Abstract
Methamphetamine (METH) is a highly addictive drug that is also neurotoxic to central dopamine (DA) systems. Although striatal DA depletions induced by METH are associated with behavioral and cognitive impairments, the link between these phenomena remains poorly understood. Previous work in both METH-pretreated animals and the 6-hydroxydopamine model of Parkinson’s disease suggests that a disruption of phasic DA signaling, which is important for learning and goal-directed behavior, may be such a link. However, prior studies used electrical stimulation to elicit phasic-like DA responses and were also performed under anesthesia, which alters DA neuron activity and presynaptic function. Here we investigated the consequences of METH-induced DA terminal loss on both electrically evoked phasic-like DA signals and so-called “spontaneous” phasic DA transients measured by voltammetry in awake rats. Not ostensibly attributable to discrete stimuli, these sub-second DA changes may play a role in enhancing reward-cue associations. METH-pretreatment reduced tissue DA content in the dorsomedial striatum and nucleus accumbens by ~55%. Analysis of phasic-like DA responses elicited by reinforcing stimulation revealed that METH pretreatment decreased their amplitude and underlying mechanisms for release and uptake to a similar degree as DA content in both striatal subregions. Most importantly, characteristics of DA transients were altered by METH-induced DA terminal loss, with amplitude and frequency decreased and duration increased. These results demonstrate for the first time that denervation of DA neurons alters naturally occurring DA transients and are consistent with diminished phasic DA signaling as a plausible mechanism linking METH-induced striatal DA depletions and cognitive deficits.
Keywords: voltammetry, psychostimulant, striatum, rat, transient
Introduction
Methamphetamine (METH) is an addictive drug that causes forebrain dopamine (DA) denervation (Seiden et al., 1976; Hotchkiss & Gibb 1980; Ricaurte et al., 1980). In humans, METH-induced loss of DA transporter binding indicative of striatal DA terminal degradation has been correlated with decreased cognitive and motor functions (Volkow et al., 2001b). Chronic METH use in addicts has also been associated with impairments in memory and attention (Simon et al., 2000; Scott et al., 2007). Consistent with this research, animal studies of METH-induced neurotoxicity have documented long-term cognitive and behavioral sequelae (Chapman et al., 2001; Schroder et al., 2003; Belcher et al., 2005; Daberkow et al., 2005; Herring et al., 2008; Izquierdo et al., 2010; O’Dell et al., 2011). However, the relationship between METH-induced DA depletion and impaired behavior and cognition remains unclear.
Phasic DA signaling, in which DA neurons burst fire in response to primary rewards or their predictors and generate transient increases in extracellular DA, is important for learning in goal-directed behavior by encoding reward prediction error and modulating synaptic plasticity (Carelli & Wightman 2004; Calabresi et al., 2007; Schultz 2007). We have recently proposed a disruption in phasic DA signaling as a putative link between METH-induced striatal DA depletion and behavioral-cognitive impairment (Howard et al., 2011). This postulate is supported by decreased phasic-like DA responses measured by voltammetry in rodent models of Parkinson’s disease (PD) (Garris et al., 1997b; Bezard et al., 2000; Bergstrom & Garris 2003) and METH-induced neurotoxicity (Howard et al., 2011; Loewinger et al., 2012), neurocomputational models predicting cognitive deficits in PD arising from diminished phasic DA transients (Frank et al., 2004; Wiecki & Frank 2010), and reduced striatal signals representing reward prediction error measured in PD patients by functional magnetic resonance imaging (Schonberg et al., 2010).
Not all evidence is consistent with this postulate. While decreases in phasic-like DA responses are associated with the absence of up-regulated DA release (Garris et al., 1997b; Bergstrom & Garris 2003; Howard et al., 2011), other studies report robust DA denervation-induced compensatory increases (Stachowiak et al., 1987; Snyder et al., 1990; Bergstrom et al., 2011), particularly in monkey (McCallum et al., 2006; Perez et al., 2008). Moreover, phasic DA decrements have only been directly observed for electrically evoked signals under anesthesia, and recent microelectrode recordings collected in the context of surgery for deep brain stimulation indicate that surviving DA neurons in awake PD patients phasically fire (Zaghloul et al., 2009) and release DA (Kishida et al., 2011), although comparisons to healthy subjects were not made. There is thus a need to establish the status of phasic DA signaling following DA denervation. Here we use voltammetry to assess the effects of METH-induced neurotoxicity on electrically evoked phasic-like DA signals and “spontaneous” DA transients in awake, freely behaving rats. Not ostensibly associated with discrete stimuli temporally, these phasic DA events may drive reward-cue associations (Robinson et al., 2002; Willuhn et al., 2010). We demonstrate for the first time under any DA-depleting condition a disruption of naturally occurring phasic DA signaling.
Materials and methods
Animals
Adult male Sprague-Dawley rats (250 to 400 g) were purchased from Harlan (Indianapolis, IN, USA) and were housed in a light- and temperature-controlled vivarium. Access to food and water was provided ad libitum. All procedures conform to the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Illinois State University.
Drugs and reagents
(±)-Methamphetamine hydrochloride was provided by the National Institute on Drug Abuse (Rockville, MD, USA). All other reagents were purchased from Sigma Chemical Company RBI-Sigma (St. Louis, MO, USA).
Neurotoxic METH regimen
Rats were placed in plastic housing tubs (50 cm length × 40 cm width × 20 cm height: 4 rats/tub). Injections of saline or (±)-methamphetamine hydrochloride (7.5 mg/kg s.c. in 0.9% saline; calculated as free base) occurred every two hours until a total of four injections were administered. Core body temperature was monitored rectally with a Thermalert TH-5 thermometer (Physitemp, Clifton, NJ, USA) every hour during injections and for two additional hours after the final injection.
In vivo DA measurements
Extracellular DA levels were monitored in the striatum of freely-behaving rats by fast-scan cyclic voltammetry (FSCV) as previously described (Garris et al., 1997a; Garris et al., 2003). Approximately two weeks following METH pretreatment, rats were anesthetized using ketamine (80 mg/kg i.p.) and xylazine (60 mg/kg i.p.) and were secured in a stereotactic frame (David Kopf Instruments, Tujunga, CA, USA). A twisted, bipolar stimulating electrode (Plastics One, Roanoke, VA, USA) was chronically implanted in the medial forebrain bundle and a guide hub was placed ipsilaterally to allow attachment of a microdrive for lowering a fresh carbon-fiber microelectrode (CFM) into the striatum during subsequent recording sessions. A Ag/AgCl reference electrode was affixed in the contralateral cortex. After recovery from surgery and at least three weeks following METH-pretreatment, animals were placed in a walled-off, open-field chamber (40 cm length × 40 cm width × 21 cm height), and a headstage and stimulating-electrode cable were connected to the implanted stimulating and reference electrodes. A fresh CFM was also connected and lowered into the striatum (+1.2 AP, +1.5 ML, -4.5 DV) (Paxinos & Watson 1986). FSCV consisted of a triangular waveform (-0.4 to 1.3 V and back, 400 V/s) applied at a rate of 10 Hz. Current measured at the peak oxidative potential for DA was converted to DA concentration based on post-calibration of the CFM using flow-injection analysis (Logman et al., 2000). Background-subtracted cyclic voltammograms were calculated by subtracting an average of baseline voltammograms collected immediately prior to the evoked response or spontaneous transient from voltammograms averaged over the peak of these events (Michael et al., 1998; Robinson et al., 2002).
