Abstract
Evidence suggests that most chemotherapeutic agents are less effective as treatment in patients with estrogen receptor-negative (ER-) breast carcinomas compared to those with estrogen receptor-positive (ER+) breast carcinomas. Moreover, African American Women (AAW) is disproportionately diagnosed with ER- breast cancer compared to their white counterparts. Novel therapies effective against ER- breast carcinomas are urgently needed to ameliorate the health disparity. Previous reports show that low concentrations (microgram/ml) of water-soluble leaf extracts of a Nigerian edible plant, V. amygdalina (VA), potently retards the proliferative activities of ER+ human breast cancerous cells (MCF-7) in vitro in a concentration-dependent fashion. However, the anti-proliferative activities of VA in either ductal or ER- carcinoma cells have not been characterized. The exposure of BT-549 to increasing concentrations of VA (10, 100, and 1000 μg/mL) inhibited cell growth by approximately 14 % (P<0.05), 22 % (p<0.05), and 50 % (p<0.005) respectively. The cell count studies were corroborated by DNA synthesis studies. Treatments of BT-549 with 10, 100, and 1000 μg/mL VA inhibited DNA synthesis in a concentration dependent fashion by 22 %, 76 % (P<0.05), and 86 % (p<0.01) respectively. BT-549 cells were insensitive to 10 and 100 nM paclitaxel (TAX) treatments. Isolation of DNA from dried VA leaves yielded approximately 12.2 and 1 kbp genomic DNA that were Eco RI-insensitive but Hind III and Bam HI-sensitive. These pieces of information may be used to enhance the safety of medicinal botanical VA through authentication, and adulteration detection.
Keywords: anti-cancer; vernonia amygdalina; activity prediction, standardization
Introduction
Cancer of the breast is the most commonly diagnosed non-skin cancer and second leading cause of cancer-related deaths in women. Breast cancer represents 15 % of new cases of all cancers [1]. An estimated 178,000 women will be diagnosed with invasive breast cancer and 40,460 women will die from the disease this year in the U.S. [1–2]. More than one-half of all breast carcinomas are estrogen receptor-positive (ER+). Tamoxifen (TAM), an anti-estrogen drug, is one of the most effective chemotherapies for ER+ breast carcinomas [3]. Paclitaxel or Taxol (TAX), an anti-microtubule agent; is effective against estrogen receptor-negative (ER-) breast carcinomas [3–4]. Health disparities exist in breast cancer mortality [2]; although the incidence of breast cancer is highest in White Women (WW), African American Women (AAW) have higher mortality rates than other racial or ethnic groups in the U.S. [2].
The gap, or disparity, has even widened in recent years. One of the reported reasons for the breast cancer disparity is that AAW are more likely to be diagnosed with ER- breast cancer (a more aggressive breast cancer, with less treatment options) than other ethnic groups [5–7]. Therefore, there is an urgent need for the discovery and development of agent(s) efficacious against ER- breast cancer to close or eliminate the breast cancer disparity gap. Vernonia amygdalina (VA) is increasingly emerging as a very strong candidate for breast cancer treatment. VA may be used alone or in combination (adjuvant) with known drugs. VA, commonly known as bitter leaf, is a shrub that peaks in height around 3 meters. It grows in several parts of Africa, including the tropics and particularly South Africa, Zimbabwe and Nigeria [8–10]. VA may be effective against amoebic dysentery [11]; gastrointestinal disorders [12–13]; microbial and parasitic activities [14–15]; hepatotoxicities [16]; and cancer [17–22].
It is very unlikely that a single molecule is responsible for such varied activities; instead multiple molecules, working alone or in combination with others, are much more likely to be responsible for each of these biological activities. The biologically-active compounds of VA are saponins and alkaloids [23]; terpenes, steroids, coumarines, flavonoids, phenolic acids, lignans, xanthones and anthraquinones [24–26]; edotides [19]; tannis and [25]; sesquiterpene lactone [17–18]. These compounds isolated from VA extracts, using various solvents of different polarity indexes, have been attributed to specific biological activities. For example: the antiplasmodial (anti-malarial activity) of VA extracts may be related to the presence of flavonoids, saponins, alkaloids [23]. Some studies have associated coumarines and flavonoids in most plants with antitumor activities in humans [26]. Other cancer-fighting agents in VA extracts may include sesquiterpene lactones (SLs) [17–18] and edotides [19].
