Abstract
We observed a slight drop in the growth of Xenopus laevis and Pseudacris triseriata larvae following acute exposure (24–48 h) during egg development to three concentrations of TCDD (0.3, 3.0, 30.0 μg/l). Our exposure protocol was modeled on a previous investigation that was designed to mimic the effects of maternal deposition of TCDD. The doses selected were consistent with known rates of maternal transfer between mother and egg using actual adult body burdens from contaminated habitats. Egg and embryonic mortality immediately following exposure increased only among 48 h X. laevis treatments. Control P. triseriata and X. laevis completed metamorphosis more quickly than TCDD-treated animals. The snout-vent length of recently transformed P. triseriata did not differ between treatments although controls were heavier than high-dosed animals. Likewise, the snout-vent length and weight of transformed X. laevis did not differ between control and TCDD treatments. These findings provide additional evidence that amphibians, including P. triseriata and X. laevis are relatively insensitive to acute exposure to TCDD during egg and embryonic development. Although the concentrations selected for this study were relatively high, they were not inconsistent with our current understanding of bioaccumulation via maternal transfer.
Keywords: 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD); polyhalogenated aromatic hydrocarbons (PHAHs); tadpole development
Introduction
In recent years many amphibian populations worldwide have experienced a dramatic and well-documented decline. Habitat loss and fragmentation [1, 2], exposure to UV-B radiation [3], infectious disease [4], pesticides [5–9], climate change [10, 11], and over-collection [12, 13], have all been linked to this decline. In addition, amphibians may be exposed to a variety of polyhalogenated aromatic hydrocarbons (PHAHs) including polychlorinated biphenyls (PCBs) and dioxins. The most potent of these compounds is 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [14], a highly toxic contaminant produced by various industrial processes [15, 16]. TCDD-induced toxicity is largely mediated through the cytoplasmic aryl hydrocarbon receptor (AhR) which is present in all vertebrates [17]. The AhR acts as a ligand-activated transcription factor involved in the metabolism of many drugs and xenobiotic compounds [18]. Upon binding in the cytoplasm, the AhR protein translocates to the nucleus, sheds its chaperone proteins and forms a heterodimer with ARNT (AhR nuclear translocator) [19–23]. This complex regulates the transcription of target genes including cytochrome CYP1A1 (P4501A1), CYP1B1, and NADPH quinone oxidoreductase by binding to cis-acting DNA elements (xenobiotic response elements; XREs) [23–25]. Additional interactions between the AhR complex and other signaling pathways may further influence gene expression [26]. Maternal exposure to TCDD in rats results in offspring with reduced body weight [30–32], genital dysmorphogenesis and decreased fertility [32]. Fish embryos and newly hatched larvae exposed to TCDD exhibit cardiovascular lesions, and suffer from pericardial and yolk sac edema, hemorrhaging and craniofacial malformations [33–35]. TCDD also acts as a potent cardiovascular teratogen in birds [36–38].
Amphibian eggs and larvae appear to be 100-1,000-fold less sensitive to TCDD-induced toxicity than other vertebrates [39, 40]. This may be linked to their ability to rapidly eliminate the compound (T1/2 = 1–7 d) during early development [40]. Amphibians also possess an AhR with a relatively low affinity for TCDD [21]. In amphibians, TCDD-mediated changes in gene expression through the AhR pathway have been linked to the apoptotic cell death of hepatocytes [41], larval erythrocytes [42] and intestinal principal cells [43]. TCDD exposure has also been shown to increase larval mortality [40] and retard growth and development [44, 45] in certain species.
The eggs and larvae of most amphibians develop in freshwater habitats where TCDD and other environmental contaminants tend to accumulate in aquatic sediments [46–47]. Anuran larvae (tadpoles) are highly specialized, herbivorous filter-feeders [48] that may ingest these sediments along with toxicant-laden algae [49]. Adult frogs and salamanders may also prey on invertebrates that emerge from contaminated waters [49]. In oviparous animals, hydrophobic compounds from the female are transferred along with lipids to the eggs [50, 51]. Concentrations of total dioxins in the eggs of Japanese frogs (R. ornativentris and R. japonica) collected from ammunition depot sites ranged from 327–2110 pg/g wet weight [52]. The eggs contained roughly twice the absolute dioxin levels detected in female frogs [52]. Kadokami et al. [52] estimate that roughly two-thirds of the dioxins present in the mother were transferred to the eggs.
Amphibian metamorphosis from an aquatic tadpole to a terrestrial adult is controlled by a surge of thyroid hormones [53, 54]. In rats, TCDD exposure has been linked to a dose-dependent depletion in serum and tissue thyroxine (T4) levels [44]. A similar drop in amphibians could delay metamorphosis and increase mortality as was observed by Gutleb et al. [55, 56] following exposure to PCB. Spring breeders including Psuedacris triseriata may be particularly susceptible because they commonly breed in shallow, temporary pools that often begin to evaporate as tadpoles near transformation. Endocrine-disrupting compounds that retard larval growth may also potentially produce smaller metamorphs that are less likely to reach sexual maturity and reproduce [57].
The primary goal of this investigation was to expose amphibian eggs and embryos to waterborne concentrations of TCDD in an effort to mimic maternal deposition. Egg and embryonic mortality following acute exposure were recorded and larval growth and development were monitored for two species of anurans. One native frog species (P. triseriata) and one exotic (Xenopus laevis), were selected for this comparative study.
Materials and Methods
Egg Collection and Exposure Protocol
All studies were conducted with the approval of the Kent State University Animal Care and Use Committee and in accordance with the Guidelines for the Use of Live Amphibians and Reptiles in Field and Laboratory Research compiled by the Society for the Study of Amphibians and Reptiles. Materials treated with TCDD including solutions, disposable laboratory supplies and animal carcasses were treated as hazardous waste and their disposal was monitored by the university safety office. A modified FETAX exposure protocol [58] was used to determine the effects of TCDD on egg and embryonic mortality and larval growth. In April, 2000 a western chorus frog egg mass (P. triseriata) was collected from a wetland in Summitt Co, Ohio. The mass was returned to the lab and 8 samples containing 75 fertilized eggs (blastula stage) were separated using a dissecting microscope and placed in glass dishes with the following solutions. TCDD obtained from Cambridge Isotope Laboratories, Inc. (98% purity) was originally cut using a 19:1 corn oil:acetone mixture specifically prepared for oral delivery to pregnant rats [59, 60]. From this stock, four solutions of equal volume (20 ml) were prepared in replicates of two (8 total) with dechlorinated water to produce the following concentrations: low, 0.3 μg/l; medium, 3.0 μg/l; high, 30.0 μg/l. Control groups received 120 μl of a straight corn oil:acetone solution matching the highest delivery volume used in this experiment. These doses were originally selected by Jung and Walker [40] as estimates of the maternal deposition of TCDD in amphibians. Because these solutions were nonmiscible in water, all treatments were incubated at room temperature (21° C) for 48 h on a shaker plate to increase the likelihood of even exposure. At the end of this period the developing embryos were removed from solution, rinsed thoroughly in clean water and transferred to larger glass containers containing 500 ml dechlorinated water. At this time the embryos were visually inspected using a dissecting microscope and the mortality for each treatment was recorded as the undeveloped eggs were discarded. The embryos were allowed to continue development and the hatched larvae were not disturbed nor fed during the first five days following exposure. On the 5th day after exposure, 30 larvae from each replicate were transferred to separate 10-gallon aquaria containing 8 L dechlorinated water. Treatment tanks were thus arranged in replicates of two containing 30 tadpoles each. These tanks were equipped with aerators, and housed in an environmental chamber at 21° C under a standard 12:12 light cycle. The water in all treatment tanks was replaced at three-day intervals throughout the developmental period. Tadpoles were fed cooked spinach offered ad libitum beginning 10 d after TCDD exposure and continuing throughout the duration of the experiment. Tadpole mortality was monitored throughout the study and dead larvae were removed, but not replaced when discovered.