Experimental design and transient analysis
A total of 5 saline- and 7 METH-pretreatment animals were used in this study. In each animal, 7 sites were recorded in the dorsomedial striatum (DMS) (-4.85 to -6.05 DV, 0.2 mm increments) and 8 in the nucleus accumbens (NAc) (-6.25 to -7.65 DV, 0.2 mm increments; Fig. 1A). At each site, a stimulus pulse (±125 μA and 2 ms each phase) train (60 Hz and 0.4 s) was applied at the beginning of each recording epoch. These signals were analyzed for specific characteristics of the evoked phasic-like DA response (see below). The remainder of the 5-min epoch was used for analysis of spontaneous DA transients. Principal component regression (PCR) was used to determine changes specific to DA in each 5-min FSCV recording (Keithley et al., 2009). At least one recording site with robust DA release in each animal was also used to collect a PCR training set, which consisted of at least 5 stimulus trains (typically 60 Hz, 24 pulses; 60 Hz, 12 pulses; 60 Hz, 6 pulses; 30 Hz, 24 pulses; 30 Hz, 12 pulses; (Heien et al., 2005). Figure 1B shows an example of the PCR output, where a FSCV recording (black trace) collected at the oxidation potential for DA (white dotted line, below) is separated into three components: changes in CFM background (drift; red), pH (blue), and DA (green). Below these traces is the composite pseudocolor plot showing all background-subtracted cyclic voltammograms collected in time. This FSCV recording was selected to show a pronounced background drift for illustrative purposes. DA transients were analyzed from the PCR output specific for DA changes, and were identified as signals greater than 5-times the root-mean-square-noise (Heien et al., 2005). Amplitude, duration (width at half amplitude), and intertransient interval (ITI or frequency-1) were determined using peak-finding software (MINI ANALYSIS; Synaptosoft, Decatur, GA, USA; Fig. 1C). All peaks identified by the signal-to-noise criterion of the PCR output were additionally verified as DA from the original FSCV record (Robinson et al., 2002).
Figure 1. Experimental design and analyses.
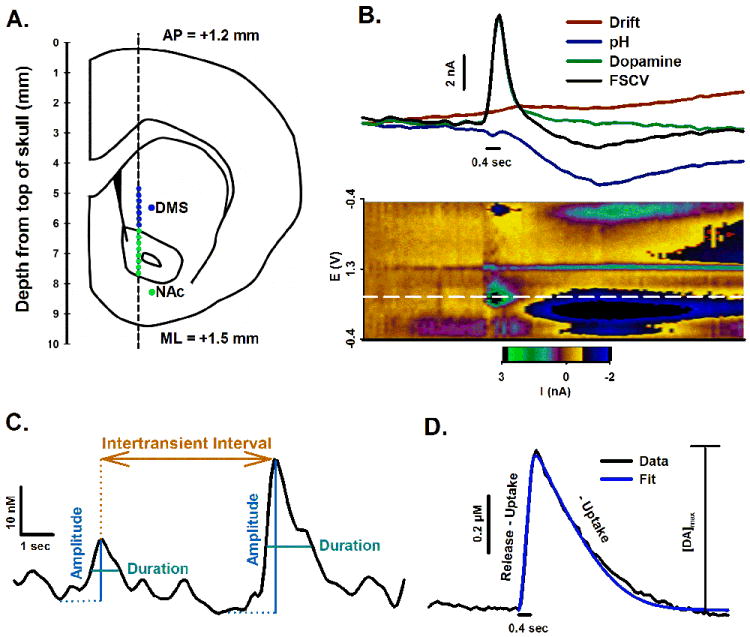
Recording locations were estimated from stereotaxic coordinates and top of skull (A). PCR separated the composite FSCV recording (black line) into individual components for CFM drift (red line), pH (blue line), and DA (green line) (B). Stimulus duration is shown by the short black line directly below traces. Underneath traces and stimulation is the pseudocolor plot, in which measured current (z axis) is plotted against applied potential (y axis) and time (x axis). The white line demarcates current collected at the peak oxidative potential for DA. Phasic DA transients were analyzed for amplitude (blue), duration (width at half amplitude, teal), and intertransient interval (frequency-1, orange) (C). Evoked phasic-like DA responses were analyzed for amplitude ([DA]max) and DA release and uptake (D). A simulation calculated from best-fit parameters for release and uptake is overlaid on an evoked DA trace. Stimulus duration is shown underneath.
Kinetic analysis
Evoked phasic-like DA signals were analyzed to determine the maximal concentration of evoked DA ([DA]max), and parameters for DA release and uptake (Fig. 3). This analysis is conceptually shown in Figure 1D. The rising phase of the electrically elicited response was considered to reflect the balance between the opposing mechanisms of release and uptake and the falling phase solely DA uptake according to (Wightman et al., 1988):
| (1) |
where [DA]p refers to the extracellular DA concentration released per each stimulus pulse, Vmax is the Michaelis-Menten term for maximal uptake rate, Km is the Michaelis-Menten term that is inversely proportional to the affinity of DA to the DA transporter, and f is the frequency of the stimulus train. Evoked responses were fit to Equation 1 using non-linear regression with a simplex minimization algorithm (Wu et al., 2001). Values for Vmax and [DA]p were determined while Km was fixed at a value of 0.2 μM.
Figure. 3. Effects of METH neurotoxicity on DA innervation and characteristics of phasic-like DA responses in the DMS and NAc.
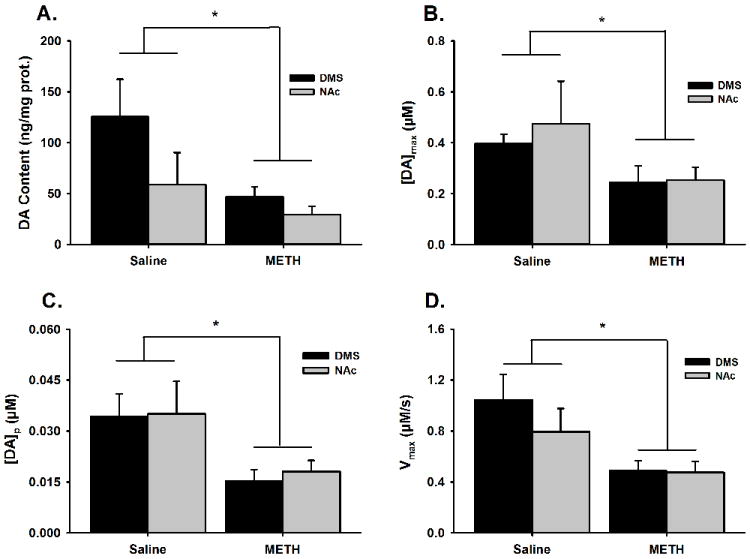
Panels show tissue DA content as an index of DA innervation (A), the amplitude of the evoked phasic-like signal or [DA]max (B), DA release or [DA]p (C), and DA uptake or Vmax (D). Data are expressed as the mean±SEM and were statistically analyzed by 2-way ANOVA. *p < 0.05 compared to saline-pretreated controls.
The time-dependent effects of electrical stimulation on [DA]p were modeled in Figure 6 using a variant of Equation 2 according to Montague et al. (2004):
| (2) |
where A0 represents the initial value of DA release (original [DA]p of Equation.1), f1 is short-term facilitation, d1 is short-term depression and d2 is long-term depression. The factors f1, d1 and d2 are initially 1, but increase (f) or decrease (d1,d2) multiplicatively by a kick factor with each stimulus pulse (1.01, 0.99, and 0.999, respectively). The factors f1, d1 and d2 also decay back to 1 in the time between stimulus pulses as governed by a time constant (4.41, 3.23 and 840 s, respectively). All values for facilitation and depression terms are from Bergstrom et al. (2011).