Taken together, there is compelling evidence to show that VA supplementation or therapy may benefit cancer patients. However, the challenge is that antagonistic relationships exist between conventional medicine (CM) and traditional medicine (TM) practitioners. This antagonism is predicated on two key issues. First, the contention is that conventional drugs are standardized and chemically-defined; the quantities and structures of the active ingredients are known. Therefore, therapeutic dosages are determinable. In contrast, traditional medicines are often native or non-purified botanical extracts with limited knowledge of their chemical compositions. Second, the issues of authentication and quality control must also be improved if TM is to gain more recognition. In this regard, we have used sample scale-up, extraction, solvent partitioning, column fractionation, profiling with an ultra violet (UV) detector and individual component spectroscopy using nuclear magnetic resonance (NMR), and the combination of HPLC and thin layer chromatography (TLC) techniques to purify VA extracts [under revision]. The objectives of the present studies are to: develop some genetic markers for the VA leaf using restriction enzyme digestion assay for authentication and adulteration detection, and to assess the anticancer activity of VA on ductal breast carcinoma cells.
Materials and Methods
Cells and Chemicals
Human Ductal Carcinoma cell line (BT-549) was a generous gift from Dr. Bryant, DVM of the Institute of Human Virology, University of Maryland School of Medicine, Baltimore, MD. RPMI 1640 Medium, Fetal Bovine Serum (FBS), and Phosphate Buffered Saline (PBS) were purchased from Gibco BRL (Grand Island, NY). [3H]-thymidine (1mCi/ml) was purchased from MP Biomedical (Solon, OH). DNA STAT-60 was purchased from Tel-Test INC (Friendwood, TX). Restriction enzymes Eco RI, Hind III, Bam HI were purchased from New England Biolabs, Inc. DNA Molecular weight marker X was purchased from Roche. ACS grade methonal and other chemicals were obtained from Sigma Chemicals Company (St. Louis, MO. USA).
Sample Collection and Preparation of Aqueous Extracts
Pesticide-free fresh VA leaves were collected in Benin City of Nigeria. The leaves were rinsed with distilled water and spread out evenly on galvanized-wire screens with the edges bent upward 2 inches on all sides. The galvanized-wire screens were placed in a specially-constructed dryer and heated to 130–140 ° F for complete dryness within 4 h. Three hundred (300) g of dried leaves was soaked in 6 L of ddH20 (1:20 w/w) overnight at 40°C before squeezing by hand to a mixture. The mixture was then filtered through clean white gauze to remove the particulate matter before filtration through a 0.45- μm filtration unit for sterilization. The resulting sample solution was lyophilized to dry powder (30 g) on a SpeedVac Concentrator (Savant SC210A), transferred into a 50 mL centrifugation tube and stored at −20° C for bioactivity assays, HPLC, TLC, NMR, UV and IR spectroscopic analyses.
Cell Proliferation Determination
For the determination of VA anti-proliferative effects, the cells were seeded in 100 mm tissue culture plates and allowed to grow to 60% confluence. Before treatment, the medium was aspirated and fresh medium was added. The cells were treated with 10, 100, 1000 μg/mL of VA. The untreated cells were used as controls. After 24 h, medium was aspirated off adherent cells and the resulting monolayer was gently washed with 5 mL of PBS (pH7.4). The cells were collected by trypsinization and re-suspended in RPMI. The cell numbers were determined by counting with a hemacytometer.
[3H] Thymidine Incorporation Studies
DNA synthesis was determined by [3H]-thymindine incorporation assays. BT-549 cells were seeded at a density of 5 × 104 in 35 mm diameter plates. BT-549 cells were allowed to grow to 60% confluence before stimulating the cells with either VA or paclitaxel for 18 h. Treatments included 10, 100, 1000 μg/mL of VA and 10 and 100 nM of TAX. Twenty microliters (2μCi/2mL) of [3H]/35 mm well was added after 18 h incubation and incubated again for 4–6 h at 37°C. All incubations were terminated by aspirating the RPMI medium and doing triplicate washes with 2 mL of cold PBS to remove excess [3H] thymidine. The addition of 2 mL/ well of 10 % cold TCA for 10 minutes at 4°C was done to fix the cells. Following fixation, the cells were washed sequentially three times with RT ddH2O and solubilized by incubation for 30 minutes with 0.5 M NaOH (2 mL/ 35mm) at 37°C. Upon solubilization, 1 mL of cell solution and 5 mL of scintillation cocktail were mixed thoroughly in each vial. Radioactivity was determined using a scintillation counter.
DNA Extraction Methods
VA leaf sample was prepared for homogenization by grinding the VA leaves into a fine powder with a mortar and pestle. To homogenize, DNA STAT 60 was added at a 50:1 ratio (w/v) and mixed to lyse the cell wall. The resulting mixture was then filtered with a strainer to remove any tissue that had not been dissolved. Filtered solution was placed in corning centrifuge tube with 0.2 mL of chloroform per 1 mL of DNA STAT-60 used for homogenization. The sample was covered and shaken vigorously for 15 seconds then left standing at room temperature (RT) for 2 minutes. Centrifugation at 4°C for 15 minutes at 12,000 g causes the homogenate to separate into two phases. The upper aqueous phase was transferred to a new corning tube and mixed with 0.5 mL of isopropanol/mL of the DNA STAT-60 used. The samples were stored at RT for 10 minutes and centrifuged at 12,000 g for 10 minutes at 4°C. The supernatant was removed and the DNA pellet was washed once with 75% ethanol at a 1:1 ratio with DNA STAT 60 used; by vortexing and subsequent centrifugation at 75000 g for 5 minutes at 4°C. The DNA pellet was air-dried briefly for 10 minutes before dissolving in water or EDTA, pH 7.