Freshly deposited X. laevis eggs were obtained in July, 2000, by injecting the dorsal lymph sac of a sexually mature female with 500 IU human chorionic gonadotropin as described by Sive et al. [61]. The eggs were fertilized directly in high salt MBS solution (pH 7.0) with sperm extracted from the testes of a mature male. The eggs were allowed to enter the second cleavage before 16 samples of 75 eggs were randomly transferred to separate glass dishes containing 1 of the 4 solutions described above. FETAX solution was substituted for dechlorinated water during the exposure. Two replicates of each of the four solutions (8 total) were incubated for 24 h while the other two sets of replicates were incubated for 48 h on a shaker plate at room temperature (21° C). After this period, the eggs were rinsed thoroughly and separated by treatment into 500 ml glass containers of dechlorinated water. The larvae were neither fed, nor disturbed until the 5th day following exposure, at which time 5 larvae were randomly selected from each treatment and individually transferred into separate (16 × 16 × 8 cm) plastic containers with 500 ml dechlorinated water. The containers were aerated and housed in an environmental chamber at standard conditions (21° C; 12:12 light cycle). Starting on day five, the larvae from each treatment were fed identical amounts of Nasco frog brittle (Nasco International, Wisconsin) throughout the developmental period and fresh water was provided at three-day intervals.
Measurements and Data Analysis
Egg mortality between treatment groups was compared separately by species for P. triseriata (48 h), and for each X. laevis (24, 48 h) trial using separate oneway ANOVAs. When these tests proved significant, the differences among specific means were evaluated using a Scheffe post-hoc test (α = 0.05). Sampling began 15 days after TCDD exposure for P. triseriata treatments and continued at 5-day intervals throughout the developmental period. During each sampling interval, five larvae were randomly selected from each treatment replicate and the snout-tail length (mm), weight (g) and the developmental stage [62] of each tadpole were recorded before returning it to its original treatment tank. A one-way MANOVA was used to ensure that the length and weight of tadpoles between treatment replicates did not significantly differ on randomly selected days of the experiment. As a result, the data from both replicates were pooled (n = 10) and the average length and weight for each treatment were calculated (±SE) and plotted for each sampling interval. The effects of both treatment and time on tadpole growth as defined by the dependent variables of length and weight were analyzed using a two-way MANOVA with the Wilks’ lambda criterion. These tests were followed by individual one-way ANOVAs and Scheffe post-hoc tests that analyzed the effects of treatment on length and weight separately for specific sampling dates.
The mortality between treatment replicates was carefully monitored during the developmental period. The tadpoles of both species were raised until those that survived reached metamorphic climax. The s-v length and weight of recently transformed P. triseriata were compared between treatments using separate one-way ANOVAs. Recently transformed animals and any remaining tadpoles that failed to reach metamorphosis by day 75 were euthanized using MS-222 (0.1%). At the end of the study, the final mortality for all treatments was recorded.
Xenopus laevis tadpoles from both 24 and 48 h trials were sampled at five-day intervals beginning seven days after exposure. The snout-tail length (mm) and developmental stage of each tadpole [63] were separately recorded and monitored for 40 days. Tadpoles that had not metamorphosed by day 75 were euthanized as described above, and the final mortality of each treatment was recorded. The effects of treatment and time on tadpole growth as defined by the dependent variable of length were analyzed using a univariate repeated-measures analysis. The Greenhouse-Geisser adjustment was used to correct for the lack of sphericity and the corresponding df and F statistics are presented. Scheffe post-hoc tests were used to evaluate the differences among treatment means on specific sampling days.
Results
Egg Mortality
After 48 h exposure, egg mortality did not differ between control and TCDD treatments for P. triseriata (F = 0.29, df =3, p = 0.83). There were also no differences in egg mortality between X. laevis treatments following 24 h exposure to TCDD (F = 2.27, df = 3, p = 0.22). Egg mortality, however, did differ between X. laevis treatments following 48 h exposure (F = 7.7, df = 3, p = 0.04) and the control eggs experienced less mortality than those exposed to high levels of TCDD (30.0 μg/l). These results are summarized in Table 1.
Table 1.
TCDD-Induced Egg Mortality.
| 24 h X. laevis | Control | Low | Medium | High |
|
| ||||
| (Replicate 1) | 6 | 9 | 12 | 12 |
| (Replicate 2) | 9 | 12 | 12 | 21 |
|
| ||||
| 48 h X. laevis | Control | Low | Medium | High |
|
| ||||
| (Replicate 1) | 9 | 15 | 18 | 24 |
| (Replicate 2) | 6 | 24 | 15 | 30 |
|
| ||||
| 48 h P. triseriata | Control | Low | Medium | High |
|
| ||||
| (Replicate 1) | 25 | 16 | 14 | 15 |
| (Replicate 2) | 10 | 17 | 20 | 10 |
Table shows the number of fertilized eggs of each species that did not complete development following 24 h and 48 h exposure to TCDD. Each treatment consists of two sets of replicates. TCDD treatment concentrations were as follows: low (0.3 μg/l), medium (3.0 μg/l), and high (30.0 μg/l). Control eggs were treated with a 120 μl of a straight corn oil:acetone solution matching the highest delivery volume used in this experiment. Egg mortality is represented as the total number of fertilized eggs (of 75 treated per receptacle) that failed to complete development.
Growth of P. triseriata
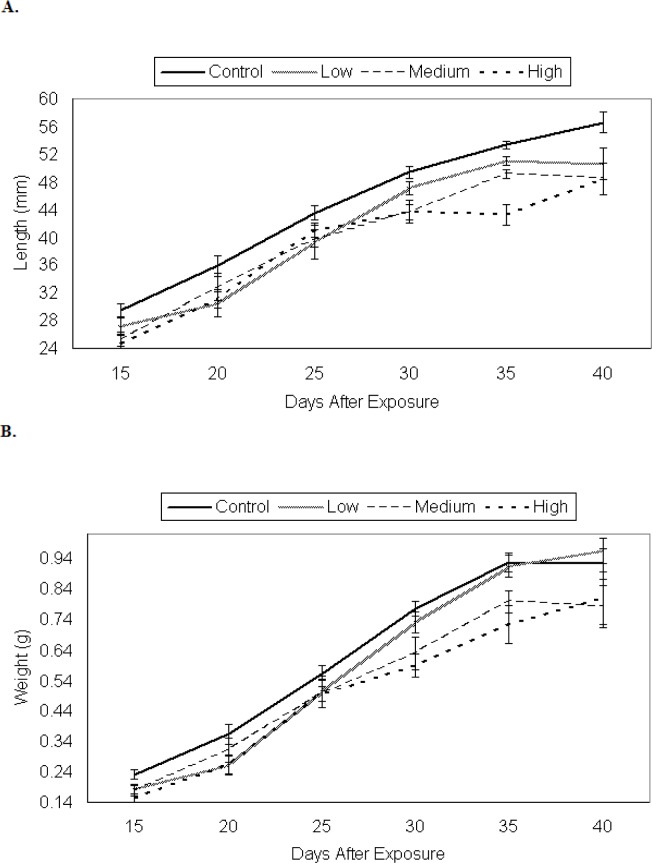
There were significant between-treatment differences in the growth of P. triseriata (Wilks’ Lambda = 0.630, F = 18.7, df = 6, 430, p < 0.001; Fig. 1a, b).