Figure 6. Analysis of the interaction between reinforcing electrical stimulation and DA transients.

Left Panels. Theoretical analysis of DA release according to Montague et al. (2004). Simulated electrically evoked DA response using Equation 2 and A0 = 0.03 μM, Km = 0.02 μM, and Vmax = 1.0 μM/s (A). Time course of variable [DA]p (B). Time course of facilitation (f1) and depression (d1 and d2) terms altering [DA]p (C). See Methods for details. Right Panels. Empirical analysis of DA transients. Transient amplitude (D). Intertransient interval (ITI) (E). Electrical stimulation was applied at 5 s of the 300-s recording epoch for characterizing DA transients; thus, 0-150 s is the stimulation bin and 150-300 s is the no-stimulation bin. Statistical analysis of the median was performed by Wilcoxon’s ranked sum test. n.s., not significant at p < 0.05.
Tissue DA Content
After recording sessions, brains were rapidly removed, chilled in a -20°C freezer for ~20 min, and then sliced fresh using a brain block and razor blades into 1-mm thick sections (Bergstrom & Garris 2003; Howard et al., 2011). Striatal portions (1 mm3) were dissected for upper and lower DMS and NAc, wet weighed, and placed in a -80°C freezer. Tissues were later homogenized in 0.1 N perchloric acid, and DA content was determined by high performance liquid chromatography using electrochemical detection (BAS 200B, Bioanalytical Systems, West Lafayette, IN, USA). The mobile phase was comprised of 0.5 g EDTA, 0.4 g octanesulfonic acid, 24.56 g monochloroacetic acid, 1.16 g sodium chloride, and 50 mL acetonitrile dissolved in 2 L of water (pH 2.8). Protein was determined colorimetrically using a commercial kit (Bio-Rad, Hercules, CA, USA). Tissue DA contents for upper and lower DMS were averaged for each animal.
Statistical analysis
Data sets were initially tested for normality by the Kolmogorov-Smirnov test. Data for tissue DA content and from electrically evoked DA responses were found to be normally distributed; hence, a parametric method was used to assess statistical differences. As such, tissue DA content, the maximal amplitude of the evoked DA response ([DA]max), DA release ([DA]p), and DA uptake (Vmax) were compared between METH- and saline-pretreated rats with 2-way ANOVA (Figs. 3A to D). In each animal, values were averaged across all recording sites in DMS or NAc. Data describing DA transients were found not to be normally distributed and could not be transformed into a normal distribution; hence, non-parametric methods were used to assess statistical differences. As such, distributions of transient amplitude, transient duration, and ITI were compared using the Kolmogorov-Smirnov two-sample test (Figs. 5A, B, D, E, G, and H) and group differences were compared by Wilcoxon’s ranked sum test of the median (Figs. 5C, F, and I and Figs. 6D and E). All DA transients collected in DMS or NAc were evaluated collectively. Statistical analyses were performed with SAS (SAS Institute Inc., Cary, NC, USA). The significance level was p ≤ 0.05.
Figure 5. Effects of METH neurotoxicity on characteristics of DA transients.
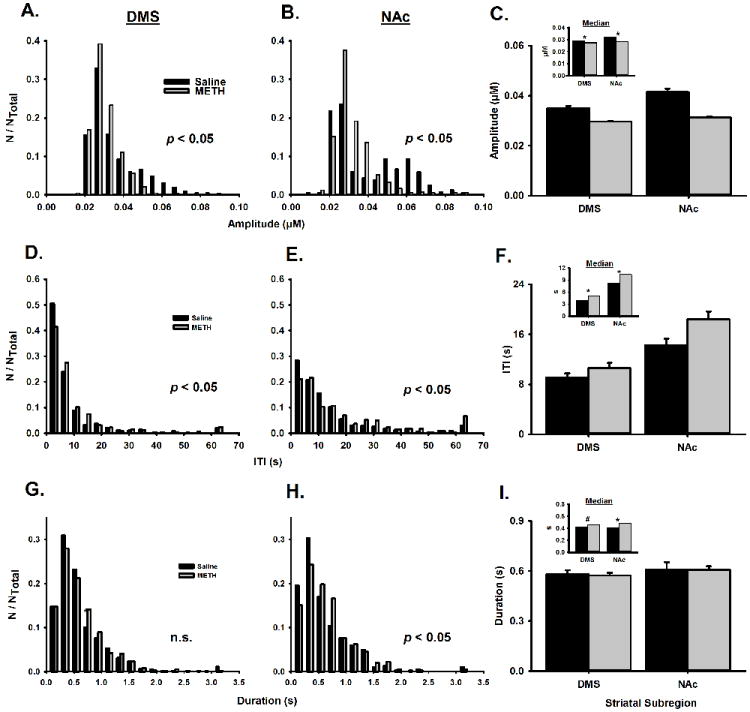
Histograms are shown for the amplitude (A and B), ITI (D and E), and duration (G and H) of DA transients collected in the DMS (left column) and NAc (right column) of saline- and METH-pretreated animals. The significance level is given for the Kolmogorow-Smirnov two-sample test. Bin size is 0.0058 μM for amplitude, 4 sec for ITI, and 0.2 sec for duration. Counts (N) in each bin are normalized to the total number of counts (NTotal). Mean±SEM and medium (INSET) are show for transient amplitude (C), ITI (F), and duration (I). Medians were statistically analyzed by Wilcoxon’s ranked sum test. *p < 0.05; #p < 0.1.
Results
Representative phasic-like DA responses
Figure 2 shows representative electrically evoked phasic-like responses collected in the DMS (Fig. 2A) and NAc (Fig. 2B). In both the DMS and NAc, METH pretreatment (right column) reduced the amplitude of the phasic-like DA response elicited by reinforcing electrical stimulation compared to saline pretreatment (left column). Voltammograms, as displayed serially in the pseudocolor plots beneath each evoked DA trace and individually in the INSET, are consistent with DA for each of the four recordings (Michael et al., 1998).
Figure 2. Effects of METH neurotoxicity on evoked phasic-like DA signals.