Endonuclease Digestion
Restriction enzymes Eco RI, Hind III, and Bam HI were used separately to digest VA and Spinacia oleracea (SO) or spinach was used as a positive control. Digestion was done for each reaction that consisted of 10X buffer, restriction enzyme, DNA sample, and water. The prepared digests were incubated at 37°C for 1h. After incubation 10X DNA loading dye was added (never to exceed 1/10 the total volume) to each tube to stop the reaction. The samples were stored at 4°C until needed.
Gel Electrophoresis
Agarose was dissolved with heat in 1X TBE buffer to prepare a 1% gel. The resulting solution was poured into a gel tray and allowed to cool for 20 minutes. The gel tray was placed in the electrophoresis apparatus and 1X TBE running buffer was added to the chamber until it covered the gel completely. The samples were loaded into the gel. The samples were run at 100 volts for 1h. The resulting gel was stained with ethidium bromide for 5 minutes in the dark, and then viewed using UV light.
Statistical Analysis
Experimental replicates within individual experiments were averaged and expressed as mean ± SD. Comparisons between means were determined by unpaired students t-test with 2 tailed P values reported, employing GraphPad statistical software package /27/. Each experiment was replicated three times with comparable results. Data were determined to be statistically significant if values were 0.05 or lower.
Results
Inhibition of BT-549 Cell Proliferation by Aqueous VA Extracts
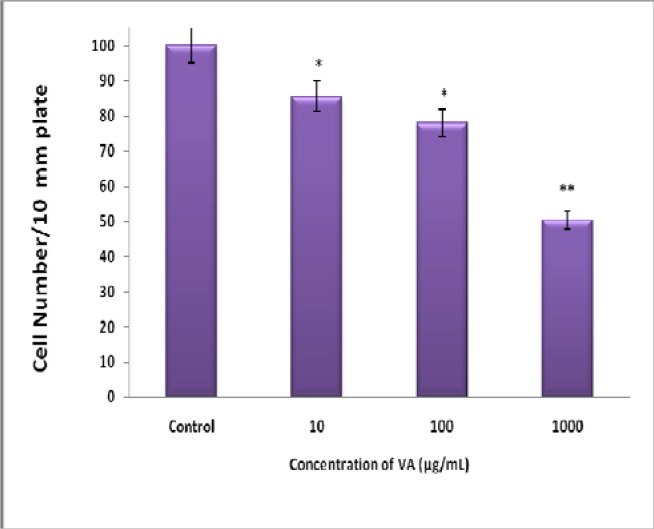
Exposure of BT-549 cells to increasing concentrations of aqueous VA abrogated cell proliferation in a concentration-dependent fashion: VA at concentrations of 10, 100, and 1000 μg/mL inhibited BT-549 cell viability (growth) by 14 % (P<0.05), 22 % (p<0.05), and 50 % (p<0.005) respectively compared to the controls.
The growth-inhibitory activity of VA was confirmed by DNA synthesis assay as DNA synthesis is a requirement for proliferating cells. Furthermore, we, and others [28–29] have previously determined that the doubling time for these cells is less than 24 h.
Inhibition of DNA synthesis by VA Aqueous extracts
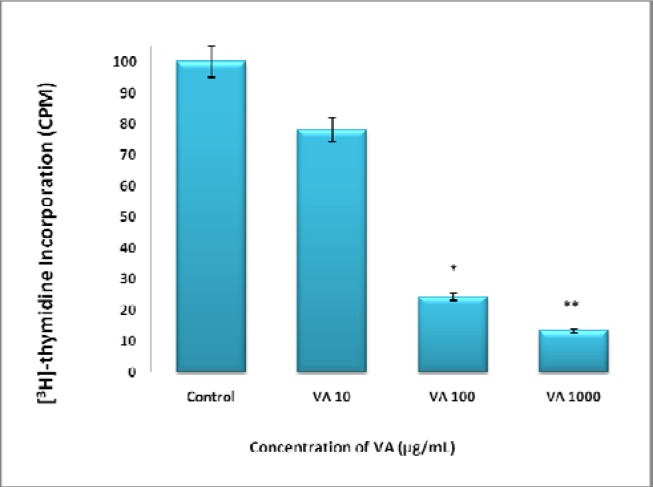
Treatment of BT-549 cells with increasing concentrations of aqueous VA abrogated DNA synthesis in a concentration-dependent fashion: VA at concentrations of 10, 100, and 1000 μg/mL inhibited BT-549 cell viability (growth) by 22 %, 76 % (P<0.05), and 86 % (p<0.01) respectively compared to the controls (Fig. 2).