Figure 1.
Larval growth of P. triseriata among control and 48 h TCDD treatments expressed as an increase in, a) total length (mm) and, b) weight (g) over time. Each data point represents the average length (± SE) of ten randomly selected larvae. Eggs were originally exposed for 48 h to waterborne concentrations of TCDD as follows: low (0.3 μg/l), medium (3.0 μg/l), and high (30.0 μg/l). Control P. triseriata were statistically longer, but not heavier than TCDD-treated larvae on the final sampling day (day 40)
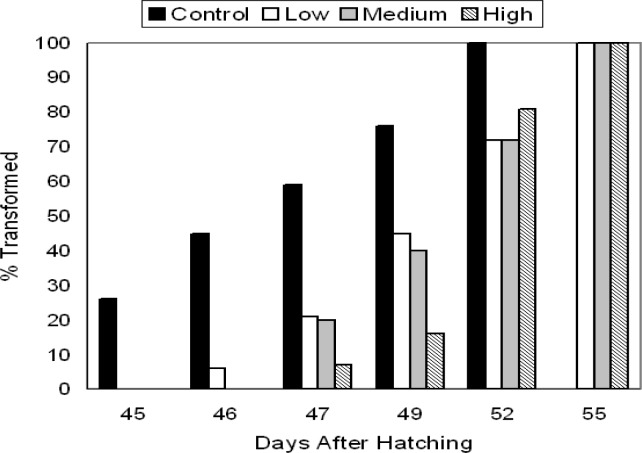
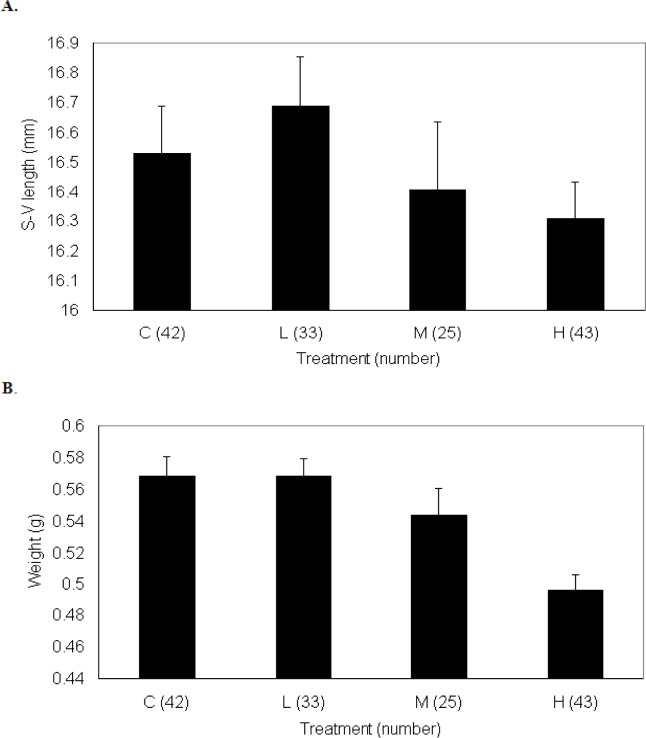
By the last sampling period (day 40), control P. triseriata were statistically longer than tadpoles from other treatments. The developmental stages for tadpoles on days 20 and 40 are shown in Table 2. Control P. triseriata also completed metamorphosis more rapidly than TCDD-treated larvae (Fig. 2). The overall s-v length of recently transformed P. triseriata did not differ between treatments (df = 3, F =1.049, p = 0.37; Fig. 3a), however, treatment differences in metamorph weight were detected (df = 3, F = 8.41, p < 0.001; Fig. 3b). Scheffe analysis indicated that high-dosed animals were statistically lighter than controls. Mortality between treatments remained low throughout the 40 day sampling period. At no time did the density between treatment tanks differ by > ± 5 tadpoles. The number of P. triseriata tadpoles that died or failed to reach metamorphosis by the conclusion of the study (day 75) were similar between treatments (18 control, 27 low, 35 medium, 17 high).
Table 2.
Developmental Stage of Larvae
| X. laevis (24 h) | X. laevis (48 h) | ||||||||
|
| |||||||||
| Day 22 | Day 22 | ||||||||
|
| |||||||||
| Stage | C | L | M | H | Stage | C | L | M | H |
| Stage 49 | - | 1 | - | 1 | Stage 49 | - | 1 | - | - |
| Stage 50 | 1 | 1 | 1 | - | Stage 50 | 1 | 1 | 2 | 2 |
| Stage 51 | 1 | 2 | 3 | 1 | Stage 51 | 3 | 2 | 2 | 2 |
| Stage 52 | 3 | 1 | 1 | 3 | Stage 52 | 1 | 1 | 1 | 1 |
| Day 32 | Day 32 | ||||||||
| Stage | C | L | M | H | Stage | C | L | M | H |
| Stage 51 | - | 1 | - | - | Stage 51 | - | - | - | - |
| Stage 52 | 1 | 1 | 1 | 1 | Stage 52 | - | 1 | 1 | 2 |
| Stage 53 | - | 2 | - | 1 | Stage 53 | 1 | 1 | - | - |
| Stage 54 | 3 | - | 3 | 2 | Stage 54 | 2 | 2 | 3 | 2 |
| Stage 55 | 1 | 1 | 1 | 1 | Stage 55 | 2 | 1 | 1 | 1 |
|
| |||||||||
| P. triseriata (48 h) | P. triseriata (48 h) | ||||||||
|
| |||||||||
| Day 20 | Day 40 | ||||||||
|
| |||||||||
| Stage | C | L | M | H | Stage | C | L | M | H |
| Stage 31 | - | 1 | 1 | 1 | Stage 37 | − | 2 | 2 | 3 |
| Stage 32 | 1 | 3 | 3 | 3 | Stage 38 | 1 | 4 | 2 | 3 |
| Stage 33 | 3 | 3 | 4 | 4 | Stage 39 | 2 | 2 | 4 | 3 |
| Stage 34 | 2 | 2 | 1 | 1 | Stage 40 | 4 | 2 | 2 | 1 |
| Stage 35 | 4 | 1 | 1 | 1 | Stage 41 | 3 | - | - | - |
Figure 2:
Time (days) required for 48 h control and TCDD-treated P. triseriata larvae to reach metamorphosis. Control P. triseriata completed metamorphosis more rapidly than TCDD- treated animals. Eggs were originally exposed for 48 h to waterborne concentrations of TCDD as follows: low (0.3 μg/l), medium (3.0 μg/l), and high (30.0 μg/l).