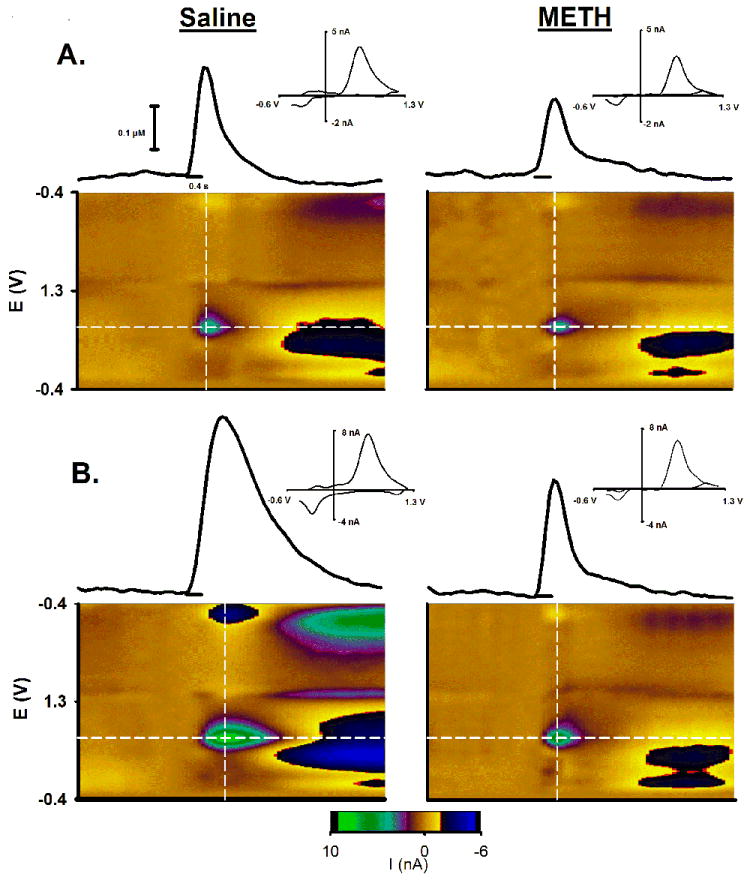
Representative evoked phasic-like DA signals in the DMS (A) and NAc (B) are shown for saline- and METH-pretreated animals (left and right columns, respectively). The FSCV recording (solid line) in the middle of each panel shows the evoked DA trace, with the pulse train demarcated by the shorter line underneath. Immediately below the trace and stimulation is the pseudocolor plot showing all background-subtracted cyclic voltammograms collected in this recording graphed sequentially in time. The evoked trace shown above the plot was collected at the peak oxidation potential for DA identified by the horizontal white line in the pseudocolor plot. Individual background subtracted cyclic voltammograms (above INSET) were determined at the apex of the evoked trace along the vertical white line in the pseudocolor plot. See Figure 1 for details of the pseudocolot plot.
DA innervation and characteristics of evoked phasic-like DA responses
Overall, in both the DMS and NAc, METH-pretreatment decreased DA innervation as indexed by tissue DA content, the amplitude of evoked phasic-like DA responses, and parameters of DA uptake and release. These data, shown in Figure 3, were analyzed by 2-way ANOVA. Figure 3A shows that METH-pretreatment significantly reduced tissue DA content to a similar degree in both striatal subregions (significant effect of pretreatment, F1,22 = 7.62, p = 0.01; no significant interaction, F1,22 = 1.61, p = 0.22), with tissue DA content being generally lower in the NAc compared to the DMS (significant effect of subregion, F1,22 = 4.61, p = 0.046). Figure 3B shows that METH-pretreatment significantly reduced the amplitude of evoked phasic-like DA responses ([DA]max) to a similar degree in both the DMS and NAc (significant effect of pretreatment, F1,24 = 4.46, p = 0.047; no significant interaction, F1,24 = 1.61, p = 0.73), with [DA]max being generally similar in the two striatal subregions (no significant effect of subregion, F1,24 = 0.24, p = 0.59). Figure 3C shows that METH-pretreatment significantly reduced DA release ([DA]p) to a similar degree in both the DMS and NAc (significant effect of pretreatment, F1,24 = 10.37, p = 0.004; no significant interaction, F1,24 = 0.03, p = 0.86), with [DA]p being generally similar in the two striatal subregions (no significant effect of subregion, F1,24 = 0.10, p = 0.75). Finally, Figure 3D shows that METH-pretreatment significantly reduced DA uptake (Vmax) to a similar degree in both the DMS and NAc (significant effect of pretreatment, F1,24 = 10.91, p = 0.004; no significant interaction, F1,24 = 0.79, p = 0.39), with Vmax being generally similar in the two striatal subregions (no significant effect of subregion, F1,24 = 0.97, p = 0.34). When expressed as a percent of mean values from saline-pretreated animals, all metrics were reduced by METH-induced neurotoxicity to a similar degree in both striatal regions (tissue DA content: DMS, 63% and NAc, 50%; [DA]max: DMS, 38% and NAc, 47%; [DA]p: DMS, 55% and NAc, 48%; Vmax: DMS, 53% and NAc, 40%).
Representative DA transients
Spontaneous DA transients were observed throughout the striatum. Figure 4 shows representative recordings collected in the DMS (Fig. 4A) and NAc (Fig. 4B). Saline- and METH-pretreated rats are displayed in left and right columns, respectively. There was good agreement between FSCV current traces (black line) collected at the peak oxidation potential for DA (horizontal white line in the pseudocolor plot) and DA levels resolved by the chemometrics approach of PCR (red line), indicating stable CFM recordings with predominately DA changes over time. DA transients (asterisks) were identified by peak-finding analysis of the PCR traces and examination of the corresponding voltammograms. In these recordings, METH-pretreatment reduced the amplitude and frequency of spontaneous DA transients in both the DMS (Fig. 4A) and NAc (Fig. 4B).
Figure 4. Effects of METH neurotoxicity on spontaneous DA transients.
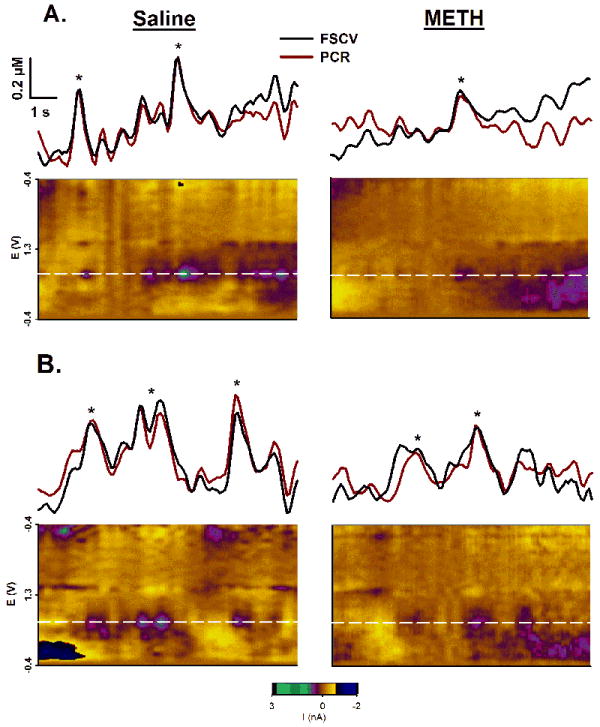
Representative recordings show spontaneous phasic DA transients in the DMS (A) and NAc (B) for saline- and METH-pretreated animals (left and right columns, respectively). See Figure 1 for details. The horizontal white line in the pseudocolor plot, demarcating measured current collected at the peak oxidative potential for DA, defines the above FSCV trace (black line) after converting current to concentration. The red line is the PCR output for the identical recording segments. *, DA transient identified by peak-finding software.
Characteristics of DA transients
After identification by PCR and the background-subtracted cyclic voltammogram, each spontaneous DA transient was analyzed for amplitude, ITI (i.e., frequency-1), and duration (Fig. 5). Histograms are shown in the left and middle columns for the DMS and NAc, respectively. Means and medians (INSET) of transient characteristics are shown in the right column. The non-parametric Kolmogorov-Smirnov two-sample test demonstrated that METH-induced neurotoxicity caused a significant change in the distribution of transient amplitude in both the DMS (p < 0.05; n = 833 for saline, n = 714 for METH; Fig. 5A) and NAc (p < 0.05; n = 384 for saline, n = 398 for METH; Fig. 5B), with a shift to the left in both striatal subregions. The non-parametric Wilcoxon’s rank sum test of the median demonstrated that METH pretreatment significantly decreased transient amplitude in the DMS (p < 0.0001, n = 833 for saline, n = 714 for METH) and NAc (p < 0.0001, n = 384 for saline, n = 398 for METH) (Fig. 5C).
