Abstract
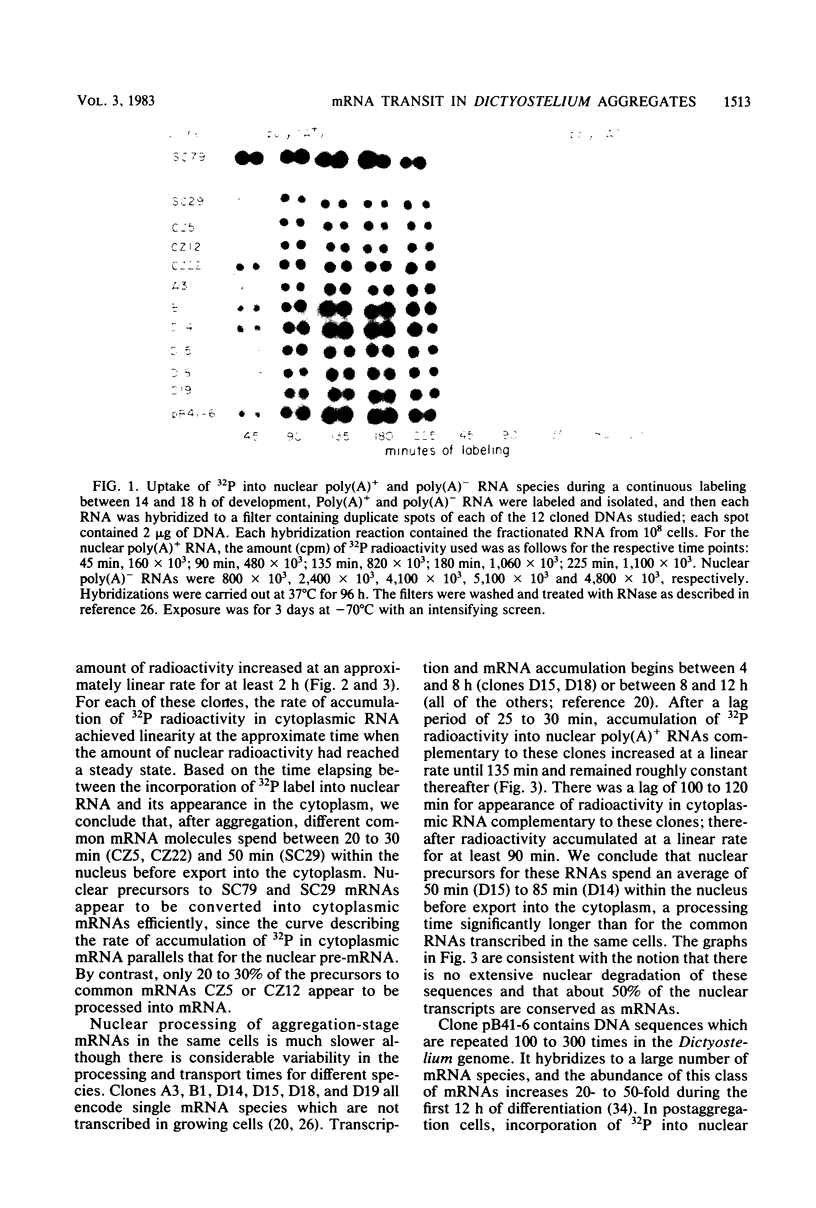
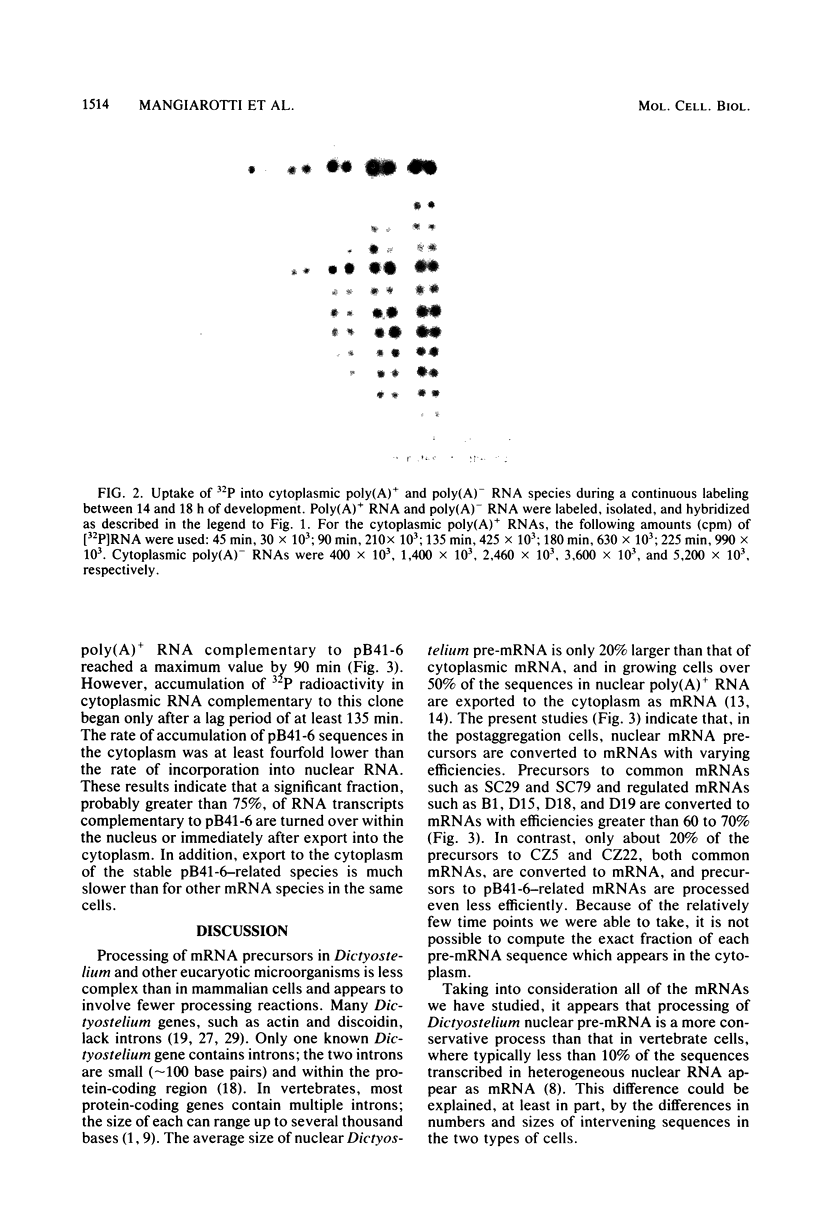
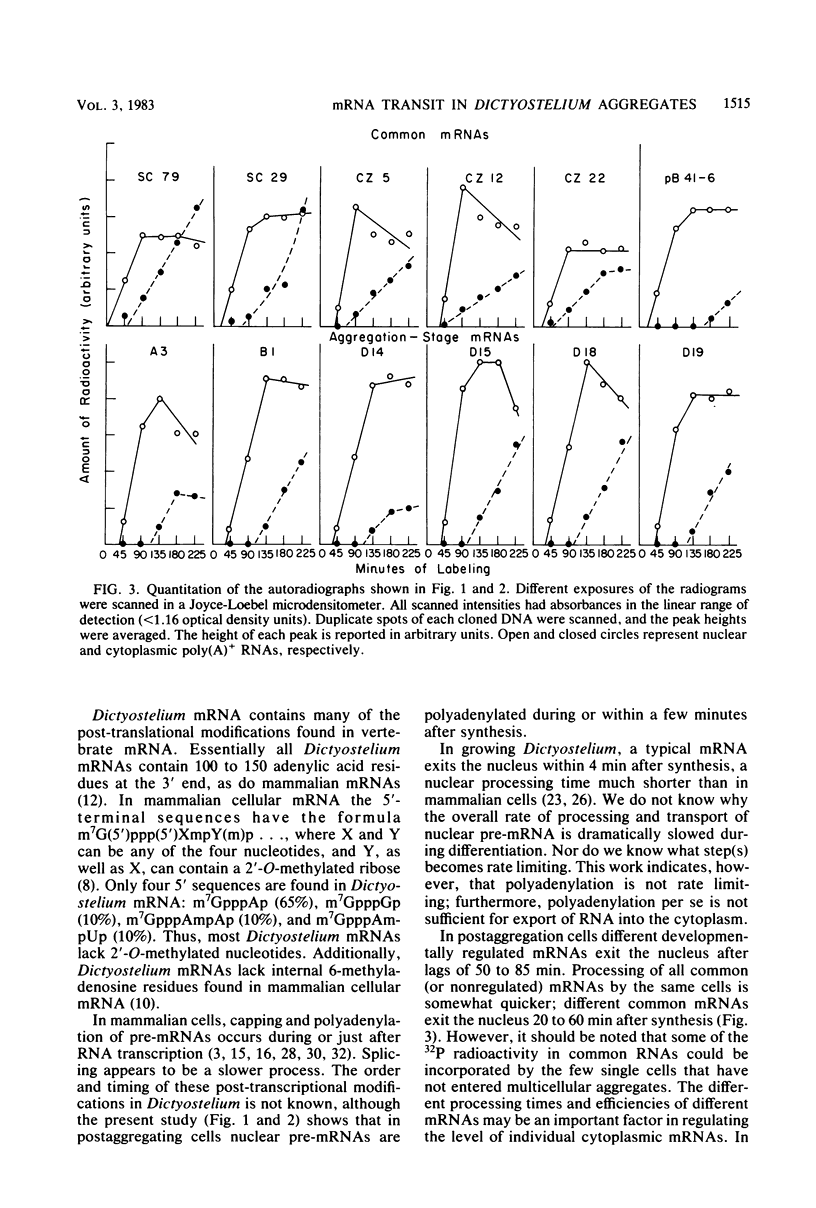
Nuclear processing of mRNA precursors in differentiating multicellular Dictyostelium discoideum aggregates is markedly slower than in growing amoebae. Thus, we have been able to determine the time of nuclear processing of individual mRNA species in postaggregating cells by following the incorporation of 32PO4 into nuclear and cytoplasmic RNA complementary to cloned cDNAs. Precursors of mRNAs synthesized during both growth and differentiation remain in the nucleus for about 25 to 60 min. By contrast, typical mRNAs which are synthesized only by postaggregative cells have nuclear processing times between 50 and 100 min. Depending on the particular mRNA, between 20 and 60% of nuclear transcripts are converted into cytoplasmic mRNA. A third class of mRNAs are transcribed from a set of repetitive DNA segments and are expressed predominantly during differentiation. Nuclear precursors of these mRNAs are extensively degraded within the nucleus or very rapidly after transport to the cytoplasm. Those sequences that are stable in the cytoplasm exit from the nucleus only after a lag of over 2 h. Thus, mRNAs encoded by different genes that are subject to different types of developmental controls display different times of transit to the cytoplasm and different efficiencies of nuclear processing. Differential nuclear processing may contribute to the regulation of the level of individual cytoplasmic mRNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson J. RNA processing and the intervening sequence problem. Annu Rev Biochem. 1979;48:1035–1069. doi: 10.1146/annurev.bi.48.070179.005131. [DOI] [PubMed] [Google Scholar]
- Alton T. H., Lodish H. F. Translational control of protein synthesis during the early stages of differentiation of the slime mold Dictyostelium discoideum. Cell. 1977 Sep;12(1):301–310. doi: 10.1016/0092-8674(77)90208-2. [DOI] [PubMed] [Google Scholar]
- Babich A., Nevins J. R., Darnell J. E., Jr Early capping of transcripts from the adenovirus major late transcription unit. Nature. 1980 Sep 18;287(5779):246–248. doi: 10.1038/287246a0. [DOI] [PubMed] [Google Scholar]