Figure 3.
Size at metamorphosis of P. triseriata control and TCDD-treated animals measured in terms of, a) snout-vent length (mm), and, b) weight (g). Eggs were originally exposed for 48 h to waterborne concentrations of TCDD as follows: low (0.3 μg/l), medium (3.0 μg/l), and high (30.0 μg/l). *The number within parentheses represents the total number of metamorphosed frogs for each treatment.
Developmental stage of control and TCDD-treated X. laevis tadpoles following either 24 h or 48 h exposure. The developmental stage is based on Nieuwkoop and Faber (1994) and is presented for sampling days 22 and 32. The developmental stage of control and TCDD-treated P. triseriata tadpoles following 48 h exposure is also presented for sampling days 20 and 40. These stages are based on Gosner (1960). All observations were made using a dissecting microscope. The TCDD treatment concentrations for both species were as follows: low (0.3 μg/l), medium (3.0 μg/l), and high (30.0 μg/l). Control eggs were treated with a 120 μl of a straight corn oil:acetone solution matching the highest delivery volume used in this experiment.
Growth of X. laevis
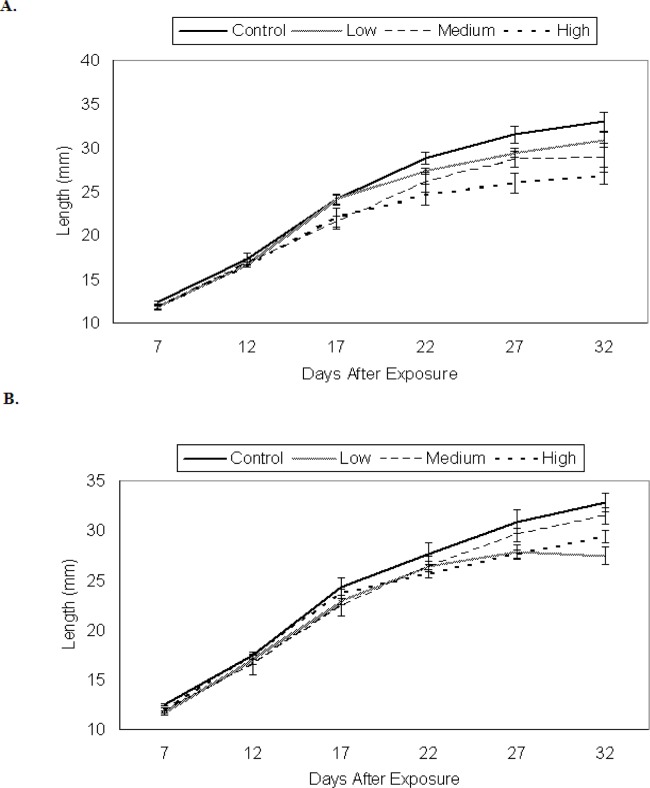
The results of the X. laevis trials also exhibited significant time-treatment interactions following both 24 h (G-G = 91, F = 4.2, df = 6.5, p = 0.002; Fig. 4a) and 48 h (G-G = 72, F = 4.5, df = 9.8, p < 0.001; Fig. 4b) exposure.
Figure 4.
Larval growth of X. laevis expressed as an increase in total length (mm) over time for, a) control and 24 h TCDD treatments and, b) control and 48 h TCDD treatments. *Each point represents the average length of the five larvae (± SE) from each treatment. Eggs were originally exposed for either 24 or 48 h to waterborne concentrations of TCDD as follows: low (0.3 μg/l), medium (3.0 μg/l), and high (30.0 μg/l). At the final sampling period (32 days post exposure), control tadpoles for both 24 and 48 h trials were significantly longer than the animals from the slowest growing treatment. For the 24 h trial, control tadpoles were significantly longer than those exposed to the high dose (30.0 μg/l) of TCDD. For the 48 h trial, control tadpoles were significantly longer that those exposed to the low dose (0.3 μg/l) of TCDD.
At the final sampling period (32 days post exposure), control tadpoles for both 24 and 48 h trials were significantly longer than the animals from the slowest growing treatment. For the 24 h trial, control tadpoles were significantly longer than those exposed to the high dose (30.0 μg/l) of TCDD. For the 48 h trial, control tadpoles were significantly longer that those exposed to the low dose (0.3 μg/l) of TCDD. No tadpoles from either trial died prior to the last sampling date (day 32). The developmental stages for tadpoles on days 22 and 32 for both 24 and 48 h trials are shown in Table 2. Although the majority of control tadpoles from both 24 h and 48 h trials completed metamorphosis (Stage 66) prior to most TCDD-treated animals, the sample size was small and the data are not reported. The s-v length and body weight of transformed X. laevis did not differ between treatments of either 24 h (df = 2, F = 2.56, p = 0.14; df = 2, F = 0.24, p = 0.79) or 48 h (df = 3, F = 0.38, p = 0.77; df = 3, F = 0.04, p = 0.99) trials. By day 75, the number of tadpoles that failed to reach metamorphosis or had died was similar between 24 h (1 control, 2 low, 1 medium and 5 high) and 48 h (2 control, 2 low, 3 medium and 3 high) treatments.
Discussion
TCDD and Egg Mortality
Jung and Walker [40] observed no increase in the mortality rates of American toad, Bufo americanus, and green frog, R. clamitans, larvae following 24 h egg-exposure to TCDD (0.3–30.0 μg/l). However, in the same study, R. pipiens eggs exposed to 3.0 μg/l TCDD for 24 h exhibited significantly increased mortality (10%) over controls < 1.5 days after hatching. Jung and Walker [40] suggested that the increased mortality among leopard frog tadpoles may have resulted from exposing the eggs at an earlier developmental period (16-cell stage) than either B. americanus or R. clamitans (neurula, stage 13). In this study, P. triseriata eggs were exposed at the intermediate blastula stage, while X. laevis eggs were exposed earlier at the two-cell stage. Unlike Jung and Walker [40], no increase in egg or early embryonic mortality was observed following 24 h (X. laevis) or 48 h (P. triseriata) exposure even to higher concentrations of TCDD (30.0 μg/l). Only X. laevis eggs exposed for 48 h experienced a statistical increase in mortality between control and TCDD treatments (Table 1). These data suggest that prolonged exposure to TCDD may increase egg and embryonic mortality among X. laevis. It is unclear whether these data reflect true species-specific differences in sensitivity to TCDD.
The AhR signaling pathway of X. laevis is particularly well documented [21, 64–67]. Lavine et al. [21] identified two AhR receptors (AhR1α, AhR1β) with a 20-fold lower-affinity for TCDD than certain strains of mice. Variations in the AhR between amphibian species and other vertebrates may better explain fluctuations in TCDD-induced toxicity [21]. For example, Gutleb et al. [56] documented a dose-dependent increase in tail and eye deformities among X. laevis but not R. temporaria larvae following long-term oral exposure to PCB. These findings suggest that ranid tadpoles may be less susceptible to PHAH-mediated malformations than X. laevis[56] and are consistent with earlier trials in which other ranid species appear relatively insensitive to TCDD exposure [68]. This apparent heightened sensitivity of X. laevis compared to other amphibian species has also been observed following larval exposure to the herbicide diuron and the organophosphate insecticide, azinphosmethyl [69, 70]. Mann and Bidwell [46] also found that feeding stage tadpoles of X. laevis were more sensitive to agricultural surfactants than four species of Australian frogs.