Conversely, METH pretreatment shifted the distribution of transient ITI to the right in both the DMS and NAc (Fig. 5D-E), indicating a decrease in the frequency of detected spontaneous DA transients. The distributions of ITI were significantly different in the DMS (Kolmogorov-Smirnov two-sample test; p < 0.05; n = 679 for saline, n = 667 for METH; Fig. 5D) and NAc (Kolmogorov-Smirnov two-sample test; p < 0.05; n = 295 for saline, n = 346 for METH; Fig 5E). METH pretreatment significantly increased transient ITI in the DMS (Wilcoxon rank sum test; p < 0.0002, n = 679 for saline, n = 667 for METH) and NAc (Wilcoxon’s rank sum test; p < 0.005, n = 295 for saline, n = 346 for METH) (Fig. 5F).
Lastly, a significant change in the distribution of transient duration was found for the NAc (Kolmogorov-Smirnov two-sample test; p < 0.05; n = 384 for saline, n = 398 for METH; Fig. 5G), but not DMS (Kolmogorov-Smirnov two-sample test; p > 0.05; n = 833 for saline, n = 714 for METH; Fig. 5H). METH pretreatment significantly increased DA transient duration in the NAc (Wilcoxon’s rank sum test; p < 0.01, n = 384 for saline, n = 398 for METH). There was a trend toward an increase in DA transient duration in the DMS (Wilcoxon’s rank sum test; p = 0.06, n = 833 for saline, n = 714 for METH) (Fig. 5I).
Interactions between electrical stimulation and DA transients
Because both evoked DA responses and DA transients were collected in each 5-min recording epoch, there is the possibility that electrical stimulation altered DA transients through an effect on DA release (Montague et al., 2004). We addressed this possibility in two ways. First, we used Equation 2 to model the time-dependent effects of electrical stimulation on [DA]p. This equation, in which [DA]p is altered by terms for short-term facilitation, and short- and long-term depression (Montague et al., 2004), simulated an evoked DA response from averaged parameters collected in the DMS of saline-pretreated animals (Fig. 6A) that mimicked the dynamics (Fig. 2A) and amplitude (Fig. 3B) of recorded signals. The electrical stimulation elicits complex, time-dependent effects on [DA]p (Fig. 6B), because facilitation and depression terms uniquely change with time (Fig. 6C). However, the maximal effect on [DA]p was a slight inhibition (2.3 %) at 20 s, which exponentially decayed thereafter (data not shown). Second, we analyzed DA transients to determine if electrical stimulation altered amplitude or ITI. To accomplish this, each 5-min recording epoch was divided into two 150-s bins. Because electrical stimulation occurred at 5 s of the first 150-s bin, this grouping allowed us to analyze for stimulation effects. This analysis showed no significant effect of electrical stimulation on transient amplitude (Wilcoxon’s rank sum test; p = 0.154, n = 859 and 878 for 0-150 and 150-300 s, respectively; Fig. 6D) or ITI (Wilcoxon’s rank sum test; p = 0.225, n = 772 and 774 for 0-150 and 150-300 s, respectively; Fig. 6E). Taken together, these theoretical and empirical results suggest minimal interaction between electrical stimulation and DA transients.
Discussion
This study is the first to report DA-denervation effects on phasic DA signaling assessed under natural conditions. We show that METH-induced striatal DA depletions altered spontaneous DA transients, highlighted by decreased amplitude and frequency. Moreover, uncompensated decreases in DA release appear to drive these deficits. Given the importance of phasic DA signaling to goal-directed behavior and reinforcement learning, the present results are consistent with the postulate that disrupted phasic DA signaling is a plausible mechanism linking between METH-induced DA denervation and behavioral-cognitive impairments.
Binge dosing regimen of METH-induced neurotoxicity
A binge dosing regimen of METH administration was used in the present study to model the long-term toxic effects of METH exposure on central monoamine systems in the human population. As recently reviewed in detail (Marshall & O’Dell 2012), this binge dosing regimen reliably mimics all reported aspects of the long-term toxic effects of METH in humans, including decreased DA and serotonin transporters (McCann et al., 1998; Volkow et al., 2001a; Sekine et al., 2006; McCann et al., 2008), decreased tissue levels of DA and serotonin and markers of such nerve terminals (Wilson et al., 1996; Moszczynska et al., 2004; Kish et al., 2009), and microglial activation (Sekine et al., 2008; Kitamura et al., 2010). Thus, we think that the findings reported herein are highly relevant to the condition of human METH abusers, including those who have been abstinent for prolonged periods of time. We readily acknowledge that the binge dosing regimen does not model aspects related to contingent administration in human METH consumption, but no animal model accurately recapitulates human consumption patterns. Moreover, only one study to date has demonstrated DA neurotoxicity with METH self-administration, and this occurred with a similar overall dose of METH used here but without serotonin neurotoxicity (Krasnova et al., 2010). Also, when directly compared using a yoked-control design, neither contingent nor non-contingent METH administration produced persistent DA depletions (Brennan et al., 2010). Taken together, these studies are consistent with the notion that the DA loss observed in rodent models of METH-induced neurotoxicity and demonstrated here reflects the amount of METH to which the animal is exposed, rather than administration contingency (Marshall & O’Dell 2012).
METH-induced neurotoxicity disrupts phasic DA signaling
METH-induced DA loss disrupted naturally occurring phasic DA signaling in both the DMS and NAc. Evidence collected here is thus consistent with previous studies performed under anesthesia, demonstrating a decreased amplitude of electrically evoked DA responses collected in rodent models of PD (Garris et al., 1997b; Bezard et al., 2000; Bergstrom & Garris 2003) and METH-induced neurotoxicity (Howard et al., 2011; Loewinger et al., 2012). All of these studies measured DA with real-time voltammetry, which exhibits sufficient temporal resolution to capture extracellular dynamics with high fidelity. Indeed, these evoked responses are classified as phasic-like, because they share the same non-plateau, peak shape of DA transients. However, other voltammetric studies report evoked responses that are either similar (Dentresangle et al., 2001) or enhanced (Perez et al., 2008) relative to control.
Consistent effects of DA depletion on phasic-like and naturally occurring DA transients are not altogether unexpected. Evoked responses can faithfully capture both the amplitude and dynamics of DA transients (Robinson et al., 2001), and we have previously used stimulation parameters mimicking DA burst firing (30 Hz, 6 pulses; (Hyland et al., 2002)) to demonstrate diminished phasic-like signals in METH-induced neurotoxicity (Howard et al., 2011). However, the magnitude of evoked phasic-like DA responses and the frequency of spontaneous DA transients change independently at different recording locations within the striatum (Wightman et al., 2007). Demonstrated to alter DA neuron function (Kelland et al., 1990; Garris et al., 2003; Sabeti et al., 2003), the use of anesthesia in previous work is also a concern. It is thus important to establish the effects of DA depletion on naturally occurring DA transients and evoked phasic-like DA signals in awake animals, as we do here. Nevertheless, there is now substantive support from animal models that partial DA depletion produces deficits in phasic DA signaling. It is interesting to speculate that a partial striatal DA loss, such as that caused by METH use in humans (Chang et al., 2007; McCann et al., 2008; Callaghan et al., 2012) and in the presymptomatic phase of PD (Hornykiewicz & Kish 1987), is also associated with disrupted phasic DA signaling in humans.