- Batts-Young B., Maizels N., Lodish H. F. Precursors of ribosomal RNA in the cellular slime mold Dictyostelium discoideum. Isolation and characterization. J Biol Chem. 1977 Jun 10;252(11):3952–3960. [PubMed] [Google Scholar]
- Blumberg D. D., Lodish H. F. Changes in the messenger RNA population during differentiation of dictyostelium discoideum. Dev Biol. 1980 Aug;78(2):285–300. doi: 10.1016/0012-1606(80)90337-1. [DOI] [PubMed] [Google Scholar]
- Blumberg D. D., Lodish H. F. Complexity of nuclear and polysomal RNAs in growing Dictyostelium discoideum cells. Dev Biol. 1980 Aug;78(2):268–284. doi: 10.1016/0012-1606(80)90336-x. [DOI] [PubMed] [Google Scholar]
- Chung S., Landfear S. M., Blumberg D. D., Cohen N. S., Lodish H. F. Synthesis and stability of developmentally regulated dictyostelium mRNAs are affected by cell--cell contact and cAMP. Cell. 1981 Jun;24(3):785–797. doi: 10.1016/0092-8674(81)90104-5. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Transcription units for mRNA production in eukaryotic cells and their DNA viruses. Prog Nucleic Acid Res Mol Biol. 1979;22:327–353. doi: 10.1016/s0079-6603(08)60803-x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Dottin R. P., Weiner A. M., Lodish F. 5' terminal nucleotide sequences of the messenger RNA's of Dictyostelium discoideum. Cell. 1976 Jun;8(2):233–244. doi: 10.1016/0092-8674(76)90007-6. [DOI] [PubMed] [Google Scholar]
- Falkenthal S., Graham M. L., Korn E. L., Lengyel J. A. Transcription, processing, and turnover of RNA from the Drosophila mobile genetic element copia. Dev Biol. 1982 Aug;92(2):294–305. doi: 10.1016/0012-1606(82)90176-2. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Jacobson A., Lodish H. F. Isolation and hybridization kinetics of messenger RNA from Dictyostelium discoideum. Nat New Biol. 1972 Oct 25;239(95):225–228. doi: 10.1038/newbio239225a0. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Kindle K., Huxley M. P. Structural organization and processing of the genetic transcript in the cellular slime mold Dictyostelium discoideum. Fed Proc. 1976 Jan;35(1):13–22. [PubMed] [Google Scholar]