Figure 2:
Inhibition of DNA synthesis of BT-549 cells by VA extracts. Log phase proliferating cells were treated for DNA synthesis with various concentrations of VA (10 or 1000 μg/mL) for 18 h followed by 6 h treatment of 1 μCi/ mL [3H] thymidine. Uptake was determined as described under materials and methods. Each data point represents the mean ± SD of three independent experiments. *P<0.05 ;**P<0.01.
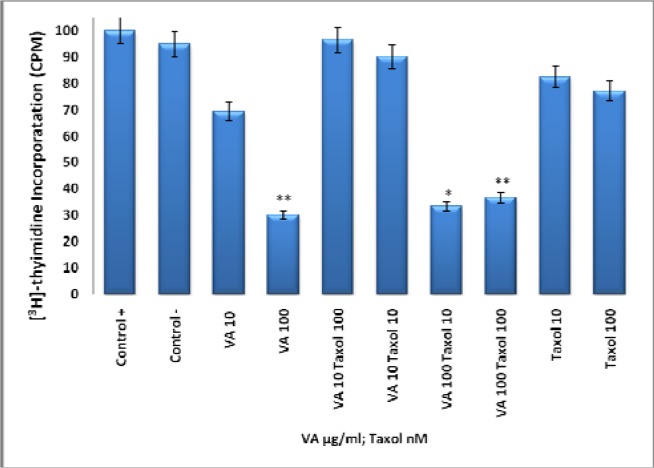
Effect of Taxol, VA, and TAX/VA Combination on DNA Synthesis
To compare the anti-proliferative effects of VA (Fig 1 and 2) to that of Taxol, cells were treated with VA, Taxol, or VA/Taxol combination. VA at10, and 100 μg/ml concentrations inhibited DNA synthesis by 30% and 70% (p <0.005) respectively compared to controls. Taxol at 10 and 100 nM did not elicit any significant effects on DNA synthesis. Furthermore, VA/TAX combination produced neither additive nor synergistic effects (Fig. 3).
Figure 1.
Inhibition of BT-549 Cell Proliferation by Aqueous VA Extracts- cells at logarithmic growth phase were treated with various concentrations (0–1000μg/mL) of VA for 24 h; followed by cell counts using a hemacytometer. Each data point represents the mean of three independent experiments. *P<0.05; **P<0.005.
Figure 3.