TCDD Effects on Larval Growth and Metamorphosis
Most studies that have found significant levels of TCDD-induced effects including higher incidents of mortality, followed prolonged periods of exposure. Mima [71] observed extensive edema and higher rates of mortality among X. laevis tadpoles that were originally exposed to 200 ppb TCDD for 5 days immediately following fertilization. Using a similar exposure protocol, Sakamoto et al. [42] found a significant rise in erythrocyte apoptosis among TCDD-treated X. laevis 12 days after fertilization. Dell’Orto et al. [45] noted a 50% increase in mortality of stage 48 X. laevis following 120 h exposure to 0.35 μg/l TCDD. Likewise, McKinney et al. [44] found that stage 51 X. laevis reared in medium (0.5 μg/l) and high (1.0 μg/l) concentrations of TCDD died 16–23 days into exposure.
In this study, there was a slight, yet significant inhibition of larval growth of both species following a shorter exposure duration (24–48 h) to various concentrations of TCDD (0.3–30.0 μg/l) (Figs. 1a,b; 4a,b). McKinney et al. [44] noted a significant dose-related decrease in the length of stage 51 X. laevis tadpoles exposed to medium (0.5 μg/l) and high (1.0 μg/l) concentrations of TCDD prior to their death. Tadpoles dosed at lower concentrations (0.1 μg/l) grew more quickly than control animals during the first 16 days of a 30 day exposure period. Dell’Orto et al. [45] also found a significant dose-related decrease in the length of X. laevis tadpoles immediately following 96 h TCDD (0.4–0.5 μg/l) exposure. Ironically, these same animals exhibited accelerated growth over controls beginning 13 days after exposure and ultimately completed metamorphosis more rapidly than treated animals. Likewise, B. americanus larvae exposed as eggs to ≥0.3 μg/l TCDD for 24 h also grew and metamorphosed more quickly and at larger body sizes than control animals [40]. This initial period of accelerated growth was not observed in this study for either species regardless of concentration or exposure duration.
Control P. triseriata tadpoles completed metamorphosis more quickly than treated animals (Fig. 2). These findings are in apparent conflict with those of past investigations [40, 44, 45]. However, of these studies, only Jung and Walker [40] utilized a similar exposure technique. In their study, radio-labeled TCDD cut with 0.7% acetone was rapidly absorbed in a linear fashion during the first 2 h of exposure and reached a plateau between 9 and 24 h. Most of the TCDD passed through the jelly coat and was detected in the embryo proper. Following 24 h exposure, they compared the concentration of TCDD detected in the eggs with the concentration of TCDD in the water to calculate the bioconcentration factor (BCF). Depending on species, the eggs absorbed between 1 and 7 times the nominal TCDD water concentration [40]. A similar exposure protocol was adopted for this study, although, TCDD was initially diluted using a corn-oil:acetone solution (19:1) specifically prepared for oral delivery to pregnant rats [59, 60]. Unfortunately, no BCFs were calculated for this study as neither the concentration of TCDD from the egg-bath solutions, nor the subsequent levels absorbed by the eggs were independently validated. The slight inhibition of larval growth across all trials suggests that the nonmiscible solution used in this study did not impede TCDD absorption. The relatively modest effects we observed may in part be linked to a similarly rapid rate of elimination [T1/2 = 1–7 d] following exposure [40].
Post-metamorphic growth normally accounts for 80–99% of total adult body mass in anurans [57]. In this study, P. triseriata that reached metamorphosis were statistically similar in length regardless of treatment (Fig. 3a). However, control metamorphs were heavier than high-dosed animals (Fig. 3b). Reduced body size in tadpoles has been correlated with slower swimming speeds and higher predation risks [72, 73]. In addition, delayed metamorphosis as observed in this study, could be particularly damaging to species such as P. triseriata that depend on rapid development to reach transformation before the shallow breeding pools evaporate. At present, the possible effects of TCDD and other endocrine-disrupting compounds on post-metamorphic growth remains poorly understood [74].
Significant concentrations of TCDD and other PHAHs have been detected in wild amphibians from contaminated habitats. Adult bullfrogs (R. catesbeiana), collected near a trichlorophenoxyacetic acid plant in Arkansas possessed TCDD levels between 87 ppt in muscle to 68,000 ppt in fat [75]. Southern toads (B. terrestris) from habitats adjacent to a Florida airbase were found to possess whole-body TCDD concentrations of 1,360 ppt after chemical spraying [76]. At a hazardous waste site in New York, whole-body concentrations of PCBs in bullfrogs reached 49.6 ppm [77], while the wet weight in livers from green frogs (R. clamitans) at a similar site in South Carolina was as high as 41.5 ppm [78]. Maternal transfer of dioxins and other PCBs to the eggs is now recognized as a major source of bioaccumulation. Roughly two-thirds of these compounds may be transferred from the female to the eggs during each spawning event [52]. Although high, the doses selected for this experiment were not unreasonable especially considering the adult body burdens of TCDD collected from contaminated habitats.
Conclusions
The results our study provide additional evidence that amphibians are relatively insensitive to acute exposure to TCDD even at relatively high concentrations [40, 45]. In fish, early-embryonic exposure to TCDD is characterized by vascular dysfunction, yolk-sac and heart edema, craniofacial malformations and a drop in growth rate [79–81]. In comparison, amphibian eggs and larvae appear to be 100–1,000-fold less sensitive to TCDD-induced toxicity than other vertebrates [39, 40]. The amphibian AhR has a relatively low affinity for TCDD [21, 23] and the compound is rapidly eliminated during embryonic development [40]. As Guttleb et al. [55] observed for PCB trials, the effects of TCDD exposure on larval amphibians is closely linked to the route, timing and duration of exposure. It is therefore unclear whether the differences in growth and development observed in this and other studies are due to variations in exposure protocol or subtle differences in the sensitivity of these species to TCDD at differing stages of development. In natural populations, amphibians may bioaccumulate TCDD and other PHAHs, through contact with contaminated sediments, the food web, and through maternal deposition. Higher body burdens during larval development could ultimately disrupt the precise neural and hormonal control mechanisms associated with metamorphosis [55]. At present, it is difficult to extrapolate the results of short-term studies such as our own to long-term population effects without conducting additional tests under more realistic ecological conditions [82].
Acknowledgments
This research was partially funded by a grant from the Declining Amphibian Population Task Force (DAPTF) and the financial support of the Department of Biological Sciences, Kent State University. We thank Travis Salisbury, Jenny Marcinkiewicz, Doug Kline and Mark Moser for their invaluable assistance. The comments from two anonymous reviewers helped to improve the quality of this manuscript. Finally, we recognize the help of D. W. Waller and thank him for the use of his cement Elvis.