- Firtel R. A., Lodish H. F. A small nuclear precursor of messenger RNA in the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1973 Sep 15;79(2):295–314. doi: 10.1016/0022-2836(73)90007-7. [DOI] [PubMed] [Google Scholar]
- Fraser N. W., Sehgal P. B., Darnell J. E., Jr Multiple discrete sites for premature RNA chain termination late in adenovirus-2 infection: enhancement by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2571–2575. doi: 10.1073/pnas.76.6.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Kessin R. H. RNA metabolsim during vegetative growth and morphogenesis of the cellular slime mold Dictyostelium discoideum. Dev Biol. 1973 Apr;31(2):242–251. doi: 10.1016/0012-1606(73)90261-3. [DOI] [PubMed] [Google Scholar]
- Kimmel A. R., Firtel R. A. Intervening sequences in a Dictyostelium gene that encodes a low abundance class mRNA. Nucleic Acids Res. 1980 Dec 11;8(23):5599–5610. doi: 10.1093/nar/8.23.5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfear S. M., Lefebvre P., Chung S., Lodish H. F. Transcriptional control of gene expression during development of Dictyostelium discoideum. Mol Cell Biol. 1982 Nov;2(11):1417–1426. doi: 10.1128/mcb.2.11.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfear S. M., Lodish H. F. A role for cyclic AMP in expression of developmentally regulated genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1044–1048. doi: 10.1073/pnas.77.2.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Jacobson A., Firtel R., Alton T., Tuchman J. Synthesis of messenger RNA and chromosome structure in the cellular slime mold. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5103–5108. doi: 10.1073/pnas.71.12.5103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Ceccarelli A., Lodish H. F. Cyclic AMP stabilizes a class of developmentally regulated Dictyostelium discoideum mRNAs. Nature. 1983 Feb 17;301(5901):616–618. doi: 10.1038/301616a0. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Chung S., Zuker C., Lodish H. F. Selection and analysis of cloned developmentally-regulated Dictyostelium discoideum genes by hybridization-competition. Nucleic Acids Res. 1981 Feb 25;9(4):947–963. doi: 10.1093/nar/9.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiarotti G., Lefebvre P., Lodish H. F. Differences in the stability of developmentally regulated mRNAs in aggregated and disaggregated Dictyostelium discoideum cells. Dev Biol. 1982 Jan;89(1):82–91. doi: 10.1016/0012-1606(82)90296-2. [DOI] [PubMed] [Google Scholar]