Effect of Taxol, VA, and TAX/VA Combination on DNA Synthesis- Log phase proliferating cells were treated for DNA synthesis with various concentrations of VA (0-1000 μg/mL) and TAX (10, 100 nM) for 18 h followed by 6 h treatment of 1μCi/ mL [3H]-thymidine. Uptake was determined as described under materials and methods. Each data point represents the mean ± SD of three independent experiments. *P<0.01; **P<0.005.
Sensitivity of VA Genomic DNA to Endoclease Digestion
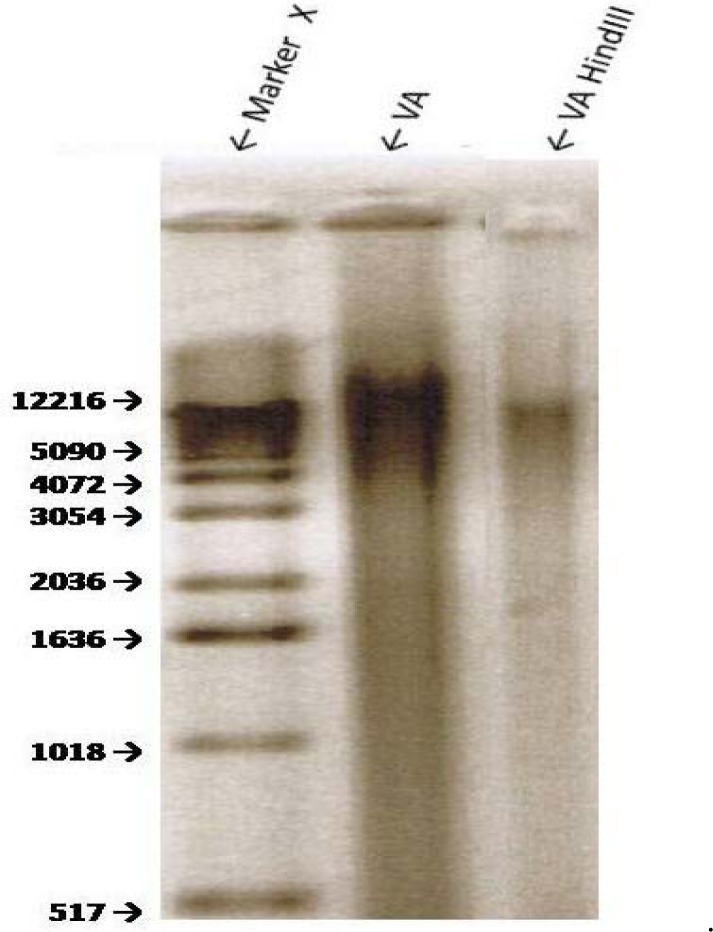
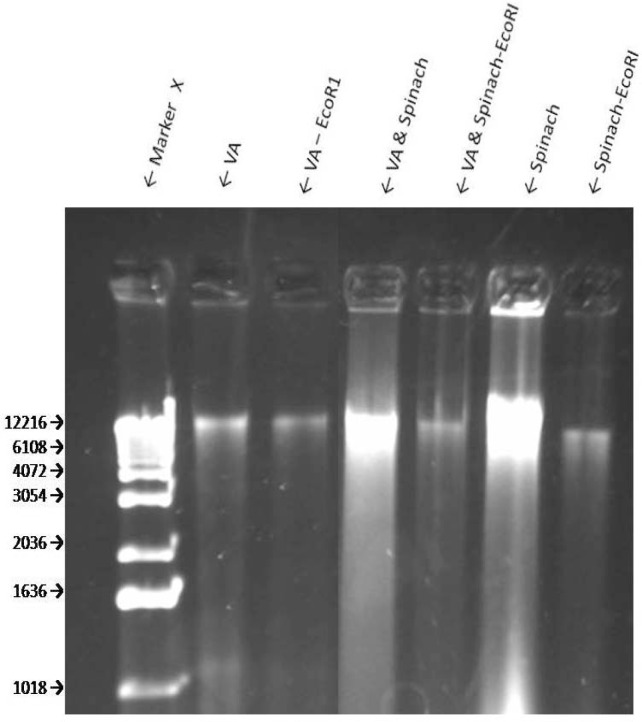
The extraction of dried VA leaves yielded approximately 12.2 and 1 K bp bands of genomic DNA (Fig 4, line 2). Treatment of the genomic DNA with BamHI produced a smaller band of approximately 1.1 kbp (Fig. 4, line 3). Digestion of the VA’s genomic DNA with Hind III resulted in at least two visible fragments: ∼ 10.4 kbp and 1.8 kbp (Fig. 5). EcoR1 digestion of VA DNA resulted in fainter 12.2 and 1 kbp bands which may suggest an incomplete digestion by EcoR1. Spinach (SO) DNA yielded DNA with a range of > 12.2 to 4.0 kbp which shows that SO contains additional and/ or larger DNA than VA; digestion with EcoRI resulted in one band similar in size to that of VA (Fig. 6), thus suggesting the sensitivity of SO DNA to EcoRI digestion.
Figure 4.
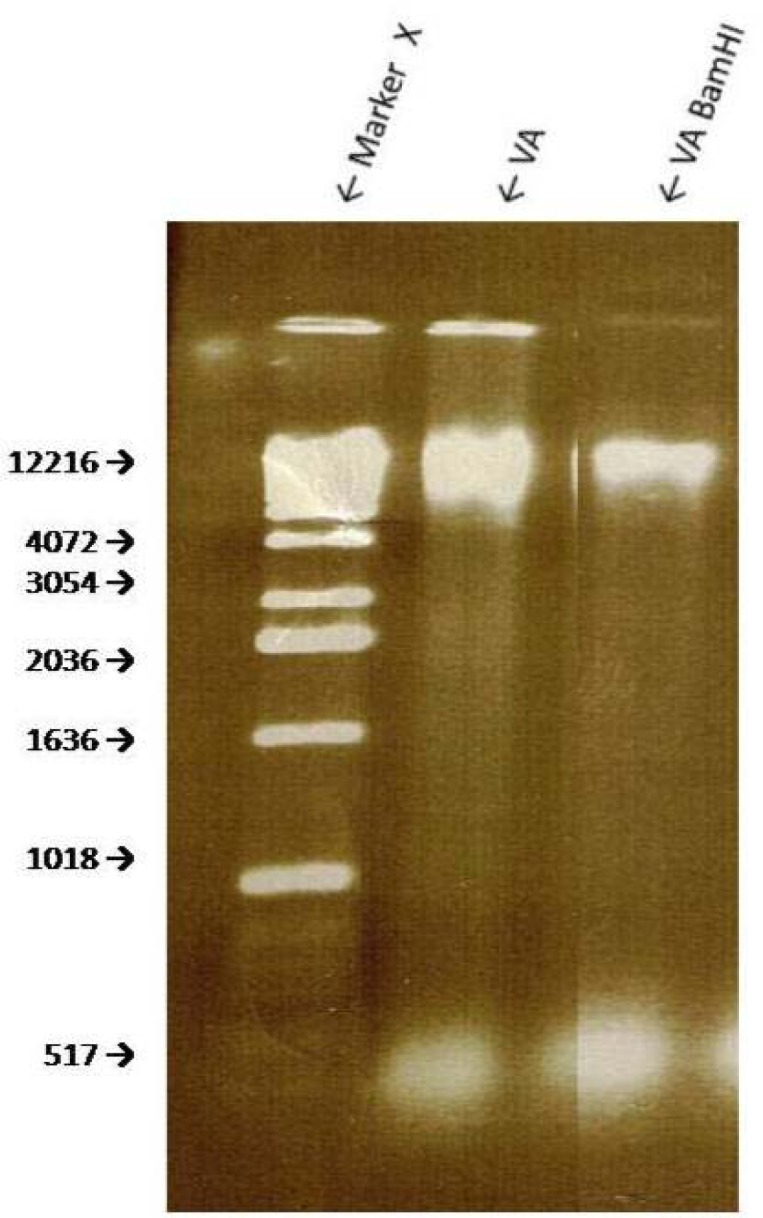
Genomic DNA digestion of VA with restriction enzyme BAM HI. Agarose gel electrophoresis of genomic DNA samples of VA and spinach were digested with restriction enzyme Bam HI. Bam HI cuts at recognition sequence G↓GATCC.