References
- 1.Davidson D, Shaffer HB, Jennings MR. Declines of the California red-legged frog: Climate, UV-B, habitat, and pesticides hypotheses. Ecological Applications. 2001;11:464–479. [Google Scholar]
- 2.Marsh DM, Trenham PC. Metapolulation dynamics and amphibian conservation. Conservation Biology. 2001;15:40–49. [Google Scholar]
- 3.Blaustein AR, Romansic JM, Kiesecker JM, Hatch AC. Ultraviolet radiation, toxic chemicals and amphibians population declines. Diversity and Distributions. 2003;9:123–140. [Google Scholar]
- 4.Daszak PA, Cunningham AA, Hyatt AD. Infectious disease and amphibian population declines. Diversity and Distributions. 2003;9:141–150. [Google Scholar]
- 5.Ankley GT, Tietge JE, Defoe DL, Jensen KM, Holcombe GW, Durhan EJ, Diamond SA. Effects of ultraviolet light and methoprene on survival and development of Rana pipiens. Environ. Toxicol. Chem. 1998;17:2530–2542. [Google Scholar]
- 6.Berrill M, Coulson D, McGillivray L, Pauli B. Toxicity of endosulfan to aquatic stages of anuran amphibians. Environ. Toxicol. Chem. 1998;17:1738–1744. [Google Scholar]
- 7.La Clair JJ, Bantle JA, Dumont J. Photoproducts and metabolites of a common insect growth regulator produce developmental deformities in Xenopus. Environ. Sci. Technol. 1998;32:1453–1461. [Google Scholar]
- 8.Mann RM, Bidwell JR. The toxicity of glyphosphate and several glyphosphate formulations to four species of southwestern Australian frogs. Arch. Environ. Contam. Toxicol. 1999;36:193–199. doi: 10.1007/s002449900460. [DOI] [PubMed] [Google Scholar]
- 9.Saka M. Acute toxicity tests on Japanese amphibian larvae using thiobencarb, a component of rice paddy herbicides. Herpetological Journal. 1999;9:73–81. [Google Scholar]
- 10.Kiesecker JM, Blaustein AR, Belden LK. Complex causes of amphibian population declines. Nature. 2001;410:681–684. doi: 10.1038/35070552. [DOI] [PubMed] [Google Scholar]
- 11.Carey C, Alexander MA. Climate change and amphibian declines: is there a link? Diversity and Distributions. 2003;9:111–121. [Google Scholar]
- 12.Jennings MR, Hayes MP. Pre-1900 overharvest of California (USA) Red-legged frogs (Rana aurora draytoni): The inducement for bullfrog (Rana catesbeiana) introduction. Herpetologica. 1985;41:94–103. [Google Scholar]
- 13.Lannoo MJ, Lank K, Waltz T, Phillips GS. An altered amphibian assemblage: Dickinson County, Iowa, 70 years after Frank Blanchard’s survey. American Midland Naturalist. 1994;131:311–319. [Google Scholar]
- 14.van den Berg M, Birnbaum L, Bosveld BTC, Brunstrom B, Cook P, Feeley M, Giesy JP, Hanberg A, Hasegawa R, Kennedy SW, et al. Toxic equivalency factors (TEFs) for PCBs, PCDDs, and PCDFs for humans and wildlife. Environ. Health Persp. 1998;106:775–792. doi: 10.1289/ehp.98106775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olie K. Chlorodibenzo-p-dioxins and chlorodibenzofurans are trace components of fly ash and flue gas of some municipal incinerators in the Netherlands. Chemosphere. 1980;9:501–522. [Google Scholar]
- 16.Environmental Protection Agency (EPA) Exposure and human health reassessment of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds: National Academy of Sciences (NAS) review draft. Accessed March 25, 2008, from http://www.epa.gov/ncea/pdfs/dioxin/nas-review/
- 17.Hahn ME, Stegeman JJ. Phylogenic distribution of the Ah receptor in non-mammalian species: Implications for dioxin toxicity and Ah receptor evolution. Chemosphere. 1992;25:931–937. [Google Scholar]
- 18.Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit. Rev. Toxicol. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman EC, Reyes H, Chu FF, Sander F, Conley LH, Brooks BA, Hankinson O. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science. 1991;252:954–958. doi: 10.1126/science.1852076. [DOI] [PubMed] [Google Scholar]
- 20.Hahn ME. Mechanisms of innate and acquired resistance to dioxin-like compounds. Rev. Toxicol. 1998;2:395–443. [Google Scholar]
- 21.Lavine JA, Rowatt AJ, Klimova T, Withington AJ, Dengler E, Beck C, Powell WH. Aryl hydrocarbon receptors in the frog Xenopus laevis: Two AhR1 paralogs exhibit low affinity for 2,3,7,8-tetrachlordibenzo-p-dioxin (TCDD) Toxicol. Sci. 2005;881:60–72. doi: 10.1093/toxsci/kfi228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vadja AM, Norris DO. Effects of steroids and dioxin (2,3,7,8-TCDD) on the developing wolffian ducts of the tiger salamander (Ambystoma tigrinum) Gen. Comp. Endocrinol. 2005;141:1–11. doi: 10.1016/j.ygcen.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann AL, King E, Dengler E, Scogin SR, Powell WH. An aryl hydrocarbon receptor repressor from Xenopus laevis: Function, expression and role in dioxin responsiveness during frog development. Toxicol. Sci. 2008;104(1):124–134. doi: 10.1093/toxsci/kfn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hankinson O. Role of coactivators in transcriptional activation by the aryl hydrocarbon receptor. Arch. Biochem. Biophys. 2005;433:379–386. doi: 10.1016/j.abb.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 25.Petrulis JR, Kusnadi A, Ramadoss P, Hollingshead B, Perdew GH. The hsp90 co-chaperone XAP2 alters importin β recognition of the bipartite nuclear localization signal of the Ah receptor and represses transcriptional activity. J. Biol. Chem. 2003;278(4):2677–2685. doi: 10.1074/jbc.M209331200. [DOI] [PubMed] [Google Scholar]
- 26.Puga A, Tomlinson CR, Xia Y. Ah receptor signals cross-talk with multiple developmental pathways. Biochem. Pharmacol. 2005;69:199–207. doi: 10.1016/j.bcp.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 27.Puga A, Marlowe J, Barnes S, Chang C, Maier A, Tan Z, Kerzee KJ, Chang X, Strobeck M, Knudsen ES. Role of the aryl hydrocarbon receptor in cell cycle regulation. Toxicol. 2002;181–182:171–177. doi: 10.1016/s0300-483x(02)00276-7. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt JV, Bradfield CA. Ah receptor signaling pathways. Annu. Rev. Cell. Dev. Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 29.Hankinson O. The aryl hydrocarbon receptor complex. Annu. Rev. Pharmacol. Toxicol. 1995;2:395–443. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 30.Birnbaum LS. Developmental effects of dioxins and related endocrine disrupting chemicals. Toxicol. Lett. 1995;35:307–340. doi: 10.1016/0378-4274(95)03592-3. [DOI] [PubMed] [Google Scholar]
