 ± 0.002 | 0.164 |
Mechanics of the respiratory system and its individual components analyzed in both C57BL/6J (C57; n=8) and C57BL/6×129 (C57×129; n=9) mice were assessed using a 1.2-second, 2.5 Hz forced oscillation maneuver and a 3-second low frequency forced oscillation of mutually prime frequencies between 1–20.5 Hz. Data analyzed by one-way ANOVA, presented as mean ± SE.
4. Discussion
This study characterizes and establishes Pdi as a useful tool to examine DIAm force during ventilatory and non-ventilatory behaviors in mice. Measurements of Pdi are important in studies examining the functional impact of respiratory and neuromuscular disease. One objective of this study was to compare Pdi in two highly utilized mouse strains (C57BL/6J and C57BL/6 × 129 mice); we determined no difference in Pdi between strains across a range of ventilatory and non-ventilatory behaviors. Furthermore, we established a novel method to determine Pdi in mice, which is based on solid-state pressure transducers that overcome technical limitations imposed by previous methods.
The most common method for determining Pdi is with dual balloon manometry, usually based on catheters spanning the DIAm surface. This method is outlined in a joint position statement by the American Thoracic and the European Respiratory Societies (ATS/ERS Statement on respiratory muscle testing, 2002). The benefits of balloon catheters relate to limited air gaps between the visceral wall (esophageal or gastric) and the balloon during measurements. Indeed, we previously used balloon catheters to conduct measurements of Pdi in rats (Mantilla et al., 2010) and cats (Sieck et al., 1989). However, air- or even fluid-based manometry systems are easily compromised when using balloons and catheters of the small-diameter needed for placement in the mouse esophagus, and the relatively small volumes generated during breathing would be dampened substantially within the system. Thus, we utilized solid state-pressure transducers of sufficient size to permit ease of placement in the mouth and provide reliable pressure measurements. This study employed transducers that were 1.33 mm in diameter (3.5 French) without complications.
This study also provides the first report of the full range of DIAm motor behaviors in mice and thus contributes to the body of literature in other species. Maximal Pdi was determined using bilateral phrenic nerve stimulation. Eupneic Pdi in the mouse, cat, and human was between 10–12% of maximal Pdi, in comparison to the hamster and rat where eupneic Pdi was between 21–27% of maximal (Mantilla et al., 2010; Sieck, 1991; Sieck, 1994; Sieck et al., 1989). During hypoxia-hypercapnia, Pdi was ~12% in mice, while both rats and cats were ~27% (Mantilla et al., 2010; Sieck et al., 1989). Of note, sneezing produced near maximal Pdi in rats (93%; (Mantilla et al., 2010) and cats (110%; (Sieck et al., 1989), however this was not the case in mice. Only 11 of 17 mice responded to chemical stimulation, and sneezing produced ~40% of maximal Pdi. Sustained tracheal occlusion was much closer to maximum in mice, accounting for ~70% of maximal Pdi, similar to ~ 63% of maximal Pdi obtained by airway occlusion in rats (Mantilla et al., 2011; Mantilla et al., 2010). In contrast, tracheal occlusion in the hamster and cat generated 43% and 50% of maximal Pdi (Sieck et al., 1989).
Previous reports document differences in the DIAm across species, including neuromuscular transmission failure and ventilatory parameters (Sieck et al., 2012). In comparison to rats, mice have reduced susceptibility to neuromuscular transmission failure (reflecting greater proportion of fatigue-resistant fibers) as well as increased minute ventilation, tidal volume, and duty cycle. Thus, it was hypothesized that mice would have a more limited reserve capacity than rats, and as such the percent of maximal Pdi generated during ventilatory behaviors in mice would be greater than in rats. However this was not the case. In addition, mice display reduced lung compliance and increased resistance compared to rats (Gomes et al., 2000). These apparently contradictory results are likely reconciled by differences in intrinsic end-expiratory pressures. The frequency-dependence of airway impedance in the mouse is indeed such that low levels of positive end-expiratory pressures can be expected to shift lung volumes to a more compliant portion of the elastance curve. These findings are thus consistent with the higher respiratory frequency in mice and the relatively small increase in Pdi generated during exposure to hypoxia-hypercapnia in mice compared to other species. The relatively larger reserve capacity for expulsive behaviors in mice is inconsistent with reduced respiratory system resistance (normalized to body weight) compared to rats (Gomes et al., 2000). Most likely, the relatively small eupneic Pdi reflects the very high respiratory frequency, consistent with the high proportion of fatigue-resistant fibers (Sieck et al., 2012).
We have established Pdi as a novel method, which can be used to examine perturbations due to respiratory complication and muscle dysfunction. Future studies will benefit from the development and characterization of Pdi DIAm motor behaviors in mice, particularly in conditions of reduced ability to perform expulsive, non-ventilatory behaviors.
Highlights.
Novel method for transdiaphragmatic pressure (Pdi) measurements in mice
Bilateral phrenic nerve stimulation elicits maximal Pdi
Ventilatory behaviors in mice generate 10–12% of maximal Pdi
Tracheal occlusion in mice generates near maximal Pdi
Acknowledgments
We would like to acknowledge Barry J. Kenny and Juan S. Medina for technical assistance in the completion of this project. This research was supported by grants from National Institute of Health RO1-HL096750 (CBM & GCS) and T32-HL105355 (SMG), and the Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol. 2000;89:908–916. doi: 10.1152/jappl.2000.89.3.908. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Seven YB, Hurtado-Palomino JN, Zhan WZ, Sieck GC. Chronic assessment of diaphragm muscle EMG activity across motor behaviors. Respir Physiol Neurobiol. 2011;177:176–182. doi: 10.1016/j.resp.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol. 2012;180:88–96. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- Sieck GC. Neural control of the inspiratory pump. NIPS. 1991;6:260–264. [Google Scholar]
- Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med. 1994;15:641–659. [PubMed] [Google Scholar]
- Watchko JF, Mayock DE, Standaert A, Woodrum DE. Postnatal changes in transdiaphragmatic pressure in piglets. Pediatr Res. 1986;20:658–661. doi: 10.1203/00006450-198607000-00016. [DOI] [PubMed] [Google Scholar]