Figure 5.
Genomic DNA digestion of VA with restriction enzyme Hind III. Agarose gel electrophoresis of genomic DNA samples of VA and spinach were digested with restriction enzyme Hind III. Hind III cuts at recognition sequence A↓AGCTT.
Figure 6:
Sensitivity of VA Genomic DNA to Endoclease Digestion- Genomic DNA digestion of VA and S. oleracea with restriction enzyme EcoRI. Agarose gel electrophoresis of genomic DNA samples of VA and spinach were digested with restriction enzyme Eco RI. Eco RI cuts at recognition sequence G↓AATTC.
Discussion
Conventional medicine (CM) is very popular in most developed countries. It is equally important to note that traditional medicine (TM) serves as a major source of healthcare for billions of people in developing countries. However, antagonistic relationships exist between CM and TM practitioners. The contention is that conventional drugs are standardized and chemically-defined; the quantities and structures of the active ingredients are known. Therefore, therapeutic dosages are determinable. In contrast, traditional medicines are often native or non-purified botanical extracts with limited knowledge of their chemical compositions.
There are data to support the medicinal benefits of plant products and extracts. This evidence is predicated on: 1) The observation and/or reports that inverse relationships exist between the consumption of vegetables and risks of developing many types of cancer [30–33] Thus, suggesting the presence of cancer-fighting phytochemicals in plants. 2) Results from cell culture and animal studies show that plant extracts may be used as treatments against many types of cancer. 3) Many highly effective anti-cancer drugs (Paclitaxel or Taxol, Vincrastine, Vinblastine, Camptothecin, Etoposides) representing four classes of anti-cancer drugs (taxanes, camptothecins, vinca, and epipodophyllotoxins) trace their origins directly or indirectly to plants [33]. Therefore, there is a consensus amongst scientists that plant products/extracts do possess active compounds of medicinal benefits. Previous studies have shown that extracts of VA inhibit the growth of cancerous cells [17–22]. Specifically, VA inhibits the growth of human ER+ carcinomas in a dose-dependent fashion in vitro [19, 21–22]. The present study corroborates the anti-cancer properties of VA previously reported by earlier investigators [17–22]. Furthermore, the present study now has extended the VA anticancer lknowledge by showing that VA may also be efficacious in ductal carcinomas that express very little or no estrogen receptor [34]. The mechanisms of VA actions include mitogen-activated protein kinases (MAPKs) or Extracellular signal-regulated kinases (ERKs) activity attenuation [21]. The combination of that fact that VA is edible [9], and has been consumed in large quantities with no reported cases of toxicity, and with emerging scientific evidence for anticancer activity makes it an attractive choice for cancer patients, at least as dietary supplements, to improve their prognosis or quality of life (QOL). This potential acceptance and subsequent increase in popularity of VA usage are also accompanied by issues of quality control and authenticity of VA products. Here, we have provided the initial steps toward the authentication protocol for VA. The extraction of dried VA leaves yielded an approximately 12.2 and 1 K bp bands of genomic DNA (Fig 6, line 2). In contrast similar treatment of SO (spinach) DNA yielded DNA bands with a range of > 12.2 to 4.0 K bp which show that SO contains more/ larger DNA than VA. Furthermore, EcoR1 digestion of VA DNA resulted in fainter 12.2 and 1 K bp bands which may suggest an incomplete digestion by EcoR1. Again, in contrast, digestion of SO DNA with EcoRI resulted in one band similar in size to that of VA (Fig. 6). Thus, suggesting the sensitivity of SO DNA to EcoRI digestion. Taken together, these studies, to the best of our knowledge, provide for the first time evidence of VA and SO genomic DNA to HindIII and EcoRI sensitivitiy respectively. This information may be used in the future as tools for VA and SO authentication.