- McKeown M., Firtel R. A. Differential expression and 5' end mapping of actin genes in Dictyostelium. Cell. 1981 Jun;24(3):799–807. doi: 10.1016/0092-8674(81)90105-7. [DOI] [PubMed] [Google Scholar]
- Nevins J. R., Darnell J. E., Jr Steps in the processing of Ad2 mRNA: poly(A)+ nuclear sequences are conserved and poly(A) addition precedes splicing. Cell. 1978 Dec;15(4):1477–1493. doi: 10.1016/0092-8674(78)90071-5. [DOI] [PubMed] [Google Scholar]
- Rowekamp W., Poole S., Firtel R. A. Analysis of the multigene family coding the developmentally regulated carbohydrate-binding protein discoidin-I in D. discoideum. Cell. 1980 Jun;20(2):495–505. doi: 10.1016/0092-8674(80)90636-4. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Harpold M., Chen-Kiang S., Darnell J. E., Jr The addition of 5' cap structures occurs early in hnRNA synthesis and prematurely terminated molecules are capped. Cell. 1980 Jan;19(1):69–78. doi: 10.1016/0092-8674(80)90389-x. [DOI] [PubMed] [Google Scholar]
- Stanfield S. W., Lengyel J. A. Small circular deoxyribonucleic acid of Drosophila melanogaster: homologous transcripts in the nucleus and cytoplasm. Biochemistry. 1980 Aug 5;19(16):3873–3877. doi: 10.1021/bi00557a036. [DOI] [PubMed] [Google Scholar]
- Weber J., Blanchard J. M., Ginsberg H., Darnell J. E., Jr Order of polyadenylic acid addition and splicing events in early adenovirus mRNA formation. J Virol. 1980 Jan;33(1):286–291. doi: 10.1128/jvi.33.1.286-291.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. G., Lloyd M. M., Devine J. M. Characterization and transcription analysis of a cloned sequence derived from a major developmentally regulated mRNA of D. discoideum. Cell. 1979 Aug;17(4):903–913. doi: 10.1016/0092-8674(79)90330-1. [DOI] [PubMed] [Google Scholar]
- Zuker C., Lodish H. F. Repetitive DNA sequences cotranscribed with developmentally regulated Dictyostelium discoideum mRNAs. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5386–5390. doi: 10.1073/pnas.78.9.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]