Mechanisms of disrupted phasic DA signaling in METH-induced neurotoxicity
Characterizing DA release and uptake from evoked DA responses and naturally occurring DA transients in identical recording locations, as we do here, provides a unique opportunity to address mechanisms underlying the METH-induced disruption of phasic DA signaling. We show here that METH-induced neurotoxicity decreases measured parameters for DA release and uptake to a similar degree as tissue DA content, which suggests that the functioning of neither presynaptic mechanism changes at the level of individual DA terminals with DA depletion. This result is thus consistent with previous reports of no compensatory up-regulation of DA release or down-regulation of DA uptake per residual DA terminal in DA-denervation models (Garris et al., 1997b; Bezard et al., 2000; Bergstrom & Garris 2003), but runs counter to other studies demonstrating compensation (Stachowiak et al., 1987; Snyder et al., 1990; Zigmond et al., 1990; Zoli et al., 1998; McCallum et al., 2006; Perez et al., 2008; Bergstrom et al., 2011). A discussion of possible explanations for these discrepant results is beyond the scope of the present study (see (Bergstrom et al., 2011).
In identical recording locations exhibiting a decrease in measured parameters for DA release and uptake reflecting a loss of DA terminals, we also show that METH-induced neurotoxicity decreases DA transient amplitude and frequency, while increasing duration. Reduced DA release is likely a major factor contributing to decreased transient amplitude. Lower frequency and/or number of action potentials within a burst might also be a contributing factor; however, phasic DA firing is unaltered in a rat model of PD, at least until DA depletions become severe (> 96%) (Hollerman & Grace 1990; Harden & Grace 1995) and much greater than that achieved with METH-induced neurotoxicity. Likewise, decreased DA uptake likely does not account for the observed decrease in transient amplitude, as decreased uptake alone would increase transient amplitude. These arguments highlight the primacy of decreased DA release to the observed reductions in transient amplitude. The greater transient duration, however, is likely linked directly to the decreased DA uptake, which alone would increase duration of the DA signal. In the absence of the decrease in uptake, in fact, the smaller amplitude METH-induced transients would exhibit shorter durations, highlighting the significance of the changes in uptake for the observed changes in transient duration. Assuming that phasic DA firing is unaltered in the setting of the partial DA loss induced by METH, decreased transient frequency seems paradoxical. However, it is quite conceivable that the reduction in transient amplitude may lower smaller transients below the level of detection, thereby decreasing apparent transient frequency.
Implications of disrupted phasic DA signaling for METH-induced neurotoxicity
Phasic DA transients respond to primary rewards and their cues (Day et al., 2007; Brown et al., 2011), encoding prediction error important for reinforcement learning (Schultz 2007), and emerge just prior to operant responding to drive goal-directed behavior (Phillips et al., 2003; Adamantidis et al., 2011). Other phasic DA transients occur in the absence of discrete stimuli (Robinson et al., 2001; Robinson et al., 2002) and are robustly augmented by abused drugs (Cheer et al., 2007; Wightman et al., 2007), which may enhance network activity (Stuber et al., 2005) and contribute to overlearning cues predicting drug availability in addiction (Hyman 2005; Willuhn et al., 2010). These so-called spontaneous DA transients may also temporally combine during intense activation to increase tonic DA levels (Stuber et al., 2005), which has been implicated in movement, cognition and motivation (Schultz 2007). A diminishment of spontaneous DA transients, as we observe here with METH-induced neurotoxicity, would thus be expected to disrupt functions associated with these phasic signals.
Acute, non-toxic administration of amphetamine activates evoked phasic-like, spontaneous, and cue-evoked DA transients (Daberkow et al., 2013). It is thus not unreasonable to speculate that METH pretreatment similarly disrupts striatal DA transients associated with rewards along with evoked phasic-like and spontaneous DA signals demonstrated in the present study. This possibility suggests that learning and memory processes of the basal ganglia might be impaired as well. Neurocomputation models predict that reduced activation of the direct “Go” pathway by diminished DA transients in PD produces deficits in reinforcement learning (Frank et al., 2004; Wiecki & Frank 2010). Selective disruption of phasic DA signaling also impairs acquisition of cue-dependent (Zweifel et al., 2009) and reward-related action-outcome (Ranaldi et al., 2011) and stimulus-response (Wang et al., 2011) learning. Moreover, our prior studies in rats with a neurotoxic METH regimen demonstrate diminished motor sequence learning (Chapman et al., 2001; Daberkow et al., 2005), consolidation of response-reversal learning (Daberkow et al., 2008; Pastuzyn et al., 2012), and formation of stimulus-response associations (Son et al., 2011). Thus, a METH-induced disruption of phasic DA signaling in the striatum could have a significant impact on basal ganglia function; however, studies directly linking altered phasic DA signaling with impaired cognitive function remain to be done.
Acknowledgments
This research was supported by National Institutes of Health grant DA 024036. CDH was supported by a Weigel grant through Phi Sigma at Illinois State University. The authors would like to thank Kim Garris and Blake Kennedy for skilled technical assistance.
Abbreviations
- CFM
Carbon fiber microelectrode
- DA
Dopamine
- DMS
Dorsomedial Striatum
- FSCV
Fast-scan cyclic voltammetry
- ITI
Intertransient interval
- METH
Methamphetamine
- NAc
Nucleus Accumbens
- PCR
Principal Component Regression
References
- Adamantidis AR, Tsai HC, Boutrel B, Zhang F, Stuber GD, Budygin EA, Tourino C, Bonci A, Deisseroth K, de LL. Optogenetic interrogation of dopaminergic modulation of the multiple phases of reward-seeking behavior. J Neurosci. 2011;31:10829–10835. doi: 10.1523/JNEUROSCI.2246-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. Impaired object recognition memory following methamphetamine, but not p-chloroamphetamine- or d-amphetamine-induced neurotoxicity. Neuropsychopharmacology. 2005;30:2026–2034. doi: 10.1038/sj.npp.1300771. [DOI] [PubMed] [Google Scholar]
- Bergstrom BP, Garris PA. “Passive stabilization” of striatal extracellular dopamine across the lesion spectrum encompassing the presymptomatic phase of Parkinson’s disease: a voltammetric study in the 6-OHDA-lesioned rat. J Neurochem. 2003;87:1224–1236. doi: 10.1046/j.1471-4159.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- Bergstrom BP, Sanberg SG, Andersson M, Mithyantha J, Carroll FI, Garris PA. Functional reorganization of the presynaptic dopaminergic terminal in parkinsonism. Neuroscience. 2011;193:310–322. doi: 10.1016/j.neuroscience.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezard E, Jaber M, Gonon F, Boireau A, Bloch B, Gross CE. Adaptive changes in the nigrostriatal pathway in response to increased 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration in the mouse. Eur J Neurosci. 2000;12:2892–2900. doi: 10.1046/j.1460-9568.2000.00180.x. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Colussi-Mas J, Carati C, Lea RA, Fitzmaurice PS, Schenk S. Methamphetamine self-administration and the effect of contingency on monoamine and metabolite tissue levels in the rat. Brain Res. 2010;1317:137–146. doi: 10.1016/j.brainres.2009.11.069. [DOI] [PubMed] [Google Scholar]