Acknowledgments
This research was supported in part by the Research Centers in Minority Institutions (RCMI)/NIH grant # 2G12RR013459-11, and in part by the National Center for Minority Health Disparities (NCMHD)/NIH grant # P20MD000534-01. Our appreciation goes to Dr. Joseph L. Bryant (University of Maryland School of Medicine, Baltimore, MD) for his technical assistance.
References and Notes
- 1.American Cancer Society. Cancer Facts & Letters. Atlanta, GA: 2007. [Google Scholar]
- 2.American Cancer Society. Cancer Statistics. 2008. [Google Scholar]
- 3.Dougherty MK, Schumaker LM, Jordan VC, Welshons WV, Curran EM, Ellis MJ, El-Ashry D. Estrogen Receptor Expression and Sensitivity to Paclitaxel in Breast Cancer. Cancer Biology & Therapy, 2004;3(5):460–467. doi: 10.4161/cbt.3.5.810. [DOI] [PubMed] [Google Scholar]
- 4.Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen Receptor A Mediates Breast Cancer Cell Resistance to Paclitaxel through Inhibition of Apoptotic Cell Death. Cancer Research. 2007;67(11) doi: 10.1158/0008-5472.CAN-06-4582. [DOI] [PubMed] [Google Scholar]
- 5.Cunningham JE, Butler WM. Racial disparities in female breast cancer in South Carolina: clinical evidence for a biological basis. Breast Cancer Research and Treatment. 2004;88(2):161–176. doi: 10.1007/s10549-004-0592-9. [DOI] [PubMed] [Google Scholar]
- 6.Ziv E, Tice J, Smith-Bindman R, Shepherd J, Cummings S, Kerlikowske K. Mammographic density and estrogen receptor status of breast cancer. Cancer Epidemiology Biomarkers and Prevention. 2004;13(12):2090–2905. [PubMed] [Google Scholar]
- 7.Woodward WA, Hwang EH, McNeese M.D, Perkins GH, Tucker SL, Strom E.A, Middleton L, Hahn K, Hortobagyi GN, Buchholz TA. African-American race is associated with a poorer overall survival rate for breast cancer patients treated with mastectomy and doxorubicin-based chemotherapy. Cancer. 2006;107(11):2662–8. doi: 10.1002/cncr.22281. [DOI] [PubMed] [Google Scholar]
- 8.Oleszek W, Igile G, Burda S, Jurzysta M. Nutritional assessment of Vernonia amygdalina leaves in growing mice. Journal of Agricultural and Food Chemistry. 1995;4:2162–2166. [Google Scholar]
- 9.Farombi EO. African indigenous plants with chemotherapeutic potentials and biotechnological approach to the production of bioactive prophylactic agents. African Journal of Biotechnology. 2003;2(12):662–671. [Google Scholar]
- 10.Erasto P, Grierson DS, Afolayan AJ. Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J Ethnopharmacology. 2006;106:117–120. doi: 10.1016/j.jep.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Moundipa PF, Kamini G, Flore M, Bilong Bilong CF, Bruchhaus I. In vitro amoebicidal activity of some medicinal plants of the Bamun region (Cameroon) African J Trad CAM, 2005;2(2):113–121. [Google Scholar]
- 12.Akah PA, Ekekwe RK. Ethnopharmacology of some of the asteraceae family used in the Nigerian traditional medicine. Fitoterapia. 1995;66:352–355. [Google Scholar]
- 13.Akah PA, Okafor CL. Blood sugar lowering effects of V. amygdalina Del in an experimental rabbit model. Phytother Res. 1992;6:171–173. [Google Scholar]
- 14.Akinpelu DA. Antimicrobial activity of Vernonia amygdalina leaves. Fitoterapi. 1999;70:432–234. [Google Scholar]
- 15.Krief S, Hladik C, Haxaire C. Ethnomedicinal and bioactive properties of plants ingested by wild chimpanzees in Uganda. Journal of Ethnopharmacology. 2005;101:1–5. doi: 10.1016/j.jep.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Iwalokun BA, Efedede BU, Alabi-Sofunde JA, Oduala T, Magbagbeola OA, Akinwande AI. Hepatoprotective and antioxidant activities of Vernonia amygdalina on acetaminophen-induced hepatic damage on mice. Journal of Medicinal Food. 2006;9(4):524–30. doi: 10.1089/jmf.2006.9.524. [DOI] [PubMed] [Google Scholar]