- 31.Pohjanvirta R, Tuomisto J. Short-term toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in laboratory animals: Effects, mechanisms, and animal models. Pharmacol. Rev. 1994;46:483–549. [PubMed] [Google Scholar]
- 32.Salisbury TB, Marcinkiewicz JL. In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-pdioxin and 2,3,4,7,8-pentachlorodibenzofuran reduces growth and disrupts reproductive parameters in female rats. Biol. Repro. 2002;66:1621–1626. doi: 10.1095/biolreprod66.6.1621. [DOI] [PubMed] [Google Scholar]
- 33.Spitsbergen JM, Walker MK, Olson JR, Peterson RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat. Toxicol. 1991;19:41–72. [Google Scholar]
- 34.Cantrell SM, Lutz LH, Tillitt DE, Hannink M. Embryotoxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD): The embryonic vasculature is a physiological target for TCDD-induced changes in DNA damage and apoptotic cell death in medaka (Oryzias latipes) Toxicol. Appl. Pharmacol. 1996;141:23–34. doi: 10.1006/taap.1996.0256. [DOI] [PubMed] [Google Scholar]
- 35.Youngchul K, Cooper KR. Toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polychlorinated biphenyls (PCBs) in the embryos and newly hatched larvae of the Japanese medaka (Oryzias latipes) Chemosphere. 1999;39(3):527–538. [Google Scholar]
- 36.Ivnitski I, Elmaoued R, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary development is preceeded by a decrease in myocyte proliferation and an increase in cardiac apoptosis. Teratology. 2001;64:201–212. doi: 10.1002/tera.1065. [DOI] [PubMed] [Google Scholar]
- 37.Ivnitski-Steele ID, Walker MK. Vascular endothelial growth factor rescues 2,3,7,8-tetrachlorodibenzo-p-dioxin inhibition of coronary vaculogenesis. Birth Defects Res. A Clin. Mol. Teratol. 2003;67:496–503. doi: 10.1002/bdra.10074. [DOI] [PubMed] [Google Scholar]
- 38.Ivnitski-Steele ID, Friggens M, Chavez M, Walker MK. 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) inhibition of coronary vasculogenesis is mediated, in part, by reduced responsiveness to endogenous angiogenic stimuli, including vascular endothelial growth factor A (VEGF-A) Birth Defects Res. A Clin. Mol. Teratol. 2005;73:440–446. doi: 10.1002/bdra.20137. [DOI] [PubMed] [Google Scholar]
- 39.Mima S, Sakamoto MK, Tanimura T. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on development in Xenopus laevis after continuous exposure from blastula to larval stage. Environ. Sci. 1993;6:221–227. [Google Scholar]
- 40.Jung RE, Walker MK. Effects of 2,3,7,8-tetrachlorodibenzo-p-Dioxin (TCDD) on Development of Anuran Amphibian Environ. Toxicol. Chem. 1997;16(2):230–240. [Google Scholar]
- 41.Sakamoto MK, Mima S, Tanimura T. A morphological study of liver lesions in Xenopus larvae exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) with special reference to apoptosis of hepatocytes. J. of Env. Path. Toxicol. Oncol. 1995;14(2):69–82. [PubMed] [Google Scholar]
- 42.Sakamoto MK, Mima S, Takahashi KP, Tanimura T. Apoptotic cell death of erythrocytes in Xenopus larvae exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Pathol. 1997;25:398–402. doi: 10.1177/019262339702500409. [DOI] [PubMed] [Google Scholar]
- 43.Sakamoto MK, Mima S, Tanimura TA. Apoptosis of the intestinal principal cells of Xenopus larvae exposed to 2,3,7,8-tetrachlorodibenzo-pdioxin. J. Environ. Pathol. Toxicol. Oncol. 1999;18:289–295. [PubMed] [Google Scholar]
- 44.McKinney JD, Fawkes J, Jordan S, Kae K, Oatley S, Coleman RE, Briner W. 2,3,7,8-Tetrachloro-p-dioxin (TCDD) as a potent and persistent thyroxine agonist: A mechanistic model for toxicity based on molecular reactivity. Env. Health Pers. 1985;61:41–53. doi: 10.1289/ehp.856141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dell’Orto N, Cantelli D, Urani C. Cellular targets in response to dioxin exposure. Chemosphere. 1998;37(14–15):2809–2821. doi: 10.1016/s0045-6535(98)00323-3. [DOI] [PubMed] [Google Scholar]
- 46.Mann RM, Bidwell JR. The acute toxicity of agricultural surfactants to the tadpoles of four Australian and two exotic frogs. Environ. Pollut. 2001;114:195–205. doi: 10.1016/s0269-7491(00)00216-5. [DOI] [PubMed] [Google Scholar]
- 47.Environmental Protection Agency (EPA) Ground water and drinking water. Technical factsheet on: Dioxin (2,3,7,8-TCDD) 2008. Accessed March 25. from http://www.epa.gov/cgi-bin/epaprintonly.cgi.
- 48.Duellman WE, Trueb L. The Biology of Amphibians. The Johns Hopkins University Press; Baltimore: 1986. Part 1 Life History: Ch. 6 Larvae; p. 167. [Google Scholar]
- 49.Huang Y, Karasov WH, Patnode KA, Jefcoate CR. Exposure of northern leopard frogs in the Green Bay ecosystem to polychlorinated biphenyls, polychlorinated dibenzo-p-dioxins, and polychlorinated dibenzofurans is measured by direct chemistry but not hepatic ethoxyresorufin-odeethylase activity. Environ. Chem. 1999;18(10):2123–2130. doi: 10.1002/etc.5620181002. [DOI] [PubMed] [Google Scholar]
- 50.Kleinow K, Baker J, Nichols J, Gobas F, Parkerton T, Muir D, Monteverdi G, Mastrodone P. Exposure, uptake and disposition of chemicals in reproduction and developmental stage of selected oviparous vertebrates. In: Di Giulio RT, Tillitt DE, editors. Reproductive and Developmental Effects of Contaminants in Oviparous Vertebrates. SEATAC Press; Pensacola, Florida: 1999. pp. 9–111. [Google Scholar]
- 51.Russell RW, Gobas FAPC, Haffner GD. Maternal transfer and in ovo exposure of organochlorines in oviparous organisms: A model and field verification. Environ. Sci. Technol. 1999;33:416–420. [Google Scholar]
- 52.Kadokami K, Takeishi M, Kuramoto M, Ono Y. Maternal transfer of organochlorine pesticides, polychlorinated dibenzo-p-dioxins, dibenzofurans, and coplanar polychlorinated buphenyls in frogs to their eggs. Chemosphere. 2004;57:383–389. doi: 10.1016/j.chemosphere.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 53.Gudernatsch JF. Feeding experiments on tadpoles. I. The influence of specific organs given as food on growth and differentiation. A contribution to the knowledge of organs with internal secretion. Arch. Entwickl. Org. 1912;35:457. [Google Scholar]
- 54.Mondou PM, Kaltenbach JC. Thyroxine concentrations in blood serum and pericardial fluid of metamorphosing tadpoles and of adult frogs. Gen. Comp. Endocrinol. 1979;39:343. doi: 10.1016/0016-6480(79)90131-x. [DOI] [PubMed] [Google Scholar]