- Brown HD, McCutcheon JE, Cone JJ, Ragozzino ME, Roitman MF. Primary food reward and reward-predictive stimuli evoke different patterns of phasic dopamine signaling throughout the striatum. Eur J Neurosci. 2011;34:1997–2006. doi: 10.1111/j.1460-9568.2011.07914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30:211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson’s disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug Alcohol Depend. 2012;120:35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wightman RM. Functional microcircuitry in the accumbens underlying drug addiction: insights from real-time signaling during behavior. Curr Opin Neurobiol. 2004;14:763–768. doi: 10.1016/j.conb.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chapman DE, Hanson GR, Kesner RP, Keefe KA. Long-term changes in basal ganglia function after a neurotoxic regimen of methamphetamine. J Pharmacol Exp Ther. 2001;296:520–527. [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Brown HD, Bunner KD, Kraniotis SA, Doellman MA, Ragozzino ME, Garris PA, Roitman MF. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. J Neurosci. 2013;33:452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daberkow DP, Kesner RP, Keefe KA. Relation between methamphetamine-induced monoamine depletions in the striatum and sequential motor learning. Pharmacol Biochem Behav. 2005;81:198–204. doi: 10.1016/j.pbb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Daberkow DP, Riedy MD, Kesner RP, Keefe KA. Effect of methamphetamine neurotoxicity on learning-induced Arc mRNA expression in identified striatal efferent neurons. Neurotox Res. 2008;14:307–315. doi: 10.1007/BF03033855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JJ, Roitman MF, Wightman RM, Carelli RM. Associative learning mediates dynamic shifts in dopamine signaling in the nucleus accumbens. Nat Neurosci. 2007;10:1020–1028. doi: 10.1038/nn1923. [DOI] [PubMed] [Google Scholar]
- Dentresangle C, Le CM, Savasta M, Leviel V. Increased extracellular DA and normal evoked DA release in the rat striatum after a partial lesion of the substantia nigra. Brain Res. 2001;893:178–185. doi: 10.1016/s0006-8993(00)03311-4. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Garris PA, Budygin EA, Phillips PE, Venton BJ, Robinson DL, Bergstrom BP, Rebec GV, Wightman RM. A role for presynaptic mechanisms in the actions of nomifensine and haloperidol. Neuroscience. 2003;118:819–829. doi: 10.1016/s0306-4522(03)00005-8. [DOI] [PubMed] [Google Scholar]
- Garris PA, Christensen JR, Rebec GV, Wightman RM. Real-time measurement of electrically evoked extracellular dopamine in the striatum of freely moving rats. J Neurochem. 1997a;68:152–161. doi: 10.1046/j.1471-4159.1997.68010152.x. [DOI] [PubMed] [Google Scholar]
- Garris PA, Walker QD, Wightman RM. Dopamine release and uptake rates both decrease in the partially denervated striatum in proportion to the loss of dopamine terminals. Brain Res. 1997b;753:225–234. doi: 10.1016/s0006-8993(97)00003-6. [DOI] [PubMed] [Google Scholar]
- Harden DG, Grace AA. Activation of dopamine cell firing by repeated L-DOPA administration to dopamine-depleted rats: its potential role in mediating the therapeutic response to L-DOPA treatment. J Neurosci. 1995;15:6157–6166. doi: 10.1523/JNEUROSCI.15-09-06157.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heien ML, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Wassum KM, Wightman RM. Real-time measurement of dopamine fluctuations after cocaine in the brain of behaving rats. Proc Natl Acad Sci U S A. 2005;102:10023–10028. doi: 10.1073/pnas.0504657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring NR, Schaefer TL, Gudelsky GA, Vorhees CV, Williams MT. Effect of +-methamphetamine on path integration learning, novel object recognition, and neurotoxicity in rats. Psychopharmacology (Berl) 2008;199:637–650. doi: 10.1007/s00213-008-1183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Grace AA. The effects of dopamine-depleting brain lesions on the electrophysiological activity of rat substantia nigra dopamine neurons. Brain Res. 1990;533:203–212. doi: 10.1016/0006-8993(90)91341-d. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O, Kish SJ. Biochemical pathophysiology of Parkinson’s disease. Adv Neurol. 1987;45:19–34. [PubMed] [Google Scholar]
- Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- Howard CD, Keefe KA, Garris PA, Daberkow DP. Methamphetamine neurotoxicity decreases phasic, but not tonic, dopaminergic signaling in the rat striatum. J Neurochem. 2011;118:668–676. doi: 10.1111/j.1471-4159.2011.07342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland BI, Reynolds JN, Hay J, Perk CG, Miller R. Firing modes of midbrain dopamine cells in the freely moving rat. Neuroscience. 2002;114:475–492. doi: 10.1016/s0306-4522(02)00267-1. [DOI] [PubMed] [Google Scholar]
- Hyman SE. Addiction: a disease of learning and memory. Am J Psychiatry. 2005;162:1414–1422. doi: 10.1176/appi.ajp.162.8.1414. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Belcher AM, Scott L, Cazares VA, Chen J, O’Dell SJ, Malvaez M, Wu T, Marshall JF. Reversal-specific learning impairments after a binge regimen of methamphetamine in rats: possible involvement of striatal dopamine. Neuropsychopharmacology. 2010;35:505–514. doi: 10.1038/npp.2009.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley RB, Heien ML, Wightman RM. Multivariate concentration determination using principal component regression with residual analysis. Trends Analyt Chem. 2009;28:1127–1136. doi: 10.1016/j.trac.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelland MD, Chiodo LA, Freeman AS. Anesthetic influences on the basal activity and pharmacological responsiveness of nigrostriatal dopamine neurons. Synapse. 1990;6:207–209. doi: 10.1002/syn.890060213. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Fitzmaurice PS, Boileau I, Schmunk GA, Ang LC, Furukawa Y, Chang LJ, Wickham DJ, Sherwin A, Tong J. Brain serotonin transporter in human methamphetamine users. Psychopharmacology (Berl) 2009;202:649–661. doi: 10.1007/s00213-008-1346-x. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Sandberg SG, Lohrenz T, Comair YG, Saez I, Phillips PE, Montague PR. Sub-second dopamine detection in human striatum. PLoS One. 2011;6:e23291. doi: 10.1371/journal.pone.0023291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura O, Takeichi T, Wang EL, Tokunaga I, Ishigami A, Kubo S. Microglial and astrocytic changes in the striatum of methamphetamine abusers. Leg Med (Tokyo) 2010;12:57–62. doi: 10.1016/j.legalmed.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Krasnova IN, Justinova Z, Ladenheim B, Jayanthi S, McCoy MT, Barnes C, Warner JE, Goldberg SR, Cadet JL. Methamphetamine self-administration is associated with persistent biochemical alterations in striatal and cortical dopaminergic terminals in the rat. PLoS One. 2010;5:e8790. doi: 10.1371/journal.pone.0008790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewinger GC, Beckert MV, Tejeda HA, Cheer JF. Methamphetamine-induced dopamine terminal deficits in the nucleus accumbens are exacerbated by reward-associated cues and attenuated by CB1 receptor antagonism. Neuropharmacology. 2012 doi: 10.1016/j.neuropharm.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logman MJ, Budygin EA, Gainetdinov RR, Wightman RM. Quantitation of in vivo measurements with carbon fiber microelectrodes. J Neurosci Methods. 2000;95:95–102. doi: 10.1016/s0165-0270(99)00155-7. [DOI] [PubMed] [Google Scholar]