- 17.Kupchan SM, Hemingway RJ, Karim A, Werner D. Tumor inhibitors. XLVII. Vernodalin and Vernomygdin, two new cytotoxic sesquiterpene lactones from Vernonia amygdalina Del. Journal of Organic chemistry. 1969;34:3908–3911. doi: 10.1021/jo01264a035. [DOI] [PubMed] [Google Scholar]
- 18.Jasaka M, Ohigashi H, Takagaki T, Nozaki H, Tada T, Hiroto M, Irie R, Huffman MA, Nishida T, Kaji M, Koshimizu K. Bitter steroid glucosides, Vernoniosides A1, A2,A3 and related B1 from possible medicinal plant-Vernonia amygdalina used by wild chimpanzees. Tetrahedron. 1992;48(4):625–632. [Google Scholar]
- 19.Izevbigie EB. Discovery of water soluble anticancer agents (Edotides) from vegetables found in Benin City, Nigeria. Exp.Biol.Med. 2003;228:293–298. doi: 10.1177/153537020322800308. [DOI] [PubMed] [Google Scholar]
- 20.Howard CB, Stevens J, Izevbigie EB, Walker A, McDaniel O. Time and dose-dependent modulation of phase 1 and phase 2 gene expression in response to treatment of MCF-7 cells with a natural anti-cancer agent. Cell. Mol. Biol. 2003;49(7):1057–1065. [PubMed] [Google Scholar]
- 21.Izevbigie EB, Bryant JL, Walker A. A novel natural inhibitor of extracellular signal-regulated kinases human breast cancer cell growth. Exp.Bio.Med. 2004;229:163–169. doi: 10.1177/153537020422900205. [DOI] [PubMed] [Google Scholar]
- 22.Opata M, Izevbigie EB. Aqueous V. amygdalina Extracts Alter MCF-7 Cell Membrane Permeability and Efflux. Int. J. Environ. Res. Public Health, 2006;3(2):174–179. doi: 10.3390/ijerph2006030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sayed MD, Zaki AY, El-Marzabai MM, Doss SL. Medicinal Plants. Phytochemistry. 1987;21:944. [Google Scholar]
- 24.Tona L, Cimanga RK, Mesia K, Musuamba CT, De Bruyne T, Apers S, Hermans N, Van Miret S, Pieters L, Totté J, Vlietink AJ. In Vitro Antiplasmodial activity of extracts and fractions of seven medicinal plants used in the Democratic Republic of Congo. Journal of Ethnophamacology. 2004;93(1):27–32. doi: 10.1016/j.jep.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Harborne JB. Phytochemical Methods. London, Chapman and Hall; New York, USA: 1973. p. 278. [Google Scholar]
- 26.Wall ME, Wani MC, Manikumar G, Abraham P, Taylor H, Huges TJ, Warner J, MacGivney R. Plant antimutagenic agents, flavonoids. Journal of Natural Products. 1998;51(6):1084–109. doi: 10.1021/np50060a006. [DOI] [PubMed] [Google Scholar]
- 27.GraphPad Software. GraphPad Software, Inc; Sand Diego, CA, USA: [Google Scholar]
- 28.Aparajita G, Akech J, Mukherjee S, Das SK. Differential expression of cholinephosphotransferase in normal and cancerous human mammary epithelial cells. Biochem. Biophys. Res. 2002;297:1043–1048. doi: 10.1016/s0006-291x(02)02332-x. Comm. [DOI] [PubMed] [Google Scholar]
- 29.Fonagy A, Swiderski C, Ostrovsky AM, Bolton W.E, Freeman J.E. Effect of nuclear P120 expression level on the proliferation capacity of breast cancer cells. Cancer Research. 1994;54:1859–1864. [PubMed] [Google Scholar]
- 30.Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. European Journal of Cancer. 2001;37(8):948–965. doi: 10.1016/s0959-8049(01)00070-3. [DOI] [PubMed] [Google Scholar]
- 31.Hennekens C, Buring JE, Mayrent SL. Epidemiology in Medicine. Vol. 3. Lippincott Williams and Wilkins; 1987. p. 36. [Google Scholar]
- 32.Peto R, Doll R, Buckley JD, Sporn MB. Can dietary beta-carotene materially reduce human cancer rates? Nature. 1981;290:201–208. doi: 10.1038/290201a0. [DOI] [PubMed] [Google Scholar]
- 33.Cragg GM, Newman DJ. International collaboration in drug discovery and development from natural sources. Pure Appl. Chem., 2005;77(11):1923–1942. [Google Scholar]
- 34.Vladusic EA, Hornby AE, Guerra-Vladusic FK, Lakins J, Lupu R. Expression and regulation of estrogen receptor beta in human breast tumors and cell lines. Oncol. Rep. 2000;7(1):157–167. doi: 10.3892/or.7.1.157. [DOI] [PubMed] [Google Scholar]