- 55.Gutleb AC, Appelman J, Bronkhorst MC, van den Berg JHJ, Spenkelink A, Brouwer A, Murk AJ. Delayed effects of pre- and early-life time exposure to polychlorinated biphenyls (PCBs) on tadpoles of two amphibian species (Xenopus laevis and Rana temporaria) Environ. Toxicol. Pharmacol. 1999;8(1):1–14. doi: 10.1016/s1382-6689(99)00023-x. [DOI] [PubMed] [Google Scholar]
- 56.Gutleb AC, Appleman J, Bronkhorst M, van den Berg JHJ, Murk AJ. Effects of oral exposure to polychlorinated biphenyls (PCBs) on the development and metamorphosis of two amphibian species (Xenopus laevis and Rana temporaria) Sci. Total Env. 2000;262:147–157. doi: 10.1016/s0048-9697(00)00598-2. [DOI] [PubMed] [Google Scholar]
- 57.Werner EE. Amphibian metamorphosis: Growth rate, predation rate and optimal size at metamorphosis. Am. Nat. 1986;128:319–341. [Google Scholar]
- 58.ASTM (American Society for Testing and Materials) ASTM E1439-98. Annual Book of ASTM Standards; Philadelphia: 1998. Standard Guide for Conducting the Frog Embryo Teratogenesis Assay—Xenopus(FETAX) [Google Scholar]
- 59.Mably TA, Moore RW, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Effects on androgenic status. Toxicol. Appl. Pharmacol. 1992;114:97–107. doi: 10.1016/0041-008x(92)90101-w. [DOI] [PubMed] [Google Scholar]
- 60.Mably TA, Moore RW, Peterson RE. In utero and lactational exposure of male rats to 2,3,7,8-tetrachlorodibenzo-p-dioxin: Effects on sexual behavior and the regulation of luteinizing hormone secretion in adulthood. Toxicol. Appl. Pharmacol. 1992;114:108–117. doi: 10.1016/0041-008x(92)90102-x. [DOI] [PubMed] [Google Scholar]
- 61.Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 2000. [Google Scholar]
- 62.Gosner N. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- 63.Nieuwkoop PD, Faber J. Normal Tables of Xenopus laevis(Daudin) Garland Publishing, Inc.; New York and London: 1994. [Google Scholar]
- 64.Fujita Y, Ohi H, Murayama N, Saguchi K, Higuchi S. Molecular cloning and sequence analysis of cDNAs coding for 3-methyl-cholanthreneinducible cytochromes P450 in Xenopus laevis liver. Arch. Biochem. Biophys. 1999;371:24–28. doi: 10.1006/abbi.1999.1425. [DOI] [PubMed] [Google Scholar]
- 65.Bollerot K, Angelier N, Coumailleau P. Molecular cloning and embryonic expression of the Xenopus Arnt gene. Mech. Dev. 2001;108:227–231. doi: 10.1016/s0925-4773(01)00488-9. [DOI] [PubMed] [Google Scholar]
- 66.Rowatt AJ, DePowell JJ, Powell WH. ARNT gene multiplicity in amphibians: Characterization of ARNT2 from the frog Xenopus laevis. J. Exp. Zool. 2003;300B:48–57. doi: 10.1002/jez.b.45. [DOI] [PubMed] [Google Scholar]
- 67.Ohi H, Fujita Y, Miyao M, Saguchi K, Murayama N, Higuchi S. Molecular cloning and expression analysis of the aryl hydrocarbon receptor of Xenopus laevis. Biochem. Biophys. Res. Commun. 2003;307:595–599. doi: 10.1016/s0006-291x(03)01244-0. [DOI] [PubMed] [Google Scholar]
- 68.Beaty PW, Holscher MA, Neal RA. Toxicology of 2,3,7,8-tetra-chlorodibenzo-p-dioxin in larvae and adult forms of Rana catesbeiana. Bull. Environ. Contam. Toxicol. 1976;16:578–581. doi: 10.1007/BF01685367. [DOI] [PubMed] [Google Scholar]
- 69.Schuytema GS, Nebeker AV, Griffis WL. Comparative toxicity of Guthion and Guthion 2S to Xenopus laevis and Pseudacris regilla tadpoles. Bull. Environ. Contam. Toxicol. 1995;54:382–388. doi: 10.1007/BF00195109. [DOI] [PubMed] [Google Scholar]
- 70.Schuytema GS, Nebeker AV. Comparative toxicity of diuron on survival and growth of Pacific treefrog, bullfrog, red-legged frog and African clawed frog embryos and tadpoles. Arch. Environ. Contam. Toxicol. 1998;34:370–376. doi: 10.1007/s002449900332. [DOI] [PubMed] [Google Scholar]
- 71.Mima S. Effects of continuous exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (2,3,7,8-TCDD) from the cleavage to the larval stageon the development of the Xenopus laevis. Acta. Med. Kinki. Univ. 1997;22:217–233. [Google Scholar]
- 72.Travis J. Variation in growth and survival of Hyla gratiosa larvae in experimental enclosures. Copeia. 1983;1:232–237. [Google Scholar]
- 73.Richards SJ, Bull CM. Size-limited predation on tadpoles of three Australian frogs. Copeia. 1990:1041. [Google Scholar]
- 74.Carey C, Bryant CJ. Possible interrelations among environmental toxicants, amphibian development, and decline of amphibian populations. Env. Health Pers. 1995;103(Supp.4):13–17. doi: 10.1289/ehp.103-1519280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Korfmacher WA, Hansen EB, Jr, Rowland KL. Tissue distribution of 2,3,7,8-TCDD in bullfrogs obtained from a 2,3,7,8-TCDD–contaminated area. Chemosphere. 1986;15:121–126. [Google Scholar]
- 76.Young AL, Cockerham LG. Fate of TCDD in field ecosystems-Assesment and significance for human exposures. In: Kamrin MA, Rodgers PW, editors. Dioxins in the Environment. Hemisphere Publ. Corp.; New York: 1985. pp. 153–171. [Google Scholar]
- 77.Watson MR, Stone WB, Okoniewski JC, Smith LM. Wildlife as monitors of the movement of polychlorinated biphenyls and other organochlorine compounds from a hazardous waste site. Trans. Northeast Fish Wildlife Conf. 1985:91–104. Hartford, Conneticut. [Google Scholar]
- 78.Fontenot LW, Noble GP, Atkins JM, Stephens MD, Cobb GP. Bioaccumulation of polychlorinated biphenyls in ranid frogs and northern water snakes from a hazardous waste site and a contaminated watershed. Chemosphere. 2000;40:803–809. doi: 10.1016/s0045-6535(99)00329-x. [DOI] [PubMed] [Google Scholar]
- 79.Cantrell SM, Joy-Schlezinger J, Stegeman JJ, Tillitt DE, Hannink M. Correlation of 2,3,7,8-tetrachlorodibenzo-p-diozin-induced apoptotic cell death in the embryonic vasculature with embryotoxicity. Toxicol. Appl. Pharmacol. 1998;148:24–34. doi: 10.1006/taap.1997.8309. [DOI] [PubMed] [Google Scholar]
- 80.Spitsbergen JM, Walker MK, Olson JR, Peterson RE. Pathologic alterations in early life stages of lake trout, Salvelinus namaycush, exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin as fertilized eggs. Aquat. Toxicol. 1990;19:41–72. [Google Scholar]
- 81.Elonen GE, Spehar RL, Holcombe G, Johnson RD, Fernandez JD, Erickson RJ, Tietge JE, Cook PM. Comparative toxicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin to seven freshwater fish species during early life-stage development. Environ. Toxicol. Chem. 1998;17(3):472–483. [Google Scholar]
- 82.Relyea RA, Mills N. Predator-induced stress makes the pesticide carbaryl more deadly to gray treefrog tadpoles. Proc. Nat. Acad. Sci. 2001;98(5):2491–2496. doi: 10.1073/pnas.031076198. [DOI] [PMC free article] [PubMed] [Google Scholar]