- Marshall JF, O’Dell SJ. Methamphetamine influences on brain and behavior: unsafe at any speed? Trends Neurosci. 2012;35:536–545. doi: 10.1016/j.tins.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum SE, Parameswaran N, Perez XA, Bao S, McIntosh JM, Grady SR, Quik M. Compensation in pre-synaptic dopaminergic function following nigrostriatal damage in primates. J Neurochem. 2006;96:960–972. doi: 10.1111/j.1471-4159.2005.03610.x. [DOI] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael D, Travis ER, Wightman RM. Color images for fast-scan CV measurements in biological systems. Anal Chem. 1998;70:586A–592A. doi: 10.1021/ac9819640. [DOI] [PubMed] [Google Scholar]
- Montague PR, McClure SM, Baldwin PR, Phillips PE, Budygin EA, Stuber GD, Kilpatrick MR, Wightman RM. Dynamic gain control of dopamine delivery in freely moving animals. J Neurosci. 2004;24:1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moszczynska A, Fitzmaurice P, Ang L, Kalasinsky KS, Schmunk GA, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Why is parkinsonism not a feature of human methamphetamine users? Brain. 2004;127:363–370. doi: 10.1093/brain/awh046. [DOI] [PubMed] [Google Scholar]
- O’Dell SJ, Feinberg LM, Marshall JF. A neurotoxic regimen of methamphetamine impairs novelty recognition as measured by a social odor-based task. Behav Brain Res. 2011;216:396–401. doi: 10.1016/j.bbr.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Pastuzyn ED, Chapman DE, Wilcox KS, Keefe KA. Altered learning and Arc-regulated consolidation of learning in striatum by methamphetamine-induced neurotoxicity. Neuropsychopharmacology. 2012;37:885–895. doi: 10.1038/npp.2011.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1986. [Google Scholar]
- Perez XA, Parameswaran N, Huang LZ, O’Leary KT, Quik M. Presynaptic dopaminergic compensation after moderate nigrostriatal damage in non-human primates. J Neurochem. 2008;105:1861–1872. doi: 10.1111/j.1471-4159.2008.05268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Ranaldi R, Kest K, Zellner MR, Lubelski D, Muller J, Cruz Y, Saliba M. The effects of VTA NMDA receptor antagonism on reward-related learning and associated c-fos expression in forebrain. Behav Brain Res. 2011;216:424–432. doi: 10.1016/j.bbr.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Robinson DL, Heien ML, Wightman RM. Frequency of dopamine concentration transients increases in dorsal and ventral striatum of male rats during introduction of conspecifics. J Neurosci. 2002;22:10477–10486. doi: 10.1523/JNEUROSCI.22-23-10477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DL, Phillips PE, Budygin EA, Trafton BJ, Garris PA, Wightman RM. Sub-second changes in accumbal dopamine during sexual behavior in male rats. Neuroreport. 2001;12:2549–2552. doi: 10.1097/00001756-200108080-00051. [DOI] [PubMed] [Google Scholar]
- Sabeti J, Gerhardt GA, Zahniser NR. Chloral hydrate and ethanol, but not urethane, alter the clearance of exogenous dopamine recorded by chronoamperometry in striatum of unrestrained rats. Neurosci Lett. 2003;343:9–12. doi: 10.1016/s0304-3940(03)00301-x. [DOI] [PubMed] [Google Scholar]
- Schonberg T, O’Doherty JP, Joel D, Inzelberg R, Segev Y, Daw ND. Selective impairment of prediction error signaling in human dorsolateral but not ventral striatum in Parkinson’s disease patients: evidence from a model-based fMRI study. Neuroimage. 2010;49:772–781. doi: 10.1016/j.neuroimage.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Schroder N, O’Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends Neurosci. 2007;30:203–210. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Matt GE, Meyer RA, Heaton RK, Atkinson JH, Grant I. Neurocognitive effects of methamphetamine: a critical review and meta-analysis. Neuropsychol Rev. 2007;17:275–297. doi: 10.1007/s11065-007-9031-0. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Fischman MW, Schuster CR. Long-term methamphetamine induced changes in brain catecholamines in tolerant rhesus monkeys. Drug Alcohol Depend. 1976;1:215–219. doi: 10.1016/0376-8716(76)90030-2. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Takei N, Yoshikawa E, Nakamura K, Futatsubashi M, Okada H, Minabe Y, Suzuki K, Iwata Y, Tsuchiya KJ, Tsukada H, Iyo M, Mori N. Brain serotonin transporter density and aggression in abstinent methamphetamine abusers. Arch Gen Psychiatry. 2006;63:90–100. doi: 10.1001/archpsyc.63.1.90. [DOI] [PubMed] [Google Scholar]
- Simon SL, Domier C, Carnell J, Brethen P, Rawson R, Ling W. Cognitive impairment in individuals currently using methamphetamine. Am J Addict. 2000;9:222–231. doi: 10.1080/10550490050148053. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Keller RW, Jr, Zigmond MJ. Dopamine efflux from striatal slices after intracerebral 6-hydroxydopamine: evidence for compensatory hyperactivity of residual terminals. J Pharmacol Exp Ther. 1990;253:867–876. [PubMed] [Google Scholar]
- Son JH, Latimer C, Keefe KA. Impaired formation of stimulus-response, but not action-outcome, associations in rats with methamphetamine-induced neurotoxicity. Neuropsychopharmacology. 2011;36:2441–2451. doi: 10.1038/npp.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak MK, Keller RW, Jr, Stricker EM, Zigmond MJ. Increased dopamine efflux from striatal slices during development and after nigrostriatal bundle damage. J Neurosci. 1987;7:1648–1654. doi: 10.1523/JNEUROSCI.07-06-01648.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Wightman RM, Carelli RM. Extinction of cocaine self-administration reveals functionally and temporally distinct dopaminergic signals in the nucleus accumbens. Neuron. 2005;46:661–669. doi: 10.1016/j.neuron.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001a;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am J Psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Wang LP, Li F, Wang D, Xie K, Wang D, Shen X, Tsien JZ. NMDA receptors in dopaminergic neurons are crucial for habit learning. Neuron. 2011;72:1055–1066. doi: 10.1016/j.neuron.2011.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson’s disease. Prog Brain Res. 2010;183:275–297. doi: 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Amatore C, Engstrom RC, Hale PD, Kristensen EW, Kuhr WG, May LJ. Real-time characterization of dopamine overflow and uptake in the rat striatum. Neuroscience. 1988;25:513–523. doi: 10.1016/0306-4522(88)90255-2. [DOI] [PubMed] [Google Scholar]
- Wightman RM, Heien ML, Wassum KM, Sombers LA, Aragona BJ, Khan AS, Ariansen JL, Cheer JF, Phillips PE, Carelli RM. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007;26:2046–2054. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Curr Top Behav Neurosci. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JM, Kalasinsky KS, Levey AI, Bergeron C, Reiber G, Anthony RM, Schmunk GA, Shannak K, Haycock JW, Kish SJ. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nat Med. 1996;2:699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- Wu Q, Reith ME, Wightman RM, Kawagoe KT, Garris PA. Determination of release and uptake parameters from electrically evoked dopamine dynamics measured by real-time voltammetry. J Neurosci Methods. 2001;112:119–133. doi: 10.1016/s0165-0270(01)00459-9. [DOI] [PubMed] [Google Scholar]
- Zaghloul KA, Blanco JA, Weidemann CT, McGill K, Jaggi JL, Baltuch GH, Kahana MJ. Human substantia nigra neurons encode unexpected financial rewards. Science. 2009;323:1496–1499. doi: 10.1126/science.1167342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ, Abercrombie ED, Berger TW, Grace AA, Stricker EM. Compensations after lesions of central dopaminergic neurons: some clinical and basic implications. Trends Neurosci. 1990;13:290–296. doi: 10.1016/0166-2236(90)90112-n. [DOI] [PubMed] [Google Scholar]
- Zoli M, Torri C, Ferrari R, Jansson A, Zini I, Fuxe K, Agnati LF. The emergence of the volume transmission concept. Brain Res Brain Res Rev. 1998;26:136–147. doi: 10.1016/s0165-0173(97)00048-9. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, Palmiter RD. